Abstract
Estuarine and adjacent inshore habitats have long been recognised as important nursery areas for fishes before they disperse to coastal habitats. Assessing nursery function supports spatial and fisheries management, yet work commonly focusses on singular habitat types. Re-considering how juvenile fish connect habitats may improve our understanding of nursery function and the scales that recruits are supplied to coastal fisheries. This study quantified the juvenile movements of two harvested fishes in south-eastern Australia, luderick (Girella tricuspidata) and yellowfin bream (Acanthopagrus australis). Acoustic tags were used to track 33 luderick and 20 yellowfin bream from seagrass meadows for up to ~400 days in Jervis Bay Marine Park. Both species had relatively small home ranges (< 7 km2) and exhibited site attachment to seagrass meadows where they were released. Most luderick and yellowfin bream were detected moving 100’s metres to kilometres to reefs adjacent to seagrass, although these movements were not habitat shifts. Rather, reef-ward movements represented repeated visits that lasted days to months before fish returned to seagrass, suggesting that these movements may be explorations in search of suitable adult habitat. Strong retention within an existing marine reserve was observed, with only five of 33 fish tagged within reserves crossing the boundary into fished waters. Overall, our results demonstrate that juvenile fish use and connect multiple habitat types during their movements. These findings support the broadening of the nursery concept from single habitats to a mosaic of functionally connected habitat patches (dubbed ‘seascape nurseries’).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estuarine and inshore habitats provide important nursery areas for fishes and are vital for sustaining many fisheries worldwide (Beck et al. 2001; Sheaves et al. 2015). Nursery areas are commonly perceived as discrete, homogenous habitats that promote juvenile survival and growth and contribute recruits to adult populations in coastal and offshore habitats (Beck et al. 2001; Heck Jnr et al. 2003; Dahlgren et al. 2006). Classically, for example, seagrass meadows are known to be nurseries for many reef-associated fish before they disperse to adult populations (Pittman and McAlpine 2003; Gillanders 2007; Sambrook et al. 2019). Whilst the nursery-role concept provides an important framework for identifying key fish habitat, defining nurseries as singular units may be too simplistic because it does not account for dynamic processes such as species movements between habitats during juvenile life stages (Sheaves et al. 2006, 2015; Nagelkerken et al. 2015; Litvin et al. 2018). To address the complexities of juvenile fish using multiple habitats, it has been suggested that the concept should view nurseries as spatially explicit seascapes of interconnected habitats, coined ‘seascape nurseries’ (Nagelkerken et al. 2015). Quantitative data on the movement of juvenile fish and connectivity between habitats are essential to properly understand whether the nursery-role concept needs to be refined, as well as quantifying the contribution of nurseries to adult populations. The ongoing losses of vegetated habitats due to human disturbances underscore the need for this information, as it may disrupt connectivity and reduce the number of recruits entering coastal fisheries (Barbier et al. 2011; Sheaves et al. 2014; Murray et al. 2022; Stamp et al. 2022; Rees et al. 2023).
Whilst previous studies have highlighted strong connectivity for juvenile fish amongst vegetated habitats, and between nursery areas and nearby coastal adult populations, there are few examples where juvenile movements have been directly quantified. Instead, current understanding on juvenile fish movements and ontogenetic habitat shifts comes largely from studies observing changes in abundances or size classes between habitats (Olds et al. 2012; Swadling et al. 2019, 2021; Berkström et al. 2020; Rees et al. 2021), natural tags such as otolith microchemistry (Gillanders 2002; Russell et al. 2021), or mark-recapture studies (Morton et al. 1993; Gray et al. 2012). For instance, Swadling et al. (2019) observed a higher abundance and diversity of fish on temperate rocky reefs close to large seagrass meadows. Such observations have been attributed to a greater number of recruits dispersing from seagrass nurseries to adjacent reefs in comparison to those farther afield. Fish may also move between habitats when they are in close proximity, to access resources and thereby increase the carrying capacity of reefs (this concept is termed landscape complementation; Dunning et al. 1992). Although such methods are useful for inferring movement pathways, they do not provide the direct measurements required to explicitly determine the spatial and temporal scales over which fish use habitats.
Acoustic telemetry provides the ability to quantify the movement, space-use and connectivity of habitats by fishes across spatiotemporal scales (Donaldson et al. 2014; Hussey et al. 2015; Taylor et al. 2017a). Studies adopting acoustic telemetry have largely focussed on tracking the movement of adult fish, providing valuable insights for species ecology and helping inform marine management and conservation (Donaldson et al. 2014; Crossin et al. 2017; Taylor et al. 2017a). Acoustic tracking of smaller fish has been limited by transmitter size, as high transmitter size to body mass ratios can have negative effects on fish health and behaviour (Jepsen et al. 2005; Brown et al. 2006; Thorstad et al. 2013). Therefore, literature describing the tracking of juvenile teleost fish movements in the marine environment has generally been restricted to species with larger (e.g., > 30 cm) body sizes (Childs et al. 2015; Taylor et al. 2017b; Murray et al. 2018; Staveley et al. 2019; Duffing Romero et al. 2021; Stamp et al. 2021). Advances in technology leading to the miniaturisation of acoustic transmitters means that tracking the movements of small marine fishes and juvenile life stages (< 20 cm) is increasing (Pursche et al. 2013, 2014; Huijbers et al. 2015; Aspillaga et al. 2021; Matley et al. 2021; Szekeres et al. 2022).
Marine reserves have been established worldwide to conserve biological diversity and social values (Spalding et al. 2008; Gaines et al. 2010; Hernandez et al. 2021), and there is growing evidence of their co-benefits for harvested fishes (Le Port et al. 2017; Goetze et al. 2021; Knott et al. 2021). To provide effective conservation benefits to fishes, marine reserves are best configured to account for the habitats used from day-to-day movements to life-cycle migrations (Kramer and Chapman 1999; Grüss et al. 2011). If reserves are too small or do not contain the requisite habitats, individuals may cross reserve boundaries and be exposed to fishing pressure, or unprotected habitats may be lost or degraded (Kramer and Chapman 1999; Grüss et al. 2011). Knowledge of the movement patterns of fishes is thus required to assist reserve design and assess effectiveness (Crossin et al. 2017; Weeks et al. 2017). In particular, information on dispersal and connectivity patterns will allow reserve design to protect species throughout their life history or support fished areas through the spill-over or emigration of individuals from protected areas (Goni et al. 2008; McCook et al. 2009; Grüss et al. 2011).
In this study, we used acoustic telemetry to quantify the juvenile movement patterns and connectivity of two common species important for recreational, commercial and indigenous fisheries in south-eastern Australia: luderick (Girellidae: Girella tricuspidata) and yellowfin bream (Sparidae: Acanthopagrus australis). Luderick are omnivores which predominantly consume green and red algae but also often feed on invertebrates, with juveniles consuming zooplankton and shifting to an adult diet at lengths of 90–100 mm (Morrison 1990; Clements and Choat 1997; Raubenheimer et al. 2005). Luderick are estimated to become reproductively active at approximately 4 years of age and lengths of ~286 mm and ~295 mm fork length for males and females, respectively (Gray et al. 2012). In comparison, yellowfin bream are primarily carnivorous benthic feeders and mature at fork lengths between 200 and 240 mm, with most juveniles becoming functional males at 2 years of age (Pollock 1985; Curley et al. 2013). For both species, seagrasses are known to be the preferred nursery habitats, and adults are commonly found on shallow coastal rocky reefs (Curley et al. 2013). The study was undertaken in a temperate Marine Park (Jervis Bay Marine Park—JBMP) which contains multiple ‘no-take’ marine reserves, and the remainder is zoned to allow recreational fishing and some forms of commercial fishing. The specific aims of this study were to determine (1) if juvenile fish exhibit small-scale site attachment within seagrass areas or if they move amongst a mosaic of patch types (i.e., seascape nurseries), (2) the occurrence and spatial scale of juvenile habitat shifts to adult populations on rocky reefs and (3) the movement of juvenile fish in relation to a no-take marine reserve within the JBMP.
Methods
Study Site and Receiver Array
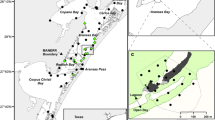
Juvenile fish movements were quantified within Jervis Bay (35° 8′ S, 150° 43′ E), a large coastal embayment in south-eastern Australia spanning 120 km2 (Fig. 1). The embayment is dominated by oceanic conditions and contains a mosaic of intertidal and subtidal rocky reef, seagrass and unconsolidated soft sediments, plus tidal creeks with seagrass, mangrove and saltmarshes also feeding into the Bay (Fig. 1). The predominant species of seagrass within the embayment is Posidonia australis, a slow growing, persistent species with large strap-like leaves (30–60 cm long and 6–14 mm wide). Populations of P. australis are listed as endangered in six NSW estuaries; however, this does not include meadows in Jervis Bay and rates of decline for the species here are relatively low (West and Glasby 2022). Other species of seagrass are also found within the Bay, although mainly within the tidal creeks, and these include Zostera muelleri subsp. capricorni and Halophila species which are both smaller and more transient than P. australis (Kilminster et al. 2015). Jervis Bay forms the central area of JBMP, which has a multiple-use zoning scheme that contains several ‘no-take’ marine reserves (hereafter termed NTMR) covering ~20% of the overall area where it is not permitted to remove or harm marine biota (Fig. 1). The remaining areas of the Bay are habitat protection zones where recreational fishing and some forms of commercial fishing are allowed and there are small areas (0.2%) designated as special purpose zones.
The Jervis Bay Marine Park acoustic array located in New South Wales, Australia. Points depict the location of acoustic receivers, and no-take marine reserves (NTMR) are shown by hatched areas. Rocky reef habitat is shown in brown and seagrass in green. The locations fish were tagged and released are included
An acoustic receiver array of 49 Vemco VR2W and VR2tx acoustic receivers was established within JBMP to passively track tagged fish (Fig. 1). Acoustic receivers were strategically placed on almost every reef within Jervis Bay, along with the seagrass and creeks where fish were tagged in the current study. This array was designed to assess movement patterns within seagrass and small estuarine nursery habitats along with the usage of adjacent rocky reef habitats and any potential ontogenetic shifts in habitat. In addition, an acoustic gate across the mouth of Jervis Bay was present to allow for the detection of tagged fish leaving or entering the Bay (Fig. 1). Acoustic receivers were separated by a minimum of 500 m and placed no farther than 300 m from the shoreline. Most of the acoustic receivers were attached to rope moorings, which consisted of sections of railway track (~50 kg) and a subsurface polystyrene buoy that maintained receivers in an upright position (hydrophones oriented to the surface). Receivers were fixed at least 1 m below the buoy and 2 m above the sea floor. The exceptions were the receivers within the creeks at depths less than 3 m, where receivers were attached to moorings with shorter ropes or on star pickets embedded into sediments.
Fish Collection and Acoustic Tagging
Fish were captured using hook and line in JBMP between June 2018 and February 2020 (Table 1). Tagging was concentrated within the Hare Bay NTMR (i.e., Hare Bay and Carama Inlet), but also occurred at other locations including Currambene Creek and Moona Moona Creek (Fig. 1). All luderick were below the size that the species reaches sexual maturity, whereas 7 of the 20 yellowfin bream were expected to be reproductively mature. We elected to tag a range of size classes (Tables 1; S1) to allow the best chance of quantifying the dispersal of individuals to adult populations, given that the size or age class the species perform ontogenetic habitat shifts is not well understood.
All fish were captured in or directly adjacent to seagrass meadows and were surgically implanted with Vemco V7-4x acoustic tags (7 mm diameter, 18 mm length and 0.7 g weight in water, ~400 d battery life: Innovasea, NS, Canada). These coded tags were programmed to randomly emit a unique signal every 180–240 s at a frequency of 69 kHz. Prior to the surgical implantation of acoustic tags, fish were placed in an aerated 50 L holding tank for a minimum of 15 min to recover from capture and were visually examined for general health and condition. Fish were then anaesthetized using 60 mg L−1 Aqui-S® solution before being transferred to a wetted cradle for surgery. A 10 mm incision was made in the ventral surface of the fish toward the rear of the peritoneal cavity in which the tag was inserted. Before surgery, all surgical equipment and acoustic tags were treated with povidone-iodine antiseptic (Betadine® solution) to prevent infection. Once the tag was inserted, the wound was sutured using one or two dissolving stitches tied with a double surgeon’s knot (Ethicon coated VICRYL, 3-0 needle). Fish were then transferred to another aerated 50 L holding tank and monitored for 30 min before release at the approximate location of capture.
Data Processing
Detection data for tagged individuals were corrected for time drift and then filtered to remove any detections that occurred less than 180 s apart, which was the minimum tag ping rate. Detections within 180 s of each other were assumed to be suspect and classified as false detections caused by tag collisions and interference from background noise (Simpfendorfer et al. 2015). Fish that were recorded on receivers for less than 10 days, had fewer than 50 detections and were not detected leaving the Bay (i.e., not detected on receivers in the gate or arrays outside of JBMP) were removed from subsequent analyses. These criteria led to the exclusion of four fish (Luderick 16, 29, 32 and Bream 2; Table S1). Additionally, data for Luderick 24 were excluded from formal analyses because it had unexpected movement patterns during the final 10 days that it was detected, which was likely due to a predation event (Fig. S3).
Data Analysis
Home-Range Estimation
To estimate the home range of juvenile fish, we used Brownian Bridge Movement Models (BBMMs). BBMMs were selected over other utilisation distribution methods because they account for the time interval and estimated pathway travelled between successive detections (Bullard 1991; Horne et al. 2007). They also incorporate location error, an important caveat in acoustic telemetry where the successful detection of an individual is dependent on them being within a receiver’s detection range (Kessel et al. 2014). Estimates of locations for the BBMMs were produced using a mean position algorithm to create centres of activity for each fish (Simpfendorfer et al. 2002). Two smoothing parameters were required to create the BBMM (Horne et al. 2007). First, the Brownian motion variance parameter (σ2m) estimates the variance in a fish’s position between two points using a maximum likelihood approach. Second, the location error was also required, and this was set as the predicted receiver detection range of 85 m based on previous range testing (Swadling et al. 2020). Home-range estimates derived from BBMMs were calculated as 50% (core home range) and 95% (home range extent) utilisation distributions.
Spatial Movement Networks
We used movement networks generated using network analysis to further explore patterns of movement and connectivity of tagged individuals within the JBMP array. Network analysis was used as it can identify movement corridors and connectivity within and between patches, which is often overlooked in typical space-use metrics (Lédée et al. 2015, 2021; Whoriskey et al. 2019). The method is embedded within graph theory and investigates relationships between nodes (acoustic receivers) that are connected by edges (fish movements), with all the combined edges represented as a network (Finn et al. 2014; Jacoby and Freeman 2016).
Directed and weighted movement networks were formed from square movement matrices. The colour of nodes (i.e., receivers) illustrated the proportion of detections recorded for a given fish, and the colour of the edges was weighted by the number of movements between nodes (i.e., connectivity). All nodes were placed in their actual (latitude and longitude) locations to assist with the interpretation of space-use and connectivity. Each network was tested against 10,000 randomly generated network structures to determine whether fish exhibited non-random movement patterns. Random networks for each individual were generated using a link re-arrangement approach via bootstrapping (Croft et al. 2011) whilst retaining the same degree of distribution from the original network. Network-level metrics (i.e., degree, betweenness, closeness and transitivity) were then calculated from each randomly generated graph and tested against metrics from the original network using a one-sample Wilcoxon signed rank test (ɑ = 0.05). Core use receivers (CUR) were quantified to further define highly visited areas within each individuals’ network following the methodology of Becker et al. (2016), which used centrality degree (i.e., the total number of ingoing/outgoing movements from a receiver) to rank the receivers within individual networks.
Relationships Between Movement, Species and Length
Generalised additive mixed models (GAMMs) were used to examine whether observed movement patterns varied between species and the fork length of fish. Yellowfin bream considered to be mature (i.e., > 220 mm) were excluded from the analyses, so that species comparisons were made for juveniles. Preliminary data exploration was performed to assess for potential outliers, normality of the data and collinearity between the explanatory variables (Zuur et al. 2009). Models were fitted with the transmitter ID as a random factor to account for within-individual replication. Akaike information criterion corrected for small sample sizes (AICc) was used to compare models, with the best fitting model containing the lowest AICc and fewest variables (Burnham and Anderson 2003). All analyses and plots were created using the statistical computing programme R (R Core Development Team 2021) and the packages adehabitatHR (Calenge 2006), igraph (Csardi and Nepusz 2006), Vtrack (Campbell et al. 2012), glatos (Holbrook et al. 2017), mgcv (Wood and Wood 2015) and ggplot2 (Wickham 2016).
Results
We tracked 33 luderick and 20 yellowfin bream between August 2018 and May 2021, recording a total of 739,584 detections within the JBMP array. There were 18 luderick (55%) and 14 yellowfin bream (70%) still being detected at the end of the study, nominated as ~400 days from deployment or the expected date the transmitters would cease functioning (Fig. 2). The number of days between the first and last detection of fish in the array (i.e., days at liberty; Table 1) was not explained by fork length or species (Table 2). Both species were often detected moving between nursery seagrass habitats and rocky reefs; however, there was no evidence of individuals permanently shifting habitats (Fig. 2). Instead, movements to reefs generally lasted days to weeks before being detected again in seagrass habitats. No fish were observed to permanently leave Jervis Bay; only one fish was detected on the gate receivers before returning to its release location on two occasions (Bream 10; Figs. 2b and S4). Multiple luderick tagged in Currambene Creek had large gaps in detection histories between December 2019 and April 2020 (Fig. 2a), and this can be attributed to the loss of a receiver over this time period.
Detections through time for tagged a luderick and b yellowfin bream at receivers located in seagrass ‘nurseries’ (blue), rocky reefs (red) or the acoustic gate (yellow). The location of tagging is represented by the shape of black dots, with squares = Hare Bay, circles = Currambene Creek, triangles = Moona Moona Creek and black diamonds = Carama Inlet. Crosses indicate the anticipated date on which battery failure occurred. Fish with no detections are not included
Species Home Ranges
Both luderick and yellowfin bream had comparatively small home ranges (Figs. 3 and 4). Core-use areas were not found to correlate with predictor variables (Table 2) and ranged from 0.019 to 0.27 km2 (mean = 0.046 km2 ± 0.05) for luderick and 0.02 to 0.39 km2 (mean = 0.165 km2 ± 0.12) for yellowfin bream. Extent home range differed between species, with juvenile yellowfin bream predicted to move over larger areas than luderick (R2 = 42.9; Table 2, Fig. 3a). Data points for the extent home range of luderick were widely spread, which can be attributed to the release locations, as all fish moving over areas greater than 0.3 km2 and detected at over 4 nodes were released in Hare Bay (Table S1; Fig. 3a). Indeed, further inspection of the home ranges revealed that there were differences between release locations, with luderick in creeks moving over smaller areas than those tagged in the seagrass meadows of Hare Bay (Fig. 4a). However, this was unsurprising given that the area of waterway is restricted in creeks. In contrast, yellowfin bream released in Carama Inlet moved over slightly larger areas than fish in Hare Bay, which was largely driven by an outlier, where one large fish (Bream 10: 284 mm FL) moved over 6.1 km2 (Fig. 4b).
Predictions from the best fitting generalised additive mixed model for a extent (95% BBMM) home ranges (km2), b number of nodes visited and c number of edges within the networks. Fitted GAMM predicted means (points) are included, and error bars represent ± SE. Symbols in a and b illustrate the different release locations, with squares = Hare Bay, circles = Currambene Creek, triangles = Moona Moona Creek and diamonds = Carama Inlet
Movement Networks: Habitat Use and Connectivity
Metrics calculated from the movement networks of all tagged fish were significantly different from random (P < 0.001), so all networks were used in the analyses. The number of nodes visited by juvenile fish was best explained by species, with yellowfin bream predicted to visit more nodes than luderick (R2 = 42.7; Table 2; Fig. 3b). Species was the best predictor for the number of edges within individual networks, with the number of edges in yellowfin bream networks being substantially higher than that of luderick networks (R2 = 45.4; Table 2; Fig. 3c).
Both species were observed to use the seagrass habitats where they were released disproportionately more than other habitats. This was evident by the high proportion of detections and number of CURs at the receiver nearest to release sites (Figs. 5 and S1–S5). It was notable that luderick released in Hare Bay were primarily detected in the eastern section of this large seagrass habitat, whereas yellowfin bream moved more widely across the seagrass in Hare Bay (Figs. 5, S1, S4 and S5). There were strong linkages between Hare Bay and Carama Inlet, with many fish detected moving in and out of this creek system (Figs. 5, S1, S4 and S5). Nine luderick and five yellowfin bream released in Hare Bay moved up to 2 km into Carama Inlet before returning to the Bay (Figs. S1 and S4). All but one yellowfin bream released in Carama Inlet were observed to egress into Hare Bay, where they showed wide-ranging movements before returning to the creek (Fig. S5). Movements into the Bay were less frequent for luderick released in creeks, with two fish from Moona Moona Creek and no fish from Currambene Creek detected in the Bay (Figs. S2 and S3).
Individual movement networks illustrating connectivity and space use for a representative luderick tagged in a Hare Bay, b Currambene Creek and c Moona Moona Creek and a representative yellowfin bream tagged in d Carama inlet and e Hare Bay. The colour of nodes illustrates the proportion of detections at a given receiver and edge colour shows the number of movements (i.e., connectivity) between receivers. Crosses on nodes denote core-use receivers, and the ‘x’ represents where the fish was released post-tagging. Black dots are receivers where fish were not detected
Individual movement networks revealed that both species frequently visited reef habitats which were directly adjacent to seagrass meadows. These reef-ward movements were predominantly observed for luderick and yellowfin bream released in the Hare Bay NTMR, where they moved 100’s of metres to kilometres from seagrass meadows to nearby rocky reefs (Figs. 5, S1, S4 and S5). Two luderick were detected egressing from Moona Moona Creek, repeatedly moving ~500 m to the reef at the mouth of this tributary (Fig. S3). There was some evidence of larger-scale movements from seagrass to reef habitats around the Bay, with one luderick and four yellowfin bream detected on reefs up to 10 km from release sites (Figs. S1, S4 and S5). Importantly, no fish were detected to permanently disperse to reef habitats and instead appeared to visit reefs for short periods of time before returning to the sites where they were released.
No-Take Marine Reserve Use
A total of 33 fish were tagged within the Hare Bay NTMR, which consisted of 13 luderick and 20 yellowfin bream (Table 1). Most fish tagged within this NTMR were never detected outside of the NTMR (Fig. S6). Only one luderick and four yellowfin bream were detected to cross the reserve boundary and spent short periods (days) outside the NTMR (Fig. S6). However, the one luderick (Luderick 5) was consistently detected outside of the NTMR over a 4-month period (March–June) before returning to Hare Bay (Fig. S6). It was notable that these movements outside of the NTMR occurred between December and March for three of the four yellowfin bream, which would be when ocean temperatures were warmest. Two yellowfin bream moved into a different NTMR, moving from Hare Bay NTMR to the Groper Coast NTMR (Figs. S4 and S5).
Discussion
Assessing the habitat use and movement of juvenile fish supports a more comprehensive understanding of fish-habitat linkages and seascape connectivity. This study offers novel insights into the movement patterns of juvenile fish belonging to two exploited species, luderick (Girella tricuspidata) and yellowfin bream (Acanthopagrus australis) in a temperate marine park. Although juveniles of both species exhibited site attachment to seagrass habitats, many fish made wide-ranging movements across seagrass meadows and to adjacent habitats. Yellowfin bream typically moved over larger areas than luderick; however, both species generally had small home ranges. Our study has indicated that there is strong connectivity between seagrass and adjacent rocky reef habitats for juveniles of these species, with a higher number of fish detected moving to reefs directly adjacent to seagrass than to reefs farther away. However, these reef-ward movements were unexpectedly not unidirectional ontogenetic habitat shifts, and fish instead made frequent visits to adjacent reefs before returning to seagrass. There was also no observed export of individuals to populations along the open coast, with no fish recorded leaving the JBMP array. Overall, these findings have important implications for the characterisation of nursery areas and provide quantitative data to support the inferred connectivity of juvenile fish from seagrass habitats to adjacent reefs made by previous observational studies (Dorenbosch et al. 2005; Olds et al. 2012; Rees et al. 2018; Swadling et al. 2019; Berkström et al. 2020).
Numerous fish species perform ontogenetic habitat shifts connecting juvenile populations in vegetated nearshore habitats to adult populations on reefs, yet the spatiotemporal scale of this dispersal has remained unclear (Pittman and McAlpine 2003; Sambrook et al. 2019; Swadling et al. 2021). We provide some of the first telemetry data demonstrating that juvenile fish move from seagrass to rocky reef habitats across relatively small spatial scales (100’s m to km’s). Contrary to predictions, however, these seagrass-reef movements were not permanent, and fish made repeated short visits to adjacent reefs before returning to the seagrass areas from which they were released. It is possible that as fish grow, they perform exploratory movements outside of their routine home ranges to adjacent reefs prior to permanently dispersing. This ‘area expansion’ behaviour where juvenile fish move to adult habitats and then return to nursery areas has been previously observed in telemetry studies (Childs et al. 2008; Huijbers et al. 2015; Murray et al. 2018; Stamp et al. 2021). For example, Murray et al. (2018) reported that juvenile Leerfish (Lichia amia) tagged in South Africa routinely made excursions out of estuaries to open coastal areas and attributed this to exploratory behaviour before a permanent ontogenetic habitat shift. Such a life-history strategy would allow later-stage juveniles to access the resources they require without the costs of permanently dispersing.
The lack of permanent dispersal over the timescale of the current study (~400 days) may suggest that many fish can remain in seagrass as adults and fewer fish perform explicit ontogenetic shifts to reef habitats than previously theorised. Indeed, this is supported by the larger mature yellowfin bream tagged in this study showing site-fidelity to seagrass habitats and previous observations of both species being present in seagrass as adults in the study system (Kiggins et al. 2018; Rees et al. 2018). It would be significant if few individuals permanently disperse to adult populations on reefs, as it would suggest that these species exist as a metapopulation, with a large proportion of the population not dispersing and contributing individuals to the larger metapopulation (i.e., not supplying recruits to coastal populations). Such a result may indicate that connectivity between inshore and coastal populations of the study species may be limited, and the depletion of coastal populations could take substantial time for stocks to rebuild from estuarine recruits. Alternatively, it could be possible that the nursery function of the habitats examined in this study may be limited, although this is unlikely. Future research quantifying the movements of these species over longer time periods is therefore needed to determine whether limited dispersal persists over multiple years. Furthermore, future work should compare the contribution of recruits to coastal populations in multiple systems to test the generality of our results, or whether intraspecific variations in movement patterns for juveniles occur, to better understand the nursery function of estuarine and inshore habitats.
The nursery-role concept has been essential for identifying key fish habitats to be included in conservation and management strategies, yet it has been argued that current definitions of nurseries may not incorporate complexities associated with seascape connectivity (Sheaves et al. 2006; Nagelkerken et al. 2015; Litvin et al. 2018). In the current study, we reveal that whilst both juvenile luderick and yellowfin bream tagged at several sites in Jervis Bay exhibit strong site attachment to small areas of seagrass habitat, they can show wide-ranging movements and connect a mosaic of different patch types. For instance, fish tracked in the Hare Bay NTMR frequently moved across large areas of seagrass, into creeks (i.e., Carama Inlet) and to rocky reefs directly adjacent to seagrass. This implies that the juvenile fish tracked in this study are not obligately bound to certain patch types (e.g., seagrass) and that they can use multiple spatially connected habitats to access food or shelter. These findings demonstrate that the current framework for defining nursery areas as discrete or singular habitats could be refined to view nurseries as a combination of habitats that fish regularly use and visit during juvenile and subadult life stages. This ‘seascape nursery’ approach offers a more realistic definition of nursery areas and would assist resource managers to identify appropriate areas for management actions (Nagelkerken et al. 2015; Sheaves et al. 2015; Bradley et al. 2019; Pittman et al. 2021).
There was strong site fidelity observed for fish released in the Hare Bay NTMR, with few individuals crossing the reserve boundary. This finding adds to the growing literature demonstrating that many fishes have small home ranges and remain inside NTMRs over relatively long time periods (Ferguson et al. 2013; Pittman et al. 2014; Harasti et al. 2015; Kendall et al. 2017; Novak et al. 2020). Strong retention within NTMRs has been previously observed for adult luderick in JBMP (Ferguson et al. 2013, 2016), along with multiple species belonging to Sparidae in Australia (Harasti et al. 2015) and abroad (Parsons et al. 2003; March et al. 2011; La Mesa et al. 2013; Abecasis et al. 2015). For example, Harasti et al. (2015) reported that juvenile and adult pink snapper (Sparidae: Chrysophrys auratus) displayed site fidelity to a NTMR in NSW for up to 1249 days. A total of five fish in the current study moved across reserve boundaries but all returned to the NTMR after spending days to months in fished waters. These movements provide evidence of spill-over from the NTMR into adjacent fished populations which can influence biodiversity, productivity and ecological functioning (McClanahan and Mangi 2000; Halpern et al. 2009; Weigel et al. 2014; Di Lorenzo et al. 2020).
The utilisation of multiple habitats by juvenile fish and the gradual increase in species home range with size have important yet rarely considered implications for marine reserve design (Nash et al. 2015; Carr et al. 2017; Balbar and Metaxas 2019). Currently, connectivity and ontogenetic habitat shifts are infrequently considered in spatial conservation strategies (but see Weeks 2017; Balbar and Metaxas 2019; Friesen et al. 2019), which is undoubtedly due to the paucity of empirical data on the movement of fishes across the seascape at various life-stages. For instance, Balbar and Metaxas (2019) reported that for 746 marine protected areas located across six countries, only 11% considered connectivity as an ecological criterion by managers designing them. In the face of limited information, marine reserves are often designed to protect a portion of the habitat in an area (i.e., habitat representation; Sala et al. 2002; Airamé et al. 2003; Rondinini 2011; Hernandez et al. 2021), with little understanding of how they are connected through processes such as animal movement. The use of multiple habitats at juvenile life stages in this study highlights that simply representing habitats within marine reserves may not allow their conservation potential to be reached. Instead, it may be more appropriate for reserve design to protect areas where there are a variety of habitats in close proximity. This strategy should incorporate connectivity and maximise conservation benefits by protecting a large proportion of the population during their movements. It would also safeguard key fish habitats (e.g., seagrasses, mangroves and saltmarshes) into the future that are currently under pressure (Waycott et al. 2009; Dunic et al. 2021; Swadling et al. 2023; Rees et al. 2023).
Conclusion
Our results demonstrate that juveniles of two co-occurring species important for fisheries show small-scale site attachment to seagrass habitats, but frequently move between different areas such as creeks and rocky reefs. The routine use of multiple habitat types by juvenile fish has important ramifications for the nursery-role concept. Specifically, these findings highlight that the concept should be refined from viewing nurseries as discrete habitat types and instead consider them as mosaics of functionally connected patches within the seascape (i.e., seascape nurseries; Nagelkerken et al. 2015). Although fish were detected moving to rocky reefs directly adjacent to seagrass at greater rates than to reefs farther away, these movements did not represent permanent dispersal to adult populations, with many individuals making repeated visits to reefs. The reef-ward movements could be driven by exploratory movements as fish grow, which would correspond to changing resource requirements, maturation and reduced predation risk (Kimirei et al. 2013; Grol et al. 2014). Overall, gaining a more quantitative understanding on nursery function and the movement patterns of juvenile fish across the seascape, including habitat-use and the spatiotemporal scale of ontogenetic habitat shifts, will help improve knowledge on species-habitat linkages and better inform managers to assist the design of conservation strategies such as marine reserves.
Data Availability
The datasets used and/or analysed in this study can be accessed through the IMOS ATF database (https://animaltracking.aodn.org.au/) under the NSW DPI Jervis Bay project. Alternatively, the data is available on request from the corresponding author.
References
Abecasis, D., and B.H. e Costa, P. Afonso, E.J. Gonçalves, and K. Erzini. 2015. Early reserve effects linked to small home ranges of a commercial fish, Diplodus sargus, Sparidae. Marine Ecology Progress Series 518: 255–266. https://doi.org/10.3354/meps11054.
Airamé, S., J.E. Dugan, K.D. Lafferty, H. Leslie, D.A. McArdle, and R.R. Warner. 2003. Applying ecological criteria to marine reserve design: A case study from the California Channel Islands. Ecological Applications 13: 170–184. https://doi.org/10.1890/1051-0761(2003)013[0170:AECTMR]2.0.CO;2.
Aspillaga, E., R. Arlinghaus, M. Martorell-Barceló, G. Follana-Berná, A. Lana, A. Campos-Candela, and J. Alós. 2021. Performance of a novel system for high-resolution tracking of marine fish societies. Animal Biotelemetry 9: 1–14. https://doi.org/10.1186/s40317-020-00224-w.
Balbar, A.C., and A. Metaxas. 2019. The current application of ecological connectivity in the design of marine protected areas. Global Ecology and Conservation 17: e00569. https://doi.org/10.1016/j.gecco.2019.e00569.
Barbier, E.B., S.D. Hacker, C. Kennedy, E.W. Koch, A.C. Stier, and B.R. Silliman. 2011. The value of estuarine and coastal ecosystem services. Ecological Monographs 81: 169–193. https://doi.org/10.1890/10-1510.1.
Beck, M.W., K.L. Heck, K.W. Able, D.L. Childers, D.B. Eggleston, B.M. Gillanders, B. Halpern, C.G. Hays, K. Hoshino, T.J. Minello, R.J. Orth, P.F. Sheridan, and M.P. Weinstein. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates: A better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas. BioScience 51: 633–641. https://doi.org/10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2.
Becker, S., J. Finn, A. Danylchuk, C. Pollock, Z. Hillis-Starr, I. Lundgren, and A. Jordaan. 2016. Influence of detection history and analytic tools on quantifying spatial ecology of a predatory fish in a marine protected area. Marine Ecology Progress Series 562: 147–161. https://doi.org/10.3354/meps11962.
Berkström, C., L. Eggertsen, W. Goodell, C.A.M.M. Cordeiro, M.B. Lucena, R. Gustafsson, S. Bandeira, N. Jiddawi, and C.E.L. Ferreira. 2020. Thresholds in seascape connectivity: The spatial arrangement of nursery habitats structure fish communities on nearby reefs. Ecography 43: 882–896. https://doi.org/10.1111/ecog.04868.
Bradley, M., R. Baker, I. Nagelkerken, and M. Sheaves. 2019. Context is more important than habitat type in determining use by juvenile fish. Landscape Ecology 34: 427–442. https://doi.org/10.1007/s10980-019-00781-3.
Brown, R.S., D.R. Geist, K.A. Deters, and A. Grassell. 2006. Effects of surgically implanted acoustic transmitters > 2% of body mass on the swimming performance, survival and growth of juvenile sockeye and Chinook salmon. Journal of Fish Biology 69: 1626–1638. https://doi.org/10.1111/j.1095-8649.2006.01227.x.
Bullard, F. 1991. Estimating the home range of an animal: A Brownian bridge approach. Chapel Hill: Thesis, University of North Carolina.
Burnham, K.P., and D. Anderson. 2003. Model selection and multi-model inference. A practical information-theoretic approach. New York, NY: Springer.
Calenge, C. 2006. The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecological Modelling 197: 516–519. https://doi.org/10.1016/j.ecolmodel.2006.03.017.
Campbell, H.A., M.E. Watts, R.G. Dwyer, and C.E. Franklin. 2012. V-Track: Software for analysing and visualising animal movement from acoustic telemetry detections. Marine and Freshwater Research 63: 815–820. https://doi.org/10.1071/MF12194.
Carr, M.H., S.P. Robinson, C. Wahle, G. Davis, S. Kroll, S. Murray, E.J. Schumacker, and M. Williams. 2017. The central importance of ecological spatial connectivity to effective coastal marine protected areas and to meeting the challenges of climate change in the marine environment. Aquatic Conservation: Marine and Freshwater Ecosystems 27: 6–29. https://doi.org/10.1002/aqc.2800.
Childs, A.R., P.D. Cowley, T.F. Næsje, and R.H. Bennett. 2015. Habitat connectivity and intra-population structure of an estuary-dependent fishery species. Marine Ecology Progress Series 537: 233–245. https://doi.org/10.3354/meps11456.
Childs, A.R., P.D. Cowley, T.F. Næsje, A.J. Booth, W.M. Potts, E.B. Thorstad, and F. Økland. 2008. Estuarine use by spotted grunter Pomadasys commersonnii in a South African estuary, as determined by acoustic telemetry. African Journal of Marine Science 30: 123–132. https://doi.org/10.2989/AJMS.2008.30.1.12.462.
Clements, K.D., and J.H. Choat. 1997. Comparison of herbivory in the closely-related marine fish genera Girella and Kyphosus. Marine Biology 127: 579–586. https://doi.org/10.1007/s002270050048.
Croft, D.P., J.R. Madden, D.W. Franks, and R. James. 2011. Hypothesis testing in animal social networks. Trends in Ecology & Evolution 26: 502–507. https://doi.org/10.1016/j.tree.2011.05.012.
Crossin, G.T., M.R. Heupel, C.M. Holbrook, N.E. Hussey, S.K. Lowerre-Barbieri, V.M. Nguyen, G.D. Raby, and S.J. Cooke. 2017. Acoustic telemetry and fisheries management. Ecological Applications 27: 1031–1049. https://doi.org/10.1002/eap.1533.
Csardi, G., and T. Nepusz. 2006. The igraph software package for complex network research. InterJournal, Complex Systems 1695: 1–9.
Curley, B.G., A.R. Jordan, W.F. Figueira, and V.C. Valenzuela. 2013. A review of the biology and ecology of key fishes targeted by coastal fisheries in south-east Australia: Identifying critical knowledge gaps required to improve spatial management. Reviews in Fish Biology and Fisheries 23: 435–458. https://doi.org/10.1007/s11160-013-9309-7.
Dahlgren, C.P., G.T. Kellison, A.J. Adams, B.M. Gillanders, M.S. Kendall, C.A. Layman, A.J. Ley, I. Nagelkerken, and J.E. Serafy. 2006. Marine nurseries and effective juvenile habitats: concepts and applications. Marine Ecology Progress Series 312: 291–295. https://doi.org/10.3354/meps312291.
Di Lorenzo, M., P. Guidetti, A. Di Franco, A. Calò, and J. Claudet. 2020. Assessing spillover from marine protected areas and its drivers: A meta-analytical approach. Fish and Fisheries 21: 906–915. https://doi.org/10.1111/faf.12469.
Dorenbosch, M., M.G.G. Grol, M.J.A. Christianen, I. Nagelkerken, and G. Van Der Velde. 2005. Indo-Pacific seagrass beds and mangroves contribute to fish density and diversity on adjacent coral reefs. Marine Ecology Progress Series 302: 63–76. https://doi.org/10.3354/meps302063.
Donaldson, M.R., S.G. Hinch, C.D. Suski, A.T. Fisk, M.R. Heupel, and S.J. Cooke. 2014. Making connections in aquatic ecosystems with acoustic telemetry monitoring. Frontiers in Ecology and the Environment 12: 565–573. https://doi.org/10.1890/130283.
Duffing Romero, M.D., J.K. Matley, J. Luo, J.S. Ault, S.J. Pittman, and R.S. Nemeth. 2021. Movement patterns of juvenile Atlantic tarpon (Megalops atlanticus) in Brewers Bay, St. Thomas, U.S., Virgin Islands. Animal Biotelemetry 9: 1–14. https://doi.org/10.1186/s40317-021-00239-x.
Dunic, J.C., C.J. Brown, R.M. Connolly, M.P. Turschwell, and I.M. Côté. 2021. Long-term declines and recovery of meadow area across the world’s seagrass bioregions. Global Change Biology 27: 4096–4109. https://doi.org/10.1111/gcb.15684.
Dunning, J.B., B.J. Danielson, and H.R. Pulliam. 1992. Ecological processes that affect populations in complex landscapes. Oikos 65: 169–175. https://doi.org/10.2307/3544901.
Ferguson, A.M., E.S. Harvey, and N.A. Knott. 2016. Herbivore abundance, site fidelity and grazing rates on temperate reefs inside and outside marine reserves. Journal of Experimental Marine Biology and Ecology 478: 96–105. https://doi.org/10.1016/j.jembe.2016.02.008.
Ferguson, A.M., E.S. Harvey, M.D. Taylor, and N.A. Knott. 2013. A herbivore knows its patch: Luderick, Girella tricuspidata, exhibit strong site fidelity on shallow subtidal reefs in a temperate marine park. PLoS ONE 8: e65838. https://doi.org/10.1371/journal.pone.0065838.
Finn, J.T., J.W. Brownscombe, C.R. Haak, S.J. Cooke, R. Cormier, T. Gagne, and A.J. Danylchuk. 2014. Applying network methods to acoustic telemetry data: Modeling the movements of tropical marine fishes. Ecological Modelling 293: 139–149. https://doi.org/10.1016/j.ecolmodel.2013.12.014.
Friesen, S.K., R. Martone, E. Rubidge, J.A. Baggio, and N.C. Ban. 2019. An approach to incorporating inferred connectivity of adult movement into marine protected area design with limited data. Ecological Applications 29: e01890. https://doi.org/10.1002/eap.1890.
Gaines, S.D., C. White, M.H. Carr, and S.R. Palumbi. 2010. Designing marine reserve networks for both conservation and fisheries management. Proceedings of the National Academy of Sciences of the United States of America 107: 18286–18293. https://doi.org/10.1073/pnas.090647310.
Gillanders, B.M. 2002. Connectivity between juvenile and adult fish populations: Do adults remain near their recruitment estuaries? Marine Ecology Progress Series 240: 215–223. https://doi.org/10.3354/meps240215.
Gillanders, B.M. 2007. Seagrasses, fish, and fisheries. In Seagrasses: Biology, Ecology and Conservation, ed. A. Larkum, R.J. Orth, and D. Carlos, 503–505. Netherlands: Springer.
Goetze, J.S., S. Wilson, B. Radford, R. Fisher, T.J. Langlois, J. Monk, N.A. Knott, H. Malcolm, L.M. Currey-Randall, D. Ierodiaconou, D. Harasti, N. Barrett, R.C. Babcock, N.E. Bosch, D. Brock, J. Claudet, J. Clough, D.V. Fairclough, M.R. Heupel, T.H. Holmes, C. Huveneers, A.R. Jordan, D. McLean, M. Meekan, D. Miller, S.J. Newman, M.J. Rees, K.E. Roberts, B.J. Saunders, C.W. Speed, M.J. Travers, E. Treml, S.K. Whitmarsh, C.B. Wakefield, and E.S. Harvey. 2021. Increased connectivity and depth improve the effectiveness of marine reserves. Global Change Biology 27: 3432–3447. https://doi.org/10.1111/gcb.15635.
Goni, R., S. Adlerstein, D. Alvarez-Berastegui, A. Forcada, O. Renones, G. Criquet, S. Polti, C. Cadiou, C. Valle, P. Lenfant, and P. Bonhomme. 2008. Spillover from six western Mediterranean marine protected areas: Evidence from artisanal fisheries. Marine Ecology Progress Series 366: 159–174. https://doi.org/10.3354/meps07532.
Gray, C.A., J.A. Haddy, J. Fearman, L.M. Barnes, W.G. Macbeth, and B.W. Kendall. 2012. Reproduction, growth and connectivity among populations of Girella tricuspidata (Pisces: Girellidae). Aquatic Biology 16: 53–68. https://doi.org/10.3354/ab00428.
Grol, M.G.G., A.L. Rypel, and I. Nagelkerken. 2014. Growth potential and predation risk drive ontogenetic shifts among nursery habitats in a coral reef fish. Marine Ecology Progress Series 502: 229–244. https://doi.org/10.3354/meps10682.
Grüss, A., D.M. Kaplan, S. Guénette, C.M. Roberts, and L.W. Botsford. 2011. Consequences of adult and juvenile movement for marine protected areas. Biological Conservation 144: 692–702. https://doi.org/10.1016/j.biocon.2010.12.015.
Halpern, B.S., S.E. Lester, and J.B. Kellner. 2009. Spillover from marine reserves and the replenishment of fished stocks. Environmental Conservation 36: 268–276. https://doi.org/10.1017/S0376892910000032.
Harasti, D., K.A. Lee, C. Gallen, J.M. Hughes, and J. Stewart. 2015. Movements, home range and site fidelity of snapper (Chrysophrys auratus) within a temperate marine protected area. PLoS ONE 10: e0142454. https://doi.org/10.1371/journal.pone.0142454.
Heck Jnr, K., G. Hays, and R.J. Orth. 2003. Critical evaluation of the nursery role hypothesis for seagrass meadows. Marine Ecology Progress Series 253: 123–136. https://doi.org/10.3354/meps253123.
Hernandez, S., C. Benham, R.L. Miller, M. Sheaves, and S. Duce. 2021. What drives modern protected area establishment in Australia? Conservation Science and Practice 3: e501. https://doi.org/10.1111/csp2.501.
Holbrook, C.M., T. Hayden, and T. Binder. 2017. glatos: a package for the great lakes acoustic telemetry observation system. R package version 0.1.3. https://gitlab.oceantrack.org/GreatLakes/glatos.
Horne, J.S., E.O. Garton, S.M. Krone, and J.S. Lewis. 2007. Analyzing animal movements using Brownian bridges. Ecology 88: 2354–2363. https://doi.org/10.1890/06-0957.1.
Huijbers, C.M., I. Nagelkerken, and C.A. Layman. 2015. Fish movement from nursery bays to coral reefs: A matter of size? Hydrobiologia 750: 89–101. https://doi.org/10.1007/s10750-014-2162-4.
Hussey, N.E., S.T. Kessel, K. Aarestrup, S.J. Cooke, P.D. Cowley, A.T. Fisk, R.G. Harcourt, K.N. Holland, S.J. Iverson, J.F. Kocik, J.E. Mills Flemming, and F.G. Whoriskey. 2015. Aquatic animal telemetry: A panoramic window into the underwater world. Science 348: 1255642. https://doi.org/10.1126/science.1255642.
Jacoby, D.M.P., and R. Freeman. 2016. Emerging network-based tools in movement ecology. Trends in Ecology & Evolution 31: 301–314. https://doi.org/10.1016/j.tree.2016.01.011.
Jepsen, N., C. Schreck, S. Clements, and E. Thorstad. 2005. A brief discussion on the 2% tag/bodymass rule of thumb. In Advances and applications – proceedings of the fifth conference on fish telemetry, ed. M.T. Spedicato, G. Lembo, and G. Marmulla, 255–259. Ustica, Italy.
Kendall, M.S., L. Siceloff, A. Winship, and M.E. Monaco. 2017. Determining conservation potential of an opportunistically defined MPA boundary using fish telemetry. Biological Conservation 211: 37–46. https://doi.org/10.1016/j.biocon.2017.05.010.
Kessel, S.T., S.J. Cooke, M.R. Heupel, N.E. Hussey, C.A. Simpfendorfer, S. Vagle, and A.T. Fisk. 2014. A review of detection range testing in aquatic passive acoustic telemetry studies. Reviews in Fish Biology and Fisheries 24: 199–218. https://doi.org/10.1007/s11160-013-9328-4.
Kiggins, R.S., N.A. Knott, and A.R. Davis. 2018. Miniature baited remote underwater video (mini-BRUV) reveals the response of cryptic fishes to seagrass cover. Environmental Biology of Fishes 101: 1717–1722. https://doi.org/10.1007/s10641-018-0823-2.
Kilminster, K., K. McMahon, M. Waycott, G.A. Kendrick, P. Scanes, L. McKenzie, K.R. O’Brien, M. Lyons, A. Ferguson, P. Maxwell, T. Glasby, and J. Udy. 2015. Unravelling complexity in seagrass systems for management: Australia as a microcosm. Science of the Total Environment 534: 97–109. https://doi.org/10.1016/j.scitotenv.2015.04.061.
Kimirei, I.A., I. Nagelkerken, M. Trommelen, P. Blankers, N. van Hoytema, D. Hoeijmakers, C.M. Huijbers, Y.D. Mgaya, and A.L. Rypel. 2013. What drives ontogenetic niche shifts of fishes in coral reef ecosystems? Ecosystems 16: 783–796. https://doi.org/10.1007/s10021-013-9645-4.
Knott, N.A., J. Williams, D. Harasti, H.A. Malcolm, M.A. Coleman, B.P. Kelaher, M.J. Rees, A. Schultz, and A. Jordan. 2021. A coherent, representative, and bioregional marine reserve network shows consistent change in rocky reef fish assemblages. Ecosphere 12: e03447. https://doi.org/10.1002/ecs2.3447.
Kramer, D.L., and M.R. Chapman. 1999. Implications of fish home range size and relocation for marine reserve function. Environmental Biology of Fishes 55: 65–79. https://doi.org/10.1023/A:1007481206399.
La Mesa, G., I. Consalvo, A. Annunziatellis, and S. Canese. 2013. Spatio-temporal movement patterns of Diplodus vulgaris (Actinopterygii, Sparidae) in a temperate marine reserve (Lampedusa, Mediterranean Sea). Hydrobiologia 720: 129–144. https://doi.org/10.1007/s10750-013-1631-5.
Le Port, A., J. Montgomery, A. Smith, A. Croucher, I. McLeod, and S. Lavery. 2017. Temperate marine protected area provides recruitment subsidies to local fisheries. Proceedings of the Royal Society B: Biological Sciences 284: 20171300. https://doi.org/10.1098/rspb.2017.1300.
Lédée, E.J.I., M.R. Heupel, M.D. Taylor, R.G. Harcourt, F.R.A. Jaine, C. Huveneers, V. Udyawer, H.A. Campbell, R.C. Babcock, X. Hoenner, et al. 2021. Continental-scale acoustic telemetry and network analysis reveal new insights into stock structure. Fish and Fisheries 22: 987–1005. https://doi.org/10.1111/faf.12565.
Lédée, E.J.I., M.R. Heupel, A.J. Tobin, D.M. Knip, and C.A. Simpfendorfer. 2015. A comparison between traditional kernel-based methods and network analysis: An example from two nearshore shark species. Animal Behaviour 103: 17–28. https://doi.org/10.1016/j.anbehav.2015.01.039.
Litvin, S.Y., M.P. Weinstein, M. Sheaves, and I. Nagelkerken. 2018. What makes nearshore habitats nurseries for nekton? An emerging view of the nursery role hypothesis. Estuaries and Coasts 41: 1539–1550. https://doi.org/10.1007/s12237-018-0383-x.
March, D., J. Alós, A. Grau, and M. Palmer. 2011. Short-term residence and movement patterns of the annular seabream Diplodus annularis in a temperate marine reserve. Estuarine, Coastal and Shelf Science 92: 581–587. https://doi.org/10.1016/j.ecss.2011.02.015.
Matley, J.K., N.V. Klinard, S.M. Larocque, A.A. Weinz, and S.F. Colborne. 2021. Space use of juvenile and subadult yellow perch (Perca flavescens) in the Detroit River using acoustic telemetry: Incorporating variable detection ranges in vegetated areas. Canadian Journal of Fisheries and Aquatic Sciences 79: 63–72. https://doi.org/10.1139/cjfas-2020-0425.
McClanahan, T.R., and S. Mangi. 2000. Spillover of exploitable fishes from a marine park and its effect on the adjacent fishery. Ecological Applications 10: 1792–1805. https://doi.org/10.1890/1051-0761(2000)010[1792:SOEFFA]2.0.CO;2.
McCook, L.J., G.R. Almany, M.L. Berumen, J.C. Day, A.L. Green, G.P. Jones, J.M. Leis, S. Planes, G.R. Russ, P.F. Sale, and S.R. Thorrold. 2009. Management under uncertainty: Guide-lines for incorporating connectivity into the protection of coral reefs. Coral Reefs 28: 353–366. https://doi.org/10.1007/s00338-008-0463-7.
Morrison, M.A. 1990. Ontogenetic shifts in the ecology of the parore, Girella tricuspidata. Auckland: University of Auckland Masters thesis.
Morton, R., I. Halliday, and D. Cameron. 1993. Movement of tagged juvenile tailor (Pomatomus saltatrix) in Moreton Bay, Queensland. Marine and Freshwater Research 44: 811–816. https://doi.org/10.1071/MF9930811.
Murray, N.J., T.A. Worthington, P. Bunting, S. Duce, V. Hagger, C.E. Lovelock, R. Lucas, M.I. Saunders, M. Sheaves, and M. Spalding. 2022. High-resolution mapping of losses and gains of Earth’s tidal wetlands. Science 376: 744–749. https://doi.org/10.1126/science.abm9583.
Murray, T.S., P.D. Cowley, R.H. Bennett, and A.R. Childs. 2018. Fish on the move: Connectivity of an estuary-dependent fishery species evaluated using a large-scale acoustic telemetry array. Canadian Journal of Fisheries and Aquatic Sciences 75: 2038–2052. https://doi.org/10.1139/cjfas-2017-0361.
Nagelkerken, I., M. Sheaves, R. Baker, and R.M. Connolly. 2015. The seascape nursery: A novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish & Fisheries 16: 362–371. https://doi.org/10.1111/faf.12057.
Nash, K.L., J.Q. Welsh, N.A.J. Graham, and D.R. Bellwood. 2015. Home-range allometry in coral reef fishes: Comparison to other vertebrates, methodological issues and management implications. Oecologia 177: 73–83. https://doi.org/10.1007/s00442-014-3152-y.
Novak, A.J., S.L. Becker, J.T. Finn, A.J. Danylchuk, C.G. Pollock, Z. Hillis-Starr, and A. Jordaan. 2020. Inferring residency and movement patterns of horse-eye jack Caranx latus in relation to a Caribbean marine protected area acoustic telemetry array. Animal Biotelemetry 8: 1–13. https://doi.org/10.1186/s40317-020-00199-8.
Olds, A.D., R.M. Connolly, K.A. Pitt, and P.S. Maxwell. 2012. Primacy of seascape connectivity effects in structuring coral reef fish assemblages. Marine Ecology Progress Series 462: 191–203. https://doi.org/10.3354/meps09849.
Parsons, D.M., R.C. Babcock, R.K.S. Hankin, T.J. Willis, J.P. Aitken, R.K. O’Dor, and G.D. Jackson. 2003. Snapper Pagrus auratus (Sparidae) home range dynamics: acoustic tagging studies in a marine reserve. Marine Ecology Progress Series 262: 253–265. https://doi.org/10.3354/meps262253.
Pittman, S., and C. McAlpine. 2003. Movements of marine fish and decapod crustaceans: Process, theory and application. Advances in Marine Biology 44: 205–294. https://doi.org/10.1016/s0065-2881(03)44004-2.
Pittman, S., K. Yates, P. Bouchet, D. Alvarez-Berastegui, S. Andréfouët, S. Bell, C. Berkström, C. Boström, C. Brown, and R. Connolly. 2021. Seascape ecology: Identifying research priorities for an emerging ocean sustainability science. Marine Ecology Progress Series 663: 1–29. https://doi.org/10.3354/meps13661.
Pittman, S.J., M.E. Monaco, A.M. Friedlander, B. Legare, R.S. Nemeth, M.S. Kendall, M. Poti, R.D. Clark, L.M. Wedding, and C. Caldow. 2014. Fish with chips: Tracking reef fish movements to evaluate size and connectivity of Caribbean marine protected areas. PLoS ONE 9: e96028. https://doi.org/10.1371/journal.pone.0096028.
Pollock, B.R. 1985. The reproductive cycle of yellowfin bream, Acanthopagrus australis (Günther), with particular reference to protandrous sex inversion. Journal of Fish Biology 26: 301–311. https://doi.org/10.1111/j.1095-8649.1985.tb04269.x.
Pursche, A.R., I.M. Suthers, and M.D. Taylor. 2014. The effect of targeted stocking on behaviour and space utilization of a released finfish. ICES Journal of Marine Science 71: 1100–1106. https://doi.org/10.1093/icesjms/fst209.
Pursche, A.R., C.T. Walsh, and M.D. Taylor. 2013. Evaluation of a novel external tag-mount for acoustic tracking of small fish. Fisheries Management and Ecology 21: 169–172. https://doi.org/10.1111/fme.12051.
R Core Development Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/.
Raubenheimer, D., W.L. Zemke-White, R.J. Phillips, and K.D. Clements. 2005. Algal macronutrients and food selection by the omnivorous marine fish Girella tricuspidata. Ecology 86: 2601–2610. https://doi.org/10.1890/04-1472.
Rees, M.J., N.A. Knott, and A.R. Davis. 2018. Habitat and seascape patterns drive spatial variability in temperate fish assemblages: Implications for marine protected areas. Marine Ecology Progress Series 607: 171–186. https://doi.org/10.3354/meps12790.
Rees, M.J., N.A. Knott, K.L. Astles, D.S. Swadling, G.J. West, A.M. Ferguson, J. Delamont, P.T. Gibson, J. Neilson, G.F. Birch, and T.M. Glasby. 2023. Cumulative effects of multiple stressors impact an endangered seagrass population and fish communities. Science of the Total Environment 904: 166706. https://doi.org/10.1016/j.scitotenv.2023.166706.
Rees, M.J., N.A. Knott, M.L. Hing, M. Hammond, J. Williams, J. Neilson, D.S. Swadling, and A. Jordan. 2021. Habitat and humans predict the distribution of juvenile and adult snapper (Sparidae: Chrysophrys auratus) along Australia’s most populated coastline. Estuarine, Coastal and Shelf Science 257: 107397. https://doi.org/10.1016/j.ecss.2021.107397.
Rondinini, C. 2011. Meeting the MPA network design principles of representativity and adequacy: developing species-area curves for habitats. Peterborough, UK: Joint Nature Conservation Committee.
Russell, A., M.D. Taylor, T.C. Barnes, D.D. Johnson, and B.M. Gillanders. 2021. Potential linkages between juvenile nurseries and exploited populations of mulloway (Argyrosomus japonicus), explored using otolith chemistry. Fisheries Research 243: 106063. https://doi.org/10.1016/j.fishres.2021.106063.
Sala, E., O. Aburto-Oropeza, G. Paredes, I. Parra, J.C. Barrera, and P.K. Dayton. 2002. A general model for designing networks of marine reserves. Science 298: 1991–1993. https://doi.org/10.1126/science.1075284.
Sambrook, K., A.S. Hoey, S. Andréfouët, G.S. Cumming, S. Duce, and M.C. Bonin. 2019. Beyond the reef: The widespread use of non-reef habitats by coral reef fishes. Fish and Fisheries 20: 903–920. https://doi.org/10.1111/faf.12383.
Sheaves, M., R. Baker, and R. Johnston. 2006. Marine nurseries and effective juvenile habitats: An alternative view. Marine Ecology Progress Series 318: 303–306. https://doi.org/10.3354/meps318303.
Sheaves, M., R. Baker, I. Nagelkerken, and R.M. Connolly. 2015. True value of estuarine and coastal nurseries for fish: Incorporating complexity and dynamics. Estuaries and Coasts 38: 401–414. https://doi.org/10.1007/s12237-014-9846-x.
Sheaves, M., J. Brookes, R. Coles, M. Freckelton, P. Groves, R. Johnston, and P. Winberg. 2014. Repair and revitalisation of Australia’s tropical estuaries and coastal wetlands: Opportunities and constraints for the reinstatement of lost function and productivity. Marine Policy 47: 23–38. https://doi.org/10.1016/j.marpol.2014.01.024.
Simpfendorfer, C.A., M.R. Heupel, and R.E. Hueter. 2002. Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Canadian Journal of Fisheries and Aquatic Sciences 59: 23–32. https://doi.org/10.1139/f01-191.
Simpfendorfer, C.A., C. Huveneers, A. Steckenreuter, K. Tattersall, X. Hoenner, R. Harcourt, and M.R. Heupel. 2015. Ghosts in the data: False detections in VEMCO pulse position modulation acoustic telemetry monitoring equipment. Animal Biotelemetry 3: 1–10. https://doi.org/10.1186/s40317-015-0094-z.
Spalding, M.D., L. Fish, and L.J. Wood. 2008. Toward representative protection of the world’s coasts and oceans—progress, gaps, and opportunities. Conservation Letters 1: 217–226. https://doi.org/10.1111/j.1755-263X.2008.00030.x.
Stamp, T., D. Clarke, S. Plenty, T. Robbins, J.E. Stewart, E. West, and E. Sheehan. 2021. Identifying juvenile and sub-adult movements to inform recovery strategies for a high value fishery—European bass (Dicentrarchus labrax). ICES Journal of Marine Science 78: 3121–3134. https://doi.org/10.1093/icesjms/fsab180.
Stamp, T., E. West, T. Robbins, S. Plenty, and E. Sheehan. 2022. Large-scale historic habitat loss in estuaries and its implications for commercial and recreational fin fisheries. ICES Journal of Marine Science 79: 1981–1991. https://doi.org/10.1093/icesjms/fsac141.
Staveley, T.A., D.M. Jacoby, D. Perry, F. van der Meijs, I. Lagenfelt, M. Cremle, and M. Gullström. 2019. Sea surface temperature dictates movement and habitat connectivity of Atlantic cod in a coastal fjord system. Ecology and Evolution 9: 9076–9086. https://doi.org/10.1002/ece3.5453.
Swadling, D.S., N.A. Knott, M.D. Taylor, M.A. Coleman, A.R. Davis, and M.J. Rees. 2021. Seascape connectivity of temperate fishes between estuarine nursery areas and open coastal reefs. Journal of Applied Ecology 59: 1406–1416. https://doi.org/10.1111/1365-2664.14157.
Swadling, D.S., N.A. Knott, M.J. Rees, and A.R. Davis. 2019. Temperate zone coastal seascapes: Seascape patterning and adjacent seagrass habitat shape the distribution of rocky reef fish assemblages. Landscape Ecology 34: 2337–2352. https://doi.org/10.1007/s10980-019-00892-x.
Swadling, D.S., N.A. Knott, M.J. Rees, H. Pederson, K.R. Adams, M.D. Taylor, and A.R. Davis. 2020. Seagrass canopies and the performance of acoustic telemetry: Implications for the interpretation of fish movements. Animal Biotelemetry 8: 1–12. https://doi.org/10.1186/s40317-020-00197-w.
Swadling, D.S., G.J. West, P.T. Gibson, R.J. Laird, and R. J. and T.M. Glasby. 2023. Don’t go breaking apart: Anthropogenic disturbances predict meadow fragmentation of an endangered seagrass. Aquatic Conservation: Marine and Freshwater Ecosystems 33: 56–69. https://doi.org/10.1002/aqc.3905.
Szekeres, P., A.V. Agberien, M. Dick, A.J. Danylchuk, and S.J. Cooke. 2022. Short-term space use of small-bodied fish in coastal flats ecosystems in The Bahamas: An acoustic telemetry study using the smallest commercially available transmitters. Environmental Biology of Fishes 106: 321–336. https://doi.org/10.1007/s10641-022-01360-3.
Taylor, M.D., R.C. Babcock, C.A. Simpfendorfer, and D.A. Crook. 2017a. Where technology meets ecology: Acoustic telemetry in contemporary Australian aquatic research and management. Marine and Freshwater Research 68: 1397–1402. https://doi.org/10.1071/MF17054.
Taylor, M.D., S.W. Laffan, A.V. Fairfax, and N.L. Payne. 2017b. Finding their way in the world: Using acoustic telemetry to evaluate relative movement patterns of hatchery-reared fish in the period following release. Fisheries Research 186: 538–543. https://doi.org/10.1016/j.fishres.2016.07.003.
Thorstad, E.B., A.H. Rikardsen, A. Alp, and F. Økland. 2013. The use of electronic tags in fish research–an overview of fish telemetry methods. Turkish Journal of Fisheries and Aquatic Sciences 13: 881–896. https://doi.org/10.4194/1303-2712-v13_5_13.
Waycott, M., C.M. Duarte, T.J. Carruthers, R.J. Orth, W.C. Dennison, S. Olyarnik, A. Calladine, J.W. Fourqurean, K.L. Heck, and A.R. Hughes. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences 106: 12377–12381. https://doi.org/10.1073/pnas.0905620106.
Weeks, R. 2017. Incorporating seascape connectivity in conservation prioritisation. PLoS ONE 12: e0182396. https://doi.org/10.1371/journal.pone.0182396.
Weeks, R., A.L. Green, E. Joseph, N. Peterson, and E. Terk. 2017. Using reef fish movement to inform marine reserve design. Journal of Applied Ecology 54: 145–152. https://doi.org/10.1111/1365-2664.12736.
Weigel, J.Y., K.O. Mannle, N.J. Bennett, E. Carter, L. Westlund, V. Burgener, Z. Hoffman, A. Simão Da Silva, E.A. Kane, and J. Sanders. 2014. Marine protected areas and fisheries: Bridging the divide. Aquatic Conservation: Marine and Freshwater Ecosystems 24: 199–215. https://doi.org/10.1002/aqc.2514.
West, G.J., and T.M. Glasby. 2022. “Interpreting long-term patterns of seagrasses abundance: How seagrass variability is dependent on genus and estuary type. Estuaries and Coasts 45: 1393–1408. https://doi.org/10.1007/s12237-021-01026-w.
Whoriskey, K., E.G. Martins, M. Auger-Méthé, L.F. Gutowsky, R.J. Lennox, S.J. Cooke, M. Power, and J. Mills Flemming. 2019. Current and emerging statistical techniques for aquatic telemetry data: A guide to analysing spatially discrete animal detections. Methods in Ecology and Evolution 10: 935–948. https://doi.org/10.1111/2041-210X.13188.
Wickham, H. 2016. ggplot2: elegant graphics for data analysis. New York, NY: Springer. https://ggplot2.tidyverse.org/.
Wood, S., and M.S. Wood. 2015. Package ‘mgcv’. R package version 1:29. https://CRAN.R-project.org/package=mgcv.
Zuur, A., E. Ieno, N. Walker, A. Saveliev, and G. Smith. 2009. Mixed effects models and extensions in ecology with R, ed. M. Gail, K. Krickeberg, J.M. Samet, A. Tsiatis, W. Wong. New York, NY: Springer.
Acknowledgements
We thank NSW Department of Primary Industries staff Dr. Martin Hing, Matthew Hammond, Mark Fackerell and Eddie Douglas for their support and assistance in the field. We also thank the numerous field volunteers who assisted this project, including Jason Delamont, Danielle Kampers, Nicola Kennedy, Dr. Kye Adams, Dr. Marie-Claire Demers, Casey O’Connor, Brittany Daniels, David Ruiz-Garcia and Douglas Reeves. We thank Greg West for discussion and review of the draft manuscript. This research was carried out under NSW Fisheries Scientific Collection Permit P01/0059(A), NSW Marine Parks Permit JBMP 18006/050623 and University of Wollongong Animal Research Authority AE14/25.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This research was funded by the NSW Department of Primary Industries, Seaworld Research and Rescue Foundation Inc. and the Winifred Violet Scott Charitable Trust (SWR/2/2018), University of Wollongong Centre for Sustainable Ecosystem Solutions, and the Ecological Society of Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Communicated by Ronald Baker
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Swadling, D.S., Knott, N.A., Taylor, M.D. et al. Consequences of Juvenile Fish Movement and Seascape Connectivity: Does the Concept of Nursery Habitat Need a Rethink?. Estuaries and Coasts 47, 607–621 (2024). https://doi.org/10.1007/s12237-023-01323-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-023-01323-6