Abstract
Acoustic telemetry was used to examine habitat use and movement of two sympatric gamefishes, red drum (Sciaenops ocellatus) and spotted seatrout (Cynoscion nebulosus), at two spatial scales (habitat and bay) within an estuarine complex. Habitat-scale tracking (~ 1 m–1 km) based on an acoustic positioning system revealed that seagrass was used extensively by both species. Red drum also commonly associated with oyster reef and boundaries between habitat types. Spatial overlap between the two species was limited and indicative of habitat partitioning; red drum were commonly observed in the shallow, inner lagoon and spotted seatrout in the deeper, open bay portion of the array. Conspicuous diel shifts were also observed for spotted seatrout; fish transitioned from seagrass to bare substrate and displayed greater rates of movement at night than day. Bay-scale (1–50+ km) tracking over a two-year period primarily showed limited movement within bays; however, directed bay-scale movements by both species were observed during winter and spring, when a small contingent of individuals moved up to 70 km from original tagging locations. Habitat use and movement were species specific and subject to temporal variation, both diel and seasonal. Habitat-scale connectivity was influenced by seascape structure and water depth, and bay-scale connectivity was generally limited, suggesting the sustainability of these fisheries is likely influenced by local conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evaluation of habitats and regions occupied by fishes during early life is needed to help prioritize conservation and restoration efforts because resources supporting these efforts are generally limited (Beck et al. 2001). Determining the relative value of habitats is often complicated by the complex arrangement of habitat types or patches within aquatic seascapes (Grober-Dunsmore et al. 2009). Habitat patches of varying sizes, shapes, and water depths can be functionally connected as a part of larger mosaics and collectively serve as essential early life habitat (Nagelkerken et al. 2014). In response, detailed assessments of habitat use and fish movement are needed to determine the relative value and functional role of habitat types or regions used during early life (Beck et al. 2001).
A variety of approaches has been used to assess habitat use and movement of estuarine fishes, and acoustic telemetry has become popular due to its improved spatial and temporal resolution over traditional techniques such as mark-recapture or fishery-independent sampling (Cunjak et al. 2005; Heupel et al. 2006). Strategically placed receivers can provide information about habitat use, residency, movement, and population connectivity. Traditionally, passive telemetry data lacked the spatial resolution needed to determine fine-scale habitat use, indicating only presence within a receiver’s detection range (Heupel et al. 2006). Currently, high-density arrays of acoustic receivers with overlapping detection ranges, commonly referred to as acoustic positioning systems, provide researchers the ability to triangulate an individual’s position with high accuracy (~1–2 m, Espinoza et al. 2011a). Moreover, data from acoustic positioning systems have been combined with high-resolution maps to elucidate habitat use and connectivity of fishes within estuarine seascapes (Espinoza et al. 2011b; Farrugia et al. 2011; Furey et al. 2013; Dance and Rooker 2015).
Acoustic telemetry was used here to examine habitat use and movement of adolescent (defined here as age 1+) red drum (Sciaenops ocellatus) and spotted seatrout (Cynoscion nebulosus) within a large estuarine complex in Texas, USA. Red drum and spotted seatrout coexist in estuaries during the first 1–3 years of life, supporting recreational fisheries of considerable economic value (Brown-Peterson et al. 2002; Wilson and Nieland 1994). The larval and early juvenile stages of both red drum and spotted seatrout are well studied (e.g., McMichael and Peters 1989; Rooker and Holt 1997; Stunz et al. 2002b; Neahr et al. 2010), and it is known that newly settled individuals are commonly associated with seagrass and salt marsh (Spartina alterniflora) (Rooker et al. 1998a, b; Stunz et al. 2002a, Dance and Rooker 2016). However, comparable information on fish-habitat relationships and movement of adolescents at different spatial scales is limited (Adams and Tremain 2000; MacRae and Cowan 2010; Dance and Rooker 2015). The aim of our study was to characterize (1) habitat-scale and short-term (daily, diel) habitat use and (2) bay-scale and long-term (seasonal) movement patterns of two sympatric estuarine fishes, spotted seatrout and red drum.
Methods
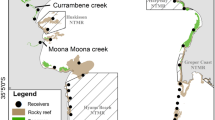
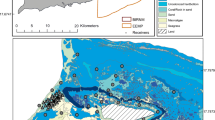
The study was conducted in a large estuarine complex comprised of a series of bays located on the central coast of Texas, USA (Fig. 1). Most of the estuary falls within the Mission-Aransas National Estuarine Research Reserve (MANERR), and seascapes are characterized by submerged habitats such as seagrasses, oyster reef, and non-vegetated substrate. The most common seagrasses, shoal grass (Halodule wrightii) and turtle grass (Thalassia testudinum), are widespread throughout, while oyster reef is mainly concentrated in Copano Bay, Carlos Bay, Mesquite Bay, and the northern region of Aransas Bay. This estuarine system receives variable but limited freshwater input, and hypersaline conditions may occur during periods of drought (Mohan and Walther 2015).
a Map of bay-scale acoustic array within a large estuarine complex along the central coast of Texas, USA. The Mission-Aransas National Estuarine Research Reserve (MANERR) extends north from the indicated boundary. b Map of Mud Island (location within Aransas Bay indicated with black box (a)) and the site of the habitat-scale array. c Layout of habitats within the habitat-scale array
Mud Island, the site of the habitat-scale array, is characterized by emergent smooth cordgrass (S. alterniflora) and black mangrove (Avicennia germinans) that shelter a shallow inner lagoon from the open expanses of Aransas Bay (Fig. 1). The array encompassed an area of approximately 145,000 m2, including a variety of habitat types and a bathymetry gradient from open bay to inner lagoon. Habitat types were classified using orthorectified satellite imagery verified by ground observation. Selected habitat boundaries as well as a grid of over 600 points were examined in the field to record habitat type and water depth. Habitats included seagrass (mixed beds with overall proportion of 71 % shoal grass H. wrightii, 25 % turtle grass T. testudinum, and 4 % manatee grass Syringodium filiforme), oyster reef, and mud or sand sediment with limited seagrass coverage (<15 %) hereafter collectively referred to as “bare” substrate. Recorded depths were corrected by tidal height following the method of Furey et al. (2013) and interpolated throughout the study site using universal kriging in the Spatial Analyst extension of ArcGIS 10.0 (ESRI, Redlands, CA). Water depth in the inner lagoon was generally less than 1 m, even during the highest tides. Water depth increased gradually on the open bay side, where the array encompassed maximum depths of 2–3 m.
Habitat-scale tracking at Mud Island was conducted using an acoustic positioning system, Vemco Positioning System (VPS); VPS uses receivers with overlapping detection ranges to triangulate individual fish positions (Espinoza et al. 2011a). Synchronization transmitters (“sync tags”; Vemco V13-1H) programmed with a random delay of 500–700 s were co-located with each receiver to calibrate and correct for time drift of the receiver internal clocks. Our habitat-scale array consisted of 20 acoustic receivers (Vemco VR2W) deployed with conservative 60–100-m spacing based on the published detection range of internal transmitters in similar estuarine environments (Dance et al. 2016). Two stationary control transmitters (Vemco V9-1H) were also deployed within the array (one in the inner lagoon and one in the open bay) for the duration of the study to monitor diel trends in detection efficiency of the system. The habitat-scale array was in place for 1 month, with tagging initiated on June 11, 2013.
Bay-scale tracking was performed with an array of 45 receivers (Vemco VR2W) distributed across the region from Corpus Christi Bay to Mesquite Bay, including a portion of the MANERR (Fig. 1). Receivers were also positioned at the two primary tidal passes connecting the system to the GoM (Packery Channel and Aransas Pass; Fig. 1). Receivers were bolted to wooden posts (e.g., channel markers) or cable-tied to polyvinyl chloride (PVC) pipe. Bay-scale tracking was conducted from May 2013 to May 2015, when expected transmitter life had expired. Receivers were serviced and downloaded semi-annually, and occasional losses occurred during the study such that 39 receivers remained at the conclusion.
Prior to tagging, both red drum and spotted seatrout were captured via hook-and-line and placed in coolers filled with seawater and supplied with pure oxygen. Transmitters were inserted through a small incision parallel to the linea alba between anal and pelvic fins, and one or two interrupted stitches with absorbable sutures (4–0 Ethicon vicryl) were used to close the wound (Reese Robillard et al. 2015). Transmitters, sutures, and surgical tools were disinfected in a benzalkonium chloride solution prior to use. Fish total length (TL) was measured to the nearest millimeter, and Hallprint dart tags offering anglers a reward for reporting recaptured fish were applied at the junction of first and second dorsal fins. Individual fish were observed for at least 15 min following surgery and released only if they exhibited normal behavior throughout.
Individuals tagged and released at the habitat-scale array (red drum n = 14; spotted seatrout n = 15) were implanted with transmitters (Vemco V9-1H) programmed with a random delay of 100–180 s for the first 20 days, which then converted to a random delay of 400–500 s (estimated battery life = 500 days). No additional spotted seatrout were tagged following the initial release group. To assess bay-scale movement, additional red drum were tagged and released at 12 locations throughout the study system (Fig. 1) in July (n = 20) and November/December (n = 20). These individuals were implanted with Vemco V9-1H transmitters programmed with a random delay of 400–500 s (estimated battery life = 530 days). Fish were assigned a year class based on age-length keys reported by Porch et al. (2002) for red drum and Nieland et al. (2002) for spotted seatrout. Age classes were designated based on estimated age at the end of the calendar year in which individuals were tagged. While red drum tagged in this study were considered immature, we acknowledge that some of the spotted seatrout were likely transitioning to sexual maturity at the time of tagging or while at large (285 mm TL at 50 % maturity; Brown-Peterson et al. 2002); therefore, for the purposes of this study, we refer to all individuals as adolescents.
Data Analysis
Two methods for position estimation were employed to assess habitat-scale movement: VPS and short-term center of activity (COA). VPS uses differences in the arrival time of a single transmission detected by three or more receivers to triangulate an animal’s positions (Espinoza et al. 2011a). Position estimates were filtered by horizontal positioning error (HPE), a dimensionless measure derived from sync tag positioning success and local environmental conditions affecting the speed of sound (Roy et al. 2014). Only estimates with an HPE <10 were used for statistical analysis. These values corresponded to actual position errors of approximately ≤2 m based on comparing HPE to known positioning error for sync tags and control transmitters (1.26 ± 0.03 m, mean ± SE). Hourly COA positions were estimated by calculating the arithmetic means of the latitude and longitude of the receiver(s) detecting a fish during each hour period as described by Simpfendorfer et al. (2002). Detections recorded in the first 2 h after release were excluded from analyses to reduce the influence of release location on fish position and allow for post-surgery acclimation to the study site. To investigate temporal variability in habitat use and movement, VPS estimates were binned by diel stage: day, night, or crepuscular (defined as 1 h before and 1 h after sunrise and sunset). Sunrise and sunset information for Port Aransas, Texas, was downloaded from the National Oceanic and Atmospheric Administration (NOAA) National Weather Service, and tidal information for Port Aransas was downloaded from the NOAA Tides and Currents database.
Habitat use was analyzed using Euclidean distance-based analysis (EDA; Conner and Plowman 2001). A minimum convex polygon that included all position estimates (calculated using either method) was used as the boundary delineating available habitat for EDA analysis. EDA ratios from VPS estimates were calculated using the distances from individual fish positions to each available habitat type compared against the distances to these habitat types for a distribution of 1000 random points (Conner and Plowman 2001). Boundaries between all habitat types (“habitat edges”) were merged and categorized as a distinct habitat type for this analysis. Ratios were calculated as the mean observed distance (from fish positions) divided by the mean expected distance (from random points) to each habitat type. A unique EDA ratio was calculated for each habitat type for each fish, retaining the individual as the experimental unit. If habitat use is completely random, the EDA ratio is expected to be equal to one, with values >1 indicating positions farther from a habitat type than expected (“less use”) and values <1 indicating positions closer to a habitat type than expected (“greater use”). Multivariate analysis of variance (MANOVA) was used to determine whether EDA ratios for each habitat type differed from a vector of 1s with a length equal to the number of habitat types investigated (Conner and Plowman 2001). When overall habitat use was non-random as indicated by a significant MANOVA test, analysis of variance (ANOVA) was employed to test each habitat type specifically for disproportionate use by comparing its mean EDA ratio to 1. The level of significance (α) was set at 0.05 for all statistical testing.
Total habitat-scale tracking duration was calculated as the number of days between the first and last detections for each fish in the habitat-scale array. Residency index was defined as the number of days an individual was detected by at least one receiver (minimum of two detections per day) divided by the maximum number of days the fish could have been detected (Afonso et al. 2009). Two sample t tests were used to test for differences in total tracking duration and residency between species. Rate of movement (ROM) was calculated as the linear distance between fish positions divided by time elapsed. Rates were only calculated if successive positions occurred within a 17-min period, the time interval required to encompass two successive detections following transmitter conversion to a random delay of 400 to 500 s (20 days post release). This restriction reduced the possibility of underestimating distance traveled due to missing locations. Because ROM data for each diel period existed only for a limited number of individuals, these data were pooled for each species. Water depth at each VPS and COA position was estimated by correcting interpolated depths derived from field observation by predicted tidal height (Furey et al. 2013), and two sample t tests were used to test for differences in depth preference between the two species.
Total bay-scale tracking duration was calculated as the number of days between release and the last known fish detection. Detections from the habitat-scale array at Mud Island were included in total tracking duration but excluded from bay-scale detection totals, and individual fish were only included in analyses if they were detected at least 10 days post release. Differences in tracking duration and number of detections between age classes of red drum were assessed using two sample t tests. For each month, individuals were classified as staying or moving. An individual was categorized as staying if detected by only one receiver during the month, with a minimum span of at least 7 days between the first and last detection. An individual was considered moving if detected by more than one receiver during the month. When individuals met either of these criteria in the same calendar month during different years, both outcomes were included in staying or moving totals.
Distance traveled between fish positions was estimated using the cost path function in ArcGIS 10.0, which calculated minimum through water distance. Total distance traveled at the bay scale was estimated as the sum of these movement distances for each individual. Final displacement was also calculated using the cost path function and estimated as the minimum through water distance between release location and the last known fish position. Analysis of covariance (ANCOVA) with days at large as a covariate was used to test for differences in total distance traveled and final displacement between age classes of red drum. The proportion of detections that occurred at an individual’s “home receiver,” defined as a receiver located within 1 km of an individual’s release site, was calculated only for individuals released at such proximity to a receiver.
Results
Habitat Scale
A total of 14 red drum (319–434 mm) and 15 spotted seatrout (240–308 mm) were tagged and released in the habitat-scale array at Mud Island (Table 1). Of these, 14 of 14 (100 %) red drum and 14 of 15 (93.3 %) spotted seatrout were detected on multiple days. Receivers recorded a total of 48,292 detections: 13,433 for red drum and 34,859 for spotted seatrout. Detections yielded 1540 VPS positions (170 red drum and 1370 spotted seatrout) and 3026 hourly COA positions (829 red drum and 2197 spotted seatrout). After data filtering for HPE and acclimation period, 167 red drum and 1310 spotted seatrout VPS positions were available for statistical analysis.
Most VPS positions for red drum were located over seagrass (52.7 %); fewer positions occurred over bare substrate (29.9 %) and oyster reef (17.4 %) (Fig. 2). Red drum COA positions indicated more even use of seagrass (40.7 %) and bare substrate (47.0 %). Fewer COA positions were located over oyster reef (12.3 %), consistent with observations for VPS positions.
Habitat-scale position estimates of red drum and spotted seatrout calculated using a Vemco positioning system (VPS) and b short-term center of activity (COA). Dashed lines indicate water depth contours, and the solid line represents the boundary delineating available habitat for analysis of habitat selection
Nearly all spotted seatrout VPS positions were located over seagrass (51.9 %) or bare substrate (48.0 %) (Fig. 2). COA positions for spotted seatrout also were almost exclusively over seagrass or bare substrate (> 99 %); however, occurrence over bare substrate (77.1 %) was higher than seagrass (22.2 %) (Fig. 2).
Analysis of EDA ratios indicated non-random habitat use by both red drum (MANOVA; p = 0.021) and spotted seatrout (MANOVA; p < 0.001). Red drum were found significantly closer than expected to seagrass (EDA = 0.39; ANOVA; p = 0.036), oyster reef (EDA = 0.40; ANOVA; p = 0.005), and habitat edge (EDA = 0.41; ANOVA; p = 0.006) (Fig. 3). Mean distance of red drum to bare substrate was not significantly different from random (EDA = 0.77; ANOVA; p = 0.529).
Mean Euclidean distance-based analysis (EDA) ratios comparing distance to each habitat type for VPS positions of red drum (n = 6) and spotted seatrout (n = 6) against distance to each habitat type for a distribution of 1000 random points. EDA ratio = 1 (represented by dashed line) indicates habitat use is random, EDA ratio <1 indicates relatively greater use, and EDA ratio >1 indicates relatively less use. Asterisks represent significant difference from expected use of each habitat type, and error bars represent ±1 standard error of the mean
Spotted seatrout were found significantly closer than expected to bare substrate (EDA = 0.44; ANOVA; p = 0.009) and significantly farther than expected from oyster reef (EDA = 1.44; ANOVA; p = 0.018). Mean distance of spotted seatrout to seagrass (EDA = 0.80; ANOVA; p = 0.553) and habitat edge (EDA = 0.80; ANOVA; p = 0.311) were not significantly different from random.
Water depth was another factor that influenced occurrence within the array, and mean water depth differed significantly between species based on both VPS (t test; p < 0.001) and COA positions (t test; p < 0.001). Mean water depth estimates (±SE) for red drum (VPS = 50.6 ± 8.5 cm; COA = 53.4 ± 5.3 cm) were shallower than for spotted seatrout (VPS = 143.6 ± 9.7 cm; COA = 132.1 ± 10.5 cm) (Fig. 4). Moreover, red drum were more frequently detected in the inner lagoon (VPS = 82.6 ± 12.7 %; COA = 85.5 ± 6.2 %) than the open bay side of the array (VPS = 17.4 ± 12.7 %; COA = 14.5 ± 6.2 %). In contrast, spotted seatrout rarely used the inner lagoon (VPS = 3.7 ± 3.4 %; COA = 26.1 ± 9.1 %) and were commonly detected on the open bay side of the array (VPS = 96.3 ± 3.4 %; COA = 73.9 ± 9.1 %).
Mean tracking duration (±SE) at the habitat scale was 20.4 ± 2.3 days for red drum and 23.4 ± 1.9 days for spotted seatrout, and mean residency index was 0.34 ± 0.08 for red drum compared to 0.56 ± 0.1 for spotted seatrout. The number of revisits to the array following absences greater than 24 h was identical for red drum (2.4 ± 0.6) and spotted seatrout (2.4 ± 0.8). No significant differences in total tracking duration (t test; p = 0.209), residency (t test; p = 0.094), and revisits (t test; p = 0.815) were observed between the two species.
Time of day influenced both habitat use and ROM for red drum and spotted seatrout. The proportion of red drum VPS positions over bare substrate was greatest during the day (46.2 %) and lowest at night (13.9 %), while the proportion over seagrass was greatest at night (75.0 %) and lowest during the day (36.9 %). Spotted seatrout VPS positions were almost exclusively located over seagrass during the day (98.0 %), but shifted primarily to bare substrate at night (80.0 %; Fig. 5). Both species also exhibited diel variability in movement, with ROM lowest during the day (red drum = 1.39 m min−1; spotted seatrout = 0.95 m min−1) and greatest at night (red drum = 2.72 m min−1; spotted seatrout = 3.4 m min−1) for both species. Position and ROM estimates during crepuscular periods were intermediate to day and night estimates.
Bay Scale
An additional 40 red drum (223–537 mm) were tagged and released for bay-scale tracking (Table 1), and overall, 44 of 54 (81.5 %) red drum and 13 of 15 (86.7 %) spotted seatrout were detected at least 10 days post release. After filtering, a total of 58,645 detections were recorded for red drum and 3175 for spotted seatrout (Fig. 6). Mean tracking duration (±SE) at the bay scale was 246.4 ± 22.5 days for red drum (pooled age classes) and 153.5 ± 27.7 days for spotted seatrout. Mean bay-scale tracking duration was similar between age-1 (272.9 ± 45.1 days) and age-2 (235.3 ± 26.0 days) red drum (t test; p = 0.488; Table 2). Maximum tracking duration for spotted seatrout was 267 days (none detected after March 2014), while 17 (31.4 %) red drum were tracked for 265 days or longer. Seven (15.9 %) red drum were tracked for a period greater than 450 days.
Seasonal movement patterns were evident for individuals of both species; directed movements were detected during winter and spring. Mean distance traveled by month for moving individuals (detected by more than one receiver) was relatively high for red drum in February (20.8 km), March (16.5 km), and April (14.6 km) and for spotted seatrout in December (15.0 km), January (21.3 km), and February (15.0 km). In addition, the proportion of detected individuals classified as moving was relatively high for red drum in November (33.3 %), December (54.1 %), and January (43.8 %), and for spotted seatrout in November/December (33.3 %), January (66.7 %), and February (100 %) (Fig. 7). While several individuals were detected at a single receiver from May to August, no individual of either species was detected moving between receivers (excluding two receivers at Mud Island) during these months (Fig. 7). Maximum total distance traveled by an individual was 72.4 km for red drum and 68.6 km for spotted seatrout. Mean distance traveled (±SE) was 11.9 ± 2.8 km for red drum and 15.5 ± 6.7 km for spotted seatrout. Age-specific differences observed for red drum (age-1 = 20.3 ± 6.6 km vs. age-2 = 8.4 ± 2.8 km) were not statistically significant (t test; p = 0.116; Table 2). Movement away from release locations was limited for both species, with mean final displacement distances of 3.0 ± 0.8 km for red drum and 1.6 ± 0.9 km for spotted seatrout.
Discussion
Red drum and spotted seatrout were commonly detected over each habitat type (seagrass, oyster reef, bare substrate) present within the Mud Island array; however, habitat use and movement patterns differed between species. Red drum showed greater use of seagrass and oyster reef than bare substrate at the habitat scale. This finding is consistent with previous investigations that indicate red drum use a variety of habitat types, but are more common in structured habitats (Bacheler et al. 2009; Dance and Rooker 2015; Fodrie et al. 2015), and this association with structured habitats both enhances foraging opportunities and reduces predation risk (Gillanders 2006; Heck and Orth 2006; Rooker et al. 1998b). Our study also demonstrated that red drum were commonly associated with habitat edges or boundaries between two different habitat types, a finding also observed by Dance and Rooker (2015). Spotted seatrout also preferred specific habitats, but were more commonly associated with bare substrate and seagrass than oyster reef. The limited use of oyster reef is likely more reflective of preferences for water depth rather than habitat type because of the shallow nature of these reefs. Association with seagrass by early juvenile spotted seatrout is well established (Flaherty-Walia et al. 2015; McMichael and Peters 1989; Neahr et al. 2010; Rooker et al. 1998a), and our results indicate that seagrass continues to be common habitat for spotted seatrout throughout adolescence, though use of this habitat was not significantly different from random.
Habitat-scale tracking results also showed a high degree of spatial separation between the two species, indicative of habitat partitioning. Habitat partitioning in fishes has previously been documented for species with overlapping home ranges and resource utilization patterns (Kinney et al. 2011; Knickle and Rose 2014; Werner et al. 1977). In this case, red drum and spotted seatrout occupied different depth zones within the array at Mud Island. Most red drum positions were located in the inner lagoon (depth <0.5 m), while spotted seatrout positions were predominantly located in the deeper, open bay regions of the array (depth >0.5 m). The mean water depth of areas used by spotted seatrout was ~1 m greater than red drum, which is substantial given the limited range of water depths (0.5–3.0 m) within this array. The difference in depth use between species may reflect foraging preference, as red drum commonly feed on macroinvertebrates in shallow seagrass and oyster reef (Scharf and Schlicht, 2000), while spotted seatrout often feed on small midwater baitfishes, which are less abundant in these shallow habitats (Baker and Sheaves 2007; Llanso et al. 1998). Given that water depth and foraging preference of estuarine fishes can vary ontogenetically and seasonally (Nunn et al. 2012), caution should be exercised when interpreting water depth use patterns from adolescents during summer months.
Temporal variability in habitat use and movement is well documented and primarily linked to foraging, avoiding predators, or both (Becker and Suthers 2014; Werner et al. 1983). Strong diel trends emerged for spotted seatrout, with individuals closely associated with seagrass (VPS = 98 %) during the day before transitioning largely to bare substrate (VPS = 80 %) at night. Moreover, ROM was markedly lower during the day (0.95 m min−1) than night (3.4 m min−1), with increased ROM or greater activity at night possibly linked to foraging (Reebs 2002). Nocturnal foraging has been previously observed for sciaenids (Facendola and Scharf 2012), and the distinct increase in ROM at night by spotted seatrout may be the result of increased foraging activity. Because foraging efficiency is often lower at night (Fraser and Metcalfe 1997), nocturnal feeding by spotted seatrout is possibly a mechanism to minimize predation risk by piscivores (e.g., dolphins Tursiops truncatus) that actively feed on estuarine fishes during diurnal and crepuscular periods (Allen et al. 2001). Furthermore, seagrass may attenuate the high-frequency sounds dolphins use to echolocate prey (Wilson et al. 2013) and further mitigate predation risk. Habitat use (63 % of positions in seagrass, 37 % bare substrate) and ROM (2.6 m min−1) for spotted seatrout during crepuscular periods was intermediate to observations during day and night, possibly indicative of the period when individuals transition between daytime sites and nocturnal foraging areas. Red drum were also more active at night than during the day, but unfortunately, the interpretation of diel variability for red drum was hampered by limited ROM estimates at night.
Our study employed complementary approaches to estimate fish position at the habitat scale, and even though the spatial and temporal resolution of the positioning methods differed, both estimated similar distributions. Because VPS requires simultaneous detections by three or more receivers, we assumed this method would provide the most accurate estimates for characterizing habitat-scale associations (Andrews et al. 2011). The challenge of using VPS at this particular site was that depths throughout the array were commonly ≤1 m, which limits detection range and therefore, opportunities for triangulation (Gjelland and Hedger 2013). In contrast, COA allowed for the inclusion of all recorded detections, generating a more holistic image of the use of the seascape by fish. The presumed limitation of COA is lower spatial resolution, because receiver locations (latitude and longitude) for all detections within a given time period are used to approximate fish position. Each positioning approach carried limitations in the context of our study; nevertheless, both methods yielded similar results with over 80 % of red drum positions located in the shallow seagrass habitat of the inner lagoon and over 70 % of spotted seatrout positions located over bare substrate in the deeper open bay portion of the array. The similarity in our results suggests that COA may be a suitable alternative to VPS when environmental conditions reduce the ability of acoustic positioning system to triangulate the location of individuals within the array.
Bay-scale movement of red drum and spotted seatrout was primarily restricted to small regions within bays, suggesting high residency. Mean final displacement of red drum and spotted seatrout from the initial tagging location was low (< 5 km), and many individuals were detected in the same area for several months. These results are consistent with previous otolith chemistry (Patterson et al. 2004; Rooker et al. 2010) and tagging (Adams and Tremain 2000; Bacheler et al. 2009a; Dance and Rooker 2015) studies, which indicate that the degree of inter-bay connectivity is low for spotted seatrout and possibly only marginally higher for red drum, with exchanges among estuaries unlikely at distances greater than 100 km. High residency and limited movement by both species suggests that conditions within the home estuary (e.g., habitat quality, environmental parameters, and fishing pressure) likely have the greatest impact on local populations.
Directed bay-scale movement of red drum and spotted seatrout followed a seasonal pattern, occurring primarily during winter and early spring (December–March). Increased bay-scale movement of red drum during this time period is not entirely surprising given that previous studies in the GoM have shown that red drum are more likely to make movements of 1 km or greater when temperatures drop below 15 °C (Dance and Rooker 2015), and temperatures below this threshold normally occur during the winter months in Texas estuaries (NOAA National Estuarine Research Reserve System station MARMBWQ). Similarly, spotted seatrout in Louisiana estuaries relocate and often travel long distances (>10 km) following the passage of cold fronts in winter (Callihan et al. 2014), which is consistent with our observations in this central Texas estuarine complex. Bacheler et al. (2009) and Ellis (2014) reported that peak movement of red drum and spotted seatrout in North Carolina occurred in the fall, and the apparent disparity in the timing of the seasonal movements between the two regions is likely due to the fact that North Carolina is near the northern limit of the range of each species, and temperatures in this region often drop below 15 °C in the fall (NOAA National Estuarine Research Reserve System station NOCLCWQ). Red drum growth is positively associated with water temperature (Lanier and Scharf 2007), and thus it is possible that increased bay-scale movement in the winter was driven in part by fish seeking a thermal refuge, because water temperatures in the estuarine complex were lowest between December and March.
Our findings clearly demonstrate that habitat- and bay-scale movement is species-specific and varies both spatially and temporally for adolescent red drum and spotted seatrout. Results suggest that structured habitat and heterogeneous habitat assemblages are important to both species, particularly evidenced by spotted seatrout use of seagrass during the day and red drum affinity to habitat edges. Red drum and spotted seatrout partitioned habitat based on depth, and it is expected that the value of specific habitat types within and between species is closely linked to this factor. Spotted seatrout exhibited distinct diel habitat use patterns, and future study is warranted to determine whether the presumed unique functions are in fact provided by non-vegetated (nocturnal feeding) and seagrass (diurnal resting) habitats. At the bay-scale, red drum and spotted seatrout showed the capacity for directed movements of at least 70 km, but inter-bay movements of this magnitude were limited for both species and occurred only during winter and early spring. The detection of most red drum and spotted seatrout within a few kilometers of their release site even after long periods of time (9+ months) suggests that estuarine population dynamics of both species are likely controlled by local processes, and management aimed at smaller spatial scales than currently used by state agencies may be beneficial.
References
Adams, D.H., and D.M. Tremain. 2000. Association of large juvenile red drum, Sciaenops ocellatus, with an estuarine creek on the Atlantic coast of Florida. Environmental Biology of Fishes 58(2): 183–194.
Afonso, P., J. Fontes, K.N. Holland, and R.S. Santos. 2009. Multi-scale patterns of habitat use in a highly mobile reef fish, the white trevally Pseudocaranx dentex, and their implications for marine reserve design. Marine Ecology Progress Series 381: 273–286.
Allen, M.C., A.J. Read, J. Gaudet, and L.S. Sayigh. 2001. Fine-scale habitat selection of foraging bottlenose dolphins Tursiops truncatus near Clearwater, Florida. Marine Ecology Progress Series 222: 253–264.
Andrews, K.S., N. Tolimieri, G.D. Williams, J.F. Samhouri, C.J. Harvey, and P.S. Levin. 2011. Comparison of fine-scale acoustic monitoring systems using home range size of a demersal fish. Marine Biology 158(10): 2377–2387.
Bacheler, N.M., L.M. Paramore, S.M. Burdick, Buckel, and J.E. Hightower. 2009. Variation in movement patterns of red drum (Sciaenops ocellatus) inferred from conventional tagging and ultrasonic telemetry. Fishery Bulletin 107(4): 405–418.
Baker, R., and M. Sheaves. 2007. Shallow-water refuge paradigm: conflicting evidence from tethering experiments in a tropical estuary. Marine Ecology Progress Series 349: 13–22.
Beck, M.W., K.L. Heck Jr., K.W. Able, D.L. Childers, D.B. Eggleston, B.M. Gillanders, B. Halpern, C.G. Hays, K. Hoshino, T.J. Minello, R.J. Orth, P.F. Sheridan, and M.P. Weinstein. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51(8): 633–641.
Becker, A., and I.M. Suthers. 2014. Predator driven diel variation in abundance and behaviour of fish in deep and shallow habitats of an estuary. Estuarine, Coastal and Shelf Science 144: 82–88.
Brown-Peterson, N.J., M.S. Peterson, D.L. Nieland, M.D. Murphy, R.G. Taylor, and J.R. Warren. 2002. Reproductive biology of female spotted seatrout, Cynoscion nebulosus, in the Gulf of Mexico: differences among estuaries? Environmental Biology of Fishes 63(4): 405–415.
Callihan, J.L., J.H. Cowan Jr., and M.D. Harbison. 2014. Sex-specific movement response of an estuarine sciaenid (Cynoscion nebulosus) to freshets. Estuaries and Coasts 38(5): 1–13.
Conner, L.M., and B.W. Plowman. 2001. Using Euclidean distances to assess nonrandom habitat use. In Radio tracking and animal populations, 275–290. San Diego, CA: Academic Press.
Cunjak, R.A., J.M. Roussel, M.A. Gray, J.P. Dietrich, D.F. Cartwright, K.R. Munkittrick, and T.D. Jardine. 2005. Using stable isotope analysis with telemetry or mark-recapture data to identify fish movement and foraging. Oecologia 144(4): 636–646.
Dance, M.A., and J.R. Rooker. 2015. Habitat- and bay-scale connectivity of sympatric fishes in an estuarine nursery. Estuarine, Coastal and Shelf Science 167: 447–457.
Dance, M.A., and J.R. Rooker. 2016. Stage-specific variability in habitat associations of juvenile red drum across a latitudinal gradient. Marine Ecology Progress Series 557: 221–235.
Dance, M.A., D.L. Moulton, N.B. Furey, and J.R. Rooker. 2016. Does transmitter placement or species affect detection efficiency of tagged animals in biotelemetry research? Fisheries Research 183: 80–85.
Ellis, T.A. 2014. Mortality and movement of spotted seatrout at its northern latitudinal limits. PhD dissertation. North Carolina State University. 260 p.
Espinoza, M., T.J. Farrugia, D.M. Webber, F. Smith, and C.G. Lowe. 2011a. Testing a new acoustic telemetry technique to quantify long-term, fine-scale movements of aquatic animals. Fisheries Research 108(2): 364–371.
Espinoza, M., T.J. Farrugia, and C.G. Lowe. 2011b. Habitat use, movements and site fidelity of the gray smooth-hound shark (Mustelus californicus gill 1863) in a newly restored southern California estuary. Journal of Experimental Marine Biology and Ecology 401(1): 63–74.
Facendola, J.J., and F.S. Scharf. 2012. Seasonal and ontogenetic variation in the diet and daily ration of estuarine red drum as derived from field-based estimates of gastric evacuation and consumption. Marine and Coastal Fisheries 4(1): 546–559.
Farrugia, T.J., M. Espinoza, and C.G. Lowe. 2011. Abundance, habitat use and movement patterns of the shovelnose guitarfish (Rhinobatos productus) in a restored southern California estuary. Marine and Freshwater Research 62(6): 648–657.
Flaherty-Walia, K.E., R.E. Matheson Jr., and R. Paperno. 2015. Juvenile spotted seatrout (Cynoscion nebulosus) habitat use in an eastern Gulf of Mexico estuary: the effects of seagrass bed architecture, seagrass species composition, and varying degrees of freshwater influence. Estuaries and Coasts 38(1): 353–366.
Fodrie, F.J., L.A. Yeager, J.H. Grabowski, C.A. Layman, G.D. Sherwood, and M.D. Kenworthy. 2015. Measuring individuality in habitat use across complex landscapes: approaches, constraints, and implications for assessing resource specialization. Oecologia 178(1): 75–87.
Fraser, N.H.C., and N.B. Metcalfe. 1997. The costs of becoming nocturnal: feeding efficiency in relation to light intensity in juvenile Atlantic salmon. Functional Ecology 11(3): 385–391.
Furey, N.B., M.A. Dance, and J.R. Rooker. 2013. Fine-scale movements and habitat use of juvenile southern flounder Paralichthys lethostigma in an estuarine seascape. Journal of Fish Biology 82(5): 1469–1483.
Gillanders, B.M. 2006. Seagrasses, fish, and fisheries. In Seagrasses: biology, ecology, and conservation, 503–505. Dordrecht: Springer.
Gjelland, K.Ø., and R.D. Hedger. 2013. Environmental influence on transmitter detection probability in biotelemetry: developing a general model of acoustic transmission. Methods in Ecology and Evolution 4(7): 665–674.
Grober-Dunsmore, R., S.J. Pittman, C. Caldow, M.S. Kendall, and T.K. Frazer. 2009. A landscape ecology approach for the study of ecological connectivity across tropical marine seascapes. In Ecological connectivity among tropical coastal ecosystems, 493–530. Dordrecht: Springer.
Heck, K.L. Jr., and R.J. Orth. 2006. Predation in seagrass beds. In Seagrasses: biology, ecology and conservation, 537–550. Dordrecht: Springer.
Heupel, M.R., J.M. Semmens, and A.J. Hobday. 2006. Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays. Marine and Freshwater Research 57(1): 1–13.
Kinney, M.J., N.E. Hussey, A.T. Fisk, A.J. Tobin, and C.A. Simpfendorfer. 2011. Communal or competitive? Stable isotope analysis provides evidence of resource partitioning within a communal shark nursery. Marine Ecology Progress Series 439: 263–276.
Knickle, D.C., and G.A. Rose. 2014. Dietary niche partitioning in sympatric gadid species in coastal Newfoundland: evidence from stomachs and CN isotopes. Environmental Biology of Fishes 97(4): 343–355.
Lanier, J.M., and F.S. Scharf. 2007. Experimental investigation of spatial and temporal variation in estuarine growth of age-0 juvenile red drum (Sciaenops ocellatus). Journal of Experimental Marine Biology and Ecology 349(1): 131–141.
Llanso, R.J., S.S. Bell, and F.E. Vose. 1998. Food habits of red drum and spotted seatrout in a restored mangrove impoundment. Estuaries 21(2): 294–306.
MacRae, P.S., and J.H. Cowan Jr. 2010. Habitat preferences of spotted seatrout, Cynoscion nebulosus, in coastal Louisiana: a step towards informing spatial management in estuarine ecosystems. The Open Fish Science Journal 3: 154–163.
McMichael, R.H., and K.M. Peters. 1989. Early life history of spotted seatrout, Cynoscion nebulosus (Pisces: Sciaenidae), in Tampa Bay, Florida. Estuaries 12(2): 98–110.
Mohan, J.A., and B.D. Walther. 2015. Spatiotemporal variation of trace elements and stable isotopes in subtropical estuaries: II. Regional, local, and seasonal salinity-element relationships. Estuaries and Coasts 38(3): 769–781.
Nagelkerken, I., M. Sheaves, R. Baker, and R.M. Connolly. 2014. The seascape nursery: a novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish and Fisheries 16(2): 362–371.
Neahr, T.A., G.W. Stunz, and T.J. Minello. 2010. Habitat use patterns of newly settled spotted seatrout in estuaries of the North-Western Gulf of Mexico. Fisheries Management and Ecology 17(5): 404–413.
Nieland, D.L., R.G. Thomas, and C.A. Wilson. 2002. Age, growth, and reproduction of spotted seatrout in Barataria Bay, Louisiana. Transactions of the American Fisheries Society 131(2): 245–259.
NOAA National Estuarine Research Reserve System (NERRS). System-wide monitoring program. Data accessed from the NOAA NERRS centralized data management office website. http://cdmo.baruch.sc.edu/. Accessed 10 March 2016.
Nunn, A.D., L.H. Tewson, and T.G. Cowx. 2012. The foraging ecology of larval and juvenile fishes. Reviews in Fish Biology and Fisheries 22(2): 377–408.
Patterson, H.M., R.S. McBride, and N. Julien. 2004. Population structure of red drum (Sciaenops ocellatus) as determined by otolith chemistry. Marine Biology 144(5): 855–862.
Porch, C.E., C.A. Wilson, and D.L. Nieland. 2002. A new growth model for red drum (Sciaenops ocellatus) that accommodates seasonal and ontogenic changes in growth rates. Fishery Bulletin 100(1): 149–152.
Reebs, S.G. 2002. Plasticity of diel and circadian activity rhythms in fishes. Reviews in Fish Biology and Fisheries 12(4): 349–371.
Reese Robillard, M.M., L.M. Payne, R.R. Vega, and G.W. Stunz. 2015. Best practices for surgically implanting acoustic transmitters in spotted seatrout. Transactions of the American Fisheries Society 144(1): 81–88.
Rooker, J.R., and S.A. Holt. 1997. Utilization of subtropical seagrass meadows by newly settled red drum Sciaenops ocellatus: patterns of distribution and growth. Marine Ecology Progress Series 158: 139–149.
Rooker, J.R., S.A. Holt, M.A. Soto, and G.J. Holt. 1998a. Postsettlement patterns of habitat use by sciaenid fishes in subtropical seagrass meadows. Estuaries 21(2): 318–327.
Rooker, J.R., G.J. Holt, and S.A. Holt. 1998b. Vulnerability of newly settled red drum (Sciaenops ocellatus) to predatory fish: is early-life survival enhanced by seagrass meadows? Marine Biology 131: 145–151.
Rooker, J.R., G.W. Stunz, S.A. Holt, and T.J. Minello. 2010. Population connectivity of red drum in the northern Gulf of Mexico. Marine Ecology Progress Series 407: 187–196.
Roy, R., J. Beguin, C. Argillier, L. Tissot, F. Smith, S. Smedbol and E. De-Oliveira. 2014. Testing the VEMCO Positioning System: spatial distribution of the probability of location and the positioning error in a reservoir. Animal Biotelemetry 2(1): 1.
Scharf, F.S., and K.K. Schlicht. 2000. Feeding habits of red drum (Sciaenops ocellatus) in Galveston Bay, Texas: seasonal diet variation and predator-prey size relationships. Estuaries 23(1): 128–139.
Simpfendorfer, C.A., M.R. Heupel, and R.E. Hueter. 2002. Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Canadian Journal of Fisheries and Aquatic Sciences 59(1): 23–32.
Stunz, G.W., T.J. Minello, and P.S. Levin. 2002. Growth of newly settled red drum Sciaenops ocellatus in different estuarine habitat types. Marine Ecology Progress Series 238: 227–236.
Werner, E.E., D.J. Hall, D.R. Laughlin, D.J. Wagner, L.A. Wilsmann, and F.C. Funk. 1977. Habitat partitioning in a freshwater fish community. Journal of the Fisheries Board of Canada 34(3): 360–370.
Werner, E.E., G.G. Mittelbach, D.J. Hall and J.F. Gilliam. 1983. Experimental tests of optimal habitat use in fish: the role of relative habitat profitability. Ecology 64(6): 1525–1539.
Wilson, C.A., and D.L. Nieland. 1994. Reproductive biology of red drum, Sciaenops ocellatus, from the neritic waters of the northern Gulf of Mexico. Fishery Bulletin 92(4): 841–850.
Wilson, C.J., P.S. Wilson, C.A. Greene, and K.H. Dunton. 2013. Seagrass meadows provide an acoustic refuge for estuarine fish. Marine Ecology Progress Series 472: 117–127.
Acknowledgements
The authors would like to thank members of the Fisheries Ecology Lab at TAMUG and Center for Sportfish Science and Conservation at TAMU-CC for assistance in the lab and field. This publication was supported in part by an Institutional Grant from the Texas Sea Grant College Program from the National Sea Grant Office, National Oceanic and Atmospheric Administration, U.S. Department of Commerce (NA10OAR4170099 to JRR). Additional funding was provided by the McDaniel Charitable Foundation (JRR) and the Harte Research Institute (GWS). The manuscript was improved by the comments of two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Charles Simenstad
Rights and permissions
About this article
Cite this article
Moulton, D.L., Dance, M.A., Williams, J.A. et al. Habitat Partitioning and Seasonal Movement of Red Drum and Spotted Seatrout. Estuaries and Coasts 40, 905–916 (2017). https://doi.org/10.1007/s12237-016-0189-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-016-0189-7