Abstract
Groundwater may be highly enriched in dissolved carbon species, but its role as a source of carbon to coastal waters is still poorly constrained. Exports of deep and shallow groundwater-derived dissolved carbon species from a small subtropical estuary (Korogoro Creek, Australia, latitude −31.0478°, longitude 153.0649°) were quantified using a radium isotope mass balance model (233Ra and 224Ra, natural groundwater tracers) under two hydrological conditions. In addition, air-water exchange of carbon dioxide and methane in the estuary was estimated. The highest carbon inputs to the estuary were from deep fresh groundwater in the wet season. Most of the dissolved carbon delivered by groundwater and exported from the estuary to the coastal ocean was in the form of dissolved inorganic carbon (DIC; 687 mmol m−2 estuary day−1; 20 mmol m−2 catchment day−1, respectively), with a large export of alkalinity (23 mmol m−2 catchment day−1). Average water to air flux of CO2 (869 mmol m−2 day−1) and CH4 (26 mmol m−2 day−1) were 5- and 43-fold higher, respectively, than the average global evasion in estuaries due to the large input of CO2- and CH4-enriched groundwater. The groundwater discharge contribution to carbon exports from the estuary for DIC, dissolved organic carbon (DOC), alkalinity, CO2, and CH4 was 22, 41, 3, 75, and 100 %, respectively. The results show that CO2 and CH4 evasion rates from small subtropical estuaries surrounded by wetlands can be extremely high and that groundwater discharge had a major role in carbon export and evasion from the estuary and therefore should be accounted for in coastal carbon budgets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuarine ecosystems provide a major pathway for carbon to travel across the land–ocean interface and are considered an important component of the global carbon cycle (Rodríguez-Murillo et al. 2015; Seitzinger et al. 2005). Estuarine carbon input and transformation processes include material exchange with surrounding environments (Hans et al. 2011) (in particular coastal wetlands (Cai 2011)), high rates of primary productivity and respiration (Bianchi 2007), benthic-pelagic coupling (Maher and Eyre 2010), freshwater inputs (Dixon et al. 2014), atmospheric exchange (Borges and Abril 2011), and groundwater discharge (Liu et al. 2014). Of these processes, groundwater exchange is probably the most poorly resolved and has received little attention until recently.
Groundwater discharge/porewater exchange has been suggested to influence carbon cycling in estuarine environments (Santos et al. 2012a; Faber et al. 2014; Wang et al. 2015). Groundwater inputs to coastal waters may be volumetrically small when compared to surface water inputs; however, due to the high concentrations of dissolved constituents, groundwater can be a major pathway for dissolved material exports between terrestrial and coastal systems (Burnett et al. 2006; Maher et al. 2013a; Liu et al. 2014). Submarine groundwater discharge (SGD) is defined as the exchange of groundwater between land and ocean regardless of its composition and scale and may be fresh terrestrial, recirculated seawater, or a combination of both (Moore 2010). Fresh groundwater delivers new water along with dissolved constituents, while recirculated saline groundwater can deliver large amounts of recycled carbon and nutrients to surface waters (Weinstein et al. 2007; Gleeson et al. 2013; Liu et al. 2014). The fresh and recirculated marine components of SGD are often well mixed, thus making quantification of their relative contribution to coastal waters complex.
Previous studies exploring SGD inputs of dissolved carbon have reported that groundwater discharge from aquifers to the estuarine and coastal zones may be a significant source of CO2 and CH4 to the atmosphere (Cai 2011; Atkins et al. 2013; Call et al. 2015). Additionally, pCO2 and CH4 in groundwater can be orders of magnitude higher than atmospheric values (Gagan et al. 2002; Cai 2003; Call et al. 2015). However, estuarine carbon budgets have rarely assessed the importance of groundwater discharge as a driver of CO2, dissolved inorganic carbon (DIC), and dissolved organic carbon (DOC). The results from these limited studies clearly demonstrate that groundwater inputs of dissolved carbon can be significant and warrant quantification in coastal carbon budgets (Porubsky et al. 2014; Wang et al. 2015).
Most previous studies related to groundwater-derived carbon cycling have been conducted in the USA and Australia; however, there is still a lack of data from a variety of ecosystems, including estuaries in the southern hemisphere (Cai 2003; Liu et al. 2012; Maher et al. 2013a). Prior studies have attempted to estimate groundwater-derived fluxes of DIC (Atkins et al. 2013), DOC (Goñi and Gardner 2003; Kim et al. 2011), alkalinity (Stewart et al. 2015; Cyronak et al. 2014), DIC and DOC (Santos et al. 2012a; Maher et al. 2013a; Porubsky et al. 2014), or DIC and alkalinity (Santos et al. 2015; Liu et al. 2014; Wang et al. 2015) into coastal waters. To our knowledge, no previous study has attempted to simultaneously quantify groundwater-derived inputs of the four main dissolved carbon species (DIC, DOC, CO2, and CH4) and alkalinity into an estuary, and the relative importance of groundwater carbon inputs to atmospheric evasion of CO2 and CH4 and carbon exports to the coastal ocean.
We hypothesize that groundwater discharge into a small subtropical estuary will be a significant pathway for carbon exported to the coastal ocean. We tested this hypothesis by performing time series measurements of carbon parameters (DIC, DOC, pCO2, pCH4), alkalinity, and 222Rn and Ra isotopes in a tidal estuary that has a simple geometry. Our objectives were to (1) estimate atmospheric CO2 and CH4 evasion rates from the estuary, (2) calculate groundwater-derived inputs to the estuary of the four main dissolved carbon species and alkalinity under contrasting hydrological conditions (wet season and dry season), (3) quantify the relative importance of meteoric fresh and recirculated saline groundwater-derived carbon inputs to the estuary, and (4) determine the relative importance of groundwater in the total carbon and alkalinity exports from the estuary to the coastal ocean. This study builds on a companion paper reporting a detailed radon and radium isotope investigation in the same system (Sadat-Noori et al. 2015).
Materials and Method
Site Description
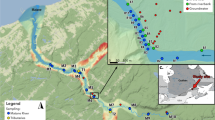
The investigation was carried out in Korogoro Creek (latitude −31.04781°, longitude 153.06492°), a small (5 km long, ~20–25 m wide, average depth 0.9 m, area 116,160 m2) subtropical tidal estuary in Hat Head, NSW, Australia (Fig. 1). The estuary has a small catchment (18 km2) which is low lying and subject to flooding by seawater during spring tides. The estuary has a residence time of around 1 day and is normally flushed during each tidal cycle, with ocean water penetrating the lower 4 km of the estuary at high tide (Ruprecht and Timms 2010). The region has an average annual rainfall of 1490 mm and experiences a mild subtropical climate all year round. January and July have the highest (26.9 °C) and lowest (11.2 °C) monthly mean air temperatures, respectively. Rainfall is highest from February to March (175.2 mm month−1) and lowest from July to September (71 mm month−1) (http://www.bom.gov.au). Our most downstream station was located at the mouth of the estuary (−31.057624°, 153.056151°) at a sandy beach environment, while the rest of the stations were surrounded by fringing mangrove vegetation.
Study site (Had Head, NSW, Australia) displaying the time series stations (green points) and groundwater sampling locations (red points). The distance between stations 1 and 2 and 2 and 3 is 1.5 km, while stations 3 and 4 are 1 km apart. The dark box represents the study site. The entire estuary length was fringed by mangroves. All carbon species were measured in Station 1, while only pCO2 data are available for stations 2–4. Image from Google Earth
Experimental Design
Two field campaigns were carried out over two hydrologically distinct seasons (wet and dry). The first set of time series data collection (wet season) was conducted from 08:45 am March 25 to 10:15 am March 27, 2013, while the second field campaign (dry season) was carried out from 08:30 pm June 6 to 10:00 am June 10, 2013. Both field campaigns were conducted around the spring tides. However, the tidal range between the semidiurnal tides varied. During the wet season, the tidal range was similar between the semidiurnal tides (~1.2 m), while in the dry season, the tidal range varied between ~0.6 and 1 m (Fig. 2) over the semidiurnal tides.
Surface water time series data from the downstream station located at the mouth of the estuary in the wet (March) and dry (June) seasons. The radon data are reported in our companion paper (Sadat-Noori et al. 2015)
During the first field campaign, we deployed automatic high frequency time series monitoring stations at four approximately equally spaced sites (~1 to 1.5 km apart) along the length of the estuary (Fig. 1). During the second field campaign, two time series monitoring stations were deployed. The station at the mouth of the estuary, here after referred to as “downstream station” continually monitored salinity, temperature, dissolved oxygen, current velocity, pCO2, pCH4, and 222Rn, during the two field campaigns, while the other stations measured the same suite of parameters with the exception of pCH4. A time series of discrete samples for DIC, DOC, and TAlk were also collected from the downstream station every hour over two consecutive tidal cycles (˃25 h) during both sampling campaigns. Groundwater samples were collected throughout the catchment (Fig. 1) by installing shallow piezometers (Charette and Allen 2006) and by sampling deep monitoring wells in the region to characterise the groundwater endmember concentration. A mass balance model was developed to evaluate fresh and saline groundwater-derived carbon and alkalinity fluxes, atmospheric exchange rates of CO2 and CH4, and to quantify carbon exports from the estuary.
Surface Water Time Series Observations
A calibrated Hydrolab automatic logger was used to measure pH (±0.02 units), salinity (±0.02 ppt), dissolved oxygen (±0.2 mg L−1), and water temperature (±0.10 °C), at 15-min intervals, at all stations, during both sampling campaigns. Depth loggers (CTD divers; Schlumberger Water Services) measured estuary depth (±0.01 m), at 10-min intervals at each of the four stations. Wind speed data were obtained online (www.wunderground.com) from a weather station at 10-m height located at South West Rocks, about 15 km away from the study site. An acoustic Doppler current profiler (ADCP; Sontek Argonaut) was installed in the middle of the estuary at the downstream site to measure current velocity and direction of flow averaged over 10-min intervals. This was combined with time-specific cross-sectional area (adjusted for tidal height) to obtain 10-min discharge estimates assuming that currents across the channel were homogenous. At the other three time series stations, current velocity was measured using Starflow Ultrasonic Doppler Flow Recorders. The estuary cross section was measured at high tide using a depth gauge at 2-m width intervals.
For determining 222Rn concentrations, hereafter referred to as “radon,” a radon-in-air monitor modified for radon-in-water (RAD 7, Durridge Co.) was used (Burnett et al. 2010 and references therein). Radon was measured every 10 min for about 40 h in the wet season and 60 h in the dry season. At the downstream station during both seasons, a cavity ring down spectrometer (Picarro G2201-i) coupled to a showerhead equilibrator was used to measure pCO2 and pCH4 at ~1 Hz (Maher et al. 2013b) with data averaged over 1-min intervals. The equilibrated air is continuously pumped in a closed-loop from the headspace of the equilibrator chamber through desiccant (Drierite), the cavity ring down spectrometer, a RAD7 and then back to the equilibrator. For measuring pCO2 at other stations, a Li-Cor 820 CO2 analyzer coupled to a RAD7 radon monitor was used (Santos et al. 2012b). CH4 partial pressure was converted to concentrations based on the solubility coefficient calculated as a function of temperature and salinity (Wiesenburg and Guinasso 1979) to allow for easy comparison with previous studies that generally use CH4 concentration rather than partial pressure.
Discrete samples were collected using a sample-rinsed 60-ml polyethylene syringe every hour for about 25 h from the downstream station in both seasons. Samples collected for DIC and DOC concentrations were filtered through 0.7 μm Whatman GF/F filters into acid-rinsed, milli-q rinsed, precombusted (4 h, 400 °C) 40-ml volatile organic carbon borosilicate vials containing 100 μl of saturated HgCl2, without any headspace or bubbles. Samples for alkalinity (TAlk) were filtered through 0.7 μm Whatman GF/F and collected in 30-ml polycarbonate vials. The samples were stored on ice until returning to the lab where they were stored at 4 °C until analysis.
Groundwater Sampling
Groundwater sampling was performed at the same time as surface water sampling in both field campaigns. Samples were collected using a push point piezometer system (Charette and Allen 2006). The tubing was thoroughly flushed with the sample prior to collecting each sample. DOC, DIC, and TAlk were sampled as per surface water methods described earlier. For radon, shallow wells ranging between 0.5 and 2 m deep were dug adjacent to the estuary near each time series station (Fig. 1), using a handheld auger at low tide. PVC pipes with 50-cm-long slotted screens were installed to allow groundwater infiltrate into the pipe. In addition, deep (5–21 m) monitoring wells installed by the NSW Office of Water located across the catchment were also sampled (Fig. 1). A peristaltic pump was used to take samples after the wells were purged (three times the casing volume). Groundwater samples are the same as those used for radium isotope and radon concentrations in Sadat-Noori et al. (2015).
Samples for CO2 and CH4 were collected in gas-tight 250-ml bottles, overflowing at least three times the bottle volume, to which 200 μL of saturated HgCl2 solution was added. A calibrated handheld YSI multiprobe was used to determine pH, temperature, DO, and salinity for each groundwater sample. A total of 27 groundwater samples were collected. Six-liter gas-tight HDPE plastic bottles were used to collect samples for groundwater radon analysis. Each six-liter bottle was connected to a RAD7 radon monitor and given at least 2 h to achieve an air-water radon equilibrium with <5 % uncertainty following well established protocols (Lee and Kim 2006). After radon analysis, the water was filtered through magnesium impregnated acrylic fibers for radium analysis (Peterson et al. 2009). Groundwater samples were classified in two classes; deep (˃5 m) and shallow (˂5 m) based on the radium data in the companion paper (Sadat-Noori et al. 2015).
Analytical Methods
Groundwater CO2 and CH4 samples were analyzed via a headspace method using a Picarro G2201-i as described by Gatland et al. (2014). Analysis for DIC and DOC concentrations were carried out following the wet oxidation method (St‐Jean 2003) with an OI Aurora 1030 W interfaced with a Therma Delta V+ Isotope Ratio Mass Spectrometer (IRMS) (Maher and Eyre 2011). TAlk (±0.2 %) was measured by Gran Titration using a Metrohm automatic titrator and 0.01 M HCl standardized to Dickson Certified Reference Material (Batch 111). Free CO2 within the estuary was determined by using the DIC and TAlk pair, and also the pCO2 and TAlk pair, using version 25 of the CO2SYS program (Pelletier et al. 2007) with the carbonic acid disassociation constants from Millero et al. (2006) and the KHSO4 constant from Dickson (1990). Radium samples were collected at the downstream time series station every hour for 30 h in wet and dry seasons, and a Radium Delayed Coincidence Counter (RaDeCC) was used for measuring 223Ra and 224Ra based on Moore and Arnold (1996). Radium data and estimated groundwater discharge rates are presented in a companion paper (Sadat-Noori et al. 2015).
Calculations
The CO2 and CH4 atmospheric exchange were estimated following Wanninkhof (1992):
where C water and C air are the partial pressure of CO2 or CH4 in the water column and in air, respectively, in units of μatm; α is the solubility coefficient, calculated as a function of temperature and salinity using the constants of Weiss (1974) for CO2 and Wiesenburg and Guinasso (1979) for CH4, k is the gas transfer velocity at the water–air interface (m day−1). The atmospheric pCO2 and pCH4 were assumed to be constant at an average of 400 and 1.8 μatm, respectively. We used an empirical equation which estimates transfer velocity as a function of water depth, current, and wind speed, which are the dominant sources of water turbulence in estuarine systems (Borges et al. 2004):
where k 600 is the transfer velocity (normalized to a Schmidt number of 600), W is the water current (cm s−1), D is water depth (m), and U 10 is the wind speed at a height of 10 m (m s−1). The Schmidt number is defined as the ratio between the kinematic viscosity to mass diffusivity. All k 600 values were corrected for the Schmidt number of CO2 and CH4 at in situ temperatures and salinities (Wanninkhof 1992), assuming a linear relationship between salinities of 0 and 35. The main uncertainty associated with quantifying air–water gas exchange results from the estimation of gas transfer velocity (k). Most previous studies have used empirical equations which calculate transfer velocity only as a function of wind speed. The model used in this study incorporates current and wind induced turbulence at the air–water interface.
The hourly estuarine export (ebb tide) and import (flood tide) of the four dissolved carbon species (DIC, DOC, free CO2, and CH4) and total alkalinity was estimated by multiplying hourly discharge rates by the carbon species concentration. Daily averages where calculated by integrating export and import rates over two tidal cycles then dividing by total time for the two tidal cycles (~25 h) to get an hourly rate, and multiplying by 24 (hours in 1 day) to obtain a daily rate. Groundwater carbon fluxes were calculated by multiplying the corresponding daily volumetric groundwater discharge in each season obtained from Sadat-Noori et al. (2015) by the median concentration of different carbon species in groundwater following Eq. (3):
where GWC flux is groundwater carbon fluxes, GWdis. is groundwater discharge in each season and GWmed. C conc. is the median concentration of different carbon species in groundwater. The median concentration was used due to the nonnormal distribution of the groundwater endmember concentrations.
A nonsteady state radium mass balance was applied to quantify fresh and saline discharging groundwater into the estuary. The model details and results are presented in a companion paper (Sadat-Noori et al. 2015). Briefly, concentrations of radium in surface water are converted into net fluxes of groundwater, discharging into the estuary over a 24-h diel cycle. Inputs to the model were groundwater, upstream 223Ra input flux during flood tide, diffusion from sediments, and desorption from suspended sediments while outputs consisted of 223Ra downstream output flux during ebb tides and the 223Ra decay.
Results
Hydrological Conditions
Contrasting hydrological conditions occurred during each field campaign. Two months prior to the time series measurements in March (wet season), the area received 612 mm of rainfall. The June 2013 (dry season) time series deployment had base flow conditions with only 57 mm of rain in the 2 months prior to field campaign. As a result, the groundwater level during March was 100 cm higher than in June. Based on the rainfall events of the area and for simplicity, we describe the first and second field campaigns as wet and dry seasons, respectively. Surface freshwater discharge (i.e., net freshwater discharge out of the mouth of the estuary) was 3 m3 s−1 in the wet season and decreased to 2.2 m3 s−1 in the dry season. Wet season had an average surface water temperature of 25.9 °C compared to 19.4 °C in the dry season. Wind speeds were on average 3.1 and 1.7 m s−1 during the wet and dry seasons, respectively (Fig. 2). Tidal range was ~1.2 m in the wet season while in the dry season the tidal range varied between 1 and 0.6 m (Fig. 2). Salinity showed a tidal trend and ranged from nearly fresh (1) to saline (up to 35) over a tidal cycle (Fig. 2). Salinity increased rapidly during the start of the flood tide just taking 2.5 h to reach 34 and dropped more slowly during ebb tide taking about 5 h to reach minimum values. Similar salinity trends were observed in both campaigns.
Groundwater Observations and Discharge Rates
Shallow and deep groundwater dissolved carbon concentrations were highly variable (Table 1). Median DIC, DOC, and TAlk in shallow groundwater were 1.1, 1.2, and 1.5 times higher than deep groundwater. Median pCO2 in deep samples (21,109 μatm) was similar to median pCO2 in shallow samples (20,924 μatm), while median CH4 concentration was 6.6 times higher in the deep samples (53 μM) than shallow (8 μM).
The discharging groundwater into the estuary surface water was separated into shallow saline and deep fresh groundwater components. Depth was used as a separation factor rather than salinity because in a tidal estuary with a short resident time salinity may not truly represent the spatial groundwater distribution along the estuary. For example, tidal pumping at the downstream station would cause high salinities in shallow groundwater, while the salinity in groundwater samples at station 4 were never higher than 5. Moreover, the 5 m indicator used as a separation was based on the fact that radium concentration was generally higher in samples collected below 5 m (see Sadat-Noori et al. 2015).
Separate discharge rates were estimated based on 223Ra and 224Ra for wet and dry seasons and deep and shallow groundwater discharge. An average of the 223Ra and 224Ra rates was used to calculate seasonal deep and shallow groundwater discharge rates which were then used to calculate groundwater-derived carbon fluxes entering estuary surface water (refer to Sadat-Noori et al. 2015 for groundwater discharge calculations). In the wet season, groundwater-derived DIC from fresh deep groundwater was 1.8 times higher than saline shallow groundwater-derived DIC. DOC and alkalinity derived from fresh deep groundwater was 1.7- and 1.3-fold higher than saline shallow groundwater-derived DOC (Table 2). Groundwater-derived free CO2 and CH4 were 2.1- and 14-fold higher from fresh deep groundwater compared to shallow. In the dry season, DIC, DOC, alkalinity, and free CO2 from saline shallow GW were 6.2, 6.3, 8.8, and 5.3 times higher than the fresh deep groundwater fluxes while CH4 fluxes were similar from both deep and shallow fluxes. All estimates of groundwater-derived carbon inputs to the estuary were higher in the wet season (Table 2).
Estuary Surface Water Time Series Measurements
Dissolved oxygen (DO) followed a clear tidal trend with the highest values at high tide (Fig. 2). DO reached 100 % saturation at high tide in both seasons and dropped to 50 and 30 % at low tide during the dry and wet seasons, respectively. Radon, pCO2 and CH4 followed a tidal trend in both seasons with the highest concentrations being recorded at low tide and lowest at high tide (Fig. 2). Radon concentrations ranged from 0.6 to 180.2 Bq m−3 with an average of 50.5 Bq m−3 in wet season and varied between 6.2 and 209.1 Bq m−3 with an average of 86.5 Bq m−3 in dry season, respectively (Fig. 2).
DOC followed a tidal trend with high concentrations at low tide and low concentrations at high tide (Fig. 2), while DIC and TAlk concentrations displayed an opposite tidal trend (high concentrations at high tide). DIC concentrations ranged from 809 to 2151 μM with an average of 1558 μM in wet conditions and 1500 μM in the dry season (Fig. 2). DOC ranged from 38 to 2158 μM with an average of 931 μM in the wet season and 668 μM in the dry season. Alkalinity varied between 840 and 2347 μM with a similar average in both seasons.
Carbon dioxide was the only carbon species with observations in multiple stations along the estuary. At the downstream station, pCO2 followed a tidal trend and was 1.5 times higher in the wet season compared to the dry season (Fig. 3). Average pCO2 at stations 2, 3, and 4 was about 14,000 μatm in the wet season. Average pCO2 for station 2 in the dry was 9549 μatm. Maximum pCO2 in surface waters was 25,130 μatm in the wet season (station 2) and 16,764 μatm in the dry season (Fig. 3). CH4 concentrations (station 1 only) ranged from 5 nM (high tide) to about 3 μM (low tide), while in the dry season, CH4 varied between 5 nM (high tide) to 4.8 μM (low tide).
CO2 and CH4 Water to Air Fluxes
Stations 1, 2, 3, and 4 had average gas transfer velocities (k 600) values of 4.6, 3.0, 4.4, and 3.1 m day−1, respectively, in the wet season while stations 1 and 2 had k 600 values of 1.7 and 2.9 m day−1, respectively, in the dry season. In the wet season, CO2 fluxes from the lower, mid and upper estuary were estimated to be 573 (station 1 average), 1505 (average of station 2 and 3), and 1650 mmol m−2 day−1 (average of stations 3 and 4), respectively. In the dry season, the average was 220 for station 1 and 1300 mmol m−2 day−1 for station 2 (no upper estuary values in dry season, due to vandalism). Wet and dry seasons had an integrated average CO2 flux of 1128 and 620 mmol m−2 day−1 (Table 3). CH4 fluxes from the downstream station were 17 and 43 mmol m−2 day−1 in wet and dry seasons, respectively (Table 3).
Estuarine Carbon Export
The Hat Head estuary exported on average 20 ± 4 and 9 ± 2 mmol C m−2 of catchment day−1 of DIC and DOC, respectively, to the coastal ocean (Table 4) based on the two field campaigns. DIC, DOC, TAlk, and free CO2 exports were 35, 80, 30, and 93 %, higher in the wet season compared to the dry season, while CH4 was 50 % higher in the dry season. Average alkalinity export (23 ± 5 mmol m−2 of catchment day−1) was similar to DIC and approximately six times higher than free CO2 (4 ± 1 mmol C m−2 of catchment day−1) and several orders of magnitude higher than CH4 (0.005 ± 0.001 mmol C m−2 of catchment day−1).
Discussion
Carbon Data Integrity
As we over-constrained the carbonate system, we investigate the reliability of our measured DIC data by comparing measured DIC concentrations with those calculated from pCO2 and TAlk (Fig. 4). Our comparison showed that calculated DIC concentrations were on average 9 % higher than measured DIC concentration in 90 % of the surface water samples. However, for fresh groundwater DIC samples, calculated and measured concentrations showed a closer agreement (Fig. 4b). The lower measured DIC concentrations may be due to CO2 losses through contact to air at the time of sampling and filtration, and/or, due to an overestimation of calculated DIC due to the contribution of organic acids to the TAlk pool (Hunt et al. 2011; Abril et al. 2015). In spite of these uncertainties, the estimated estuary DIC export rates are similar using both calculated and measured DIC, with only 8 and 6 % higher export rate using the calculated DIC values in the wet and dry season, respectively (Table 4).
Carbon Water to Air Fluxes
Water to air CO2 and CH4 fluxes over the study period show that Hat Head estuary was a source of CO2 (620 to 1128 mmol m−2 day−1) and CH4 to the atmosphere (17 to 43 mmol m−2 day−1) (Table 3). Atkins et al. (2013) reported similarly high CO2 fluxes of 800 mmol m−2 day−1 in the upper North Creek Estuary, NSW, Australia, with a smaller k value of 2.8 m day−1. Frankignoulle et al. (1998) found that nine European estuaries had a mean CO2 flux of 170 mmol m−2 day−1 using a k value of 1.9 m day−1. The average CO2 emission from ten Brazilian estuaries was reported to be 55 ± 45 mmol m−2 day−1 with pCO2 varying between 168 and 8638 μatm (Noriega and Araujo 2014). We calculated fluxes based on empirical models of k similar to Atkins et al. (2013) and Noriega and Araujo (2014), while Frankignoulle et al. (1998) used the floating chamber method. The global average pCO2 in upper estuaries is estimated to be 3033 ± 1078 μatm with a corresponding atmospheric CO2 flux of 188 ± 70 mmol m−2 day−1 (Chen et al. 2012). In our case, the CO2 water to air flux from the upper estuary (1650 mmol m−2 day−1) was an order of magnitude higher than the estimated global average. The high fluxes in this study are likely to be directly related to groundwater inputs (see below).
Several previous studies have utilized a fixed time series measuring station usually located at the mouth of the estuary to estimate CO2 and/or CH4 flux for the entire area of the estuary (Bouillon et al. 2007; Maher et al. 2013a). While time series measurements have the advantage of capturing temporal variation with very high resolution, they may not be representative of estuary-wide fluxes due to the inability to account for spatial variation. Maher et al. (2015) suggested that multiple time series stations or a combination of both time series and survey methods may be required to adequately constrain the variability of estuarine CO2 and CH4 fluxes at the estuary scale. Here, we simultaneously deployed four fixed time series stations approximately 1.5 km apart along the length of the estuary (~5 km) to cover both temporal and spatial variability, thus providing a more robust estimate of the estuary-wide CO2 dynamics. A similar sampling strategy could not be applied to CH4 and other carbon species due to logistic reasons.
Our multistation approach demonstrated the importance of spatial variability in estuarine pCO2, when calculating estuary-wide fluxes. We estimated CO2 fluxes using four automated measuring stations in the wet season and two stations in the dry season. If we would have only used a single station at the mouth of the estuary, CO2 fluxes upscaled to the entire estuary would be underestimated by 50 and 65 % during the wet and dry seasons, respectively (573 and 220 mmol m−2 day−1, for wet and dry seasons), however, still an order of magnitude higher than the global average estimate for lower estuaries (19–59 mmol m−2 day−1) (Borges and Abril 2011; Cai 2011; Chen et al. 2012). This was calculated following Eq. (1) by assuming that the downstream station represented a partial pressure of CO2 and k value for the entire estuary (i.e., the downstream flux was applied to the entire estuary area). By using the four stations, the estuary could be fragmented, with each section having a site-specific set of partial pressures and piston velocities. On the other hand, if sampling had been conducted only in the upstream section of the estuary, CO2 fluxes would be overestimated considerably. This clearly demonstrates the importance of collecting spatial data from the lower, middle, and upper parts of estuarine systems to be able to estimate a more realistic water–air flux of CO2 as suggested by previous studies (Wang and Cai 2004; Cai 2011; Maher et al. 2015).
Average CH4 fluxes from Hat Head estuary were high, averaging 26 mmol m−2 day−1 (Table 3). These fluxes are about 43 times higher than the higher end of average global methane flux estimates, from tidal estuaries, which range between 0.04 and 0.6 mmol m−2 day−1 (Borges and Abril 2011). Ferrón et al. (2007) and Zhang et al. (2008) reported an annual average CH4 flux of 0.66 and 0.61 mmol m−2 day−1 from tidal estuaries in Bay of Cádiz, SW Spain, and Changjiang, China, while Nirmal Rajkumar et al. (2008) reported CH4 fluxes of 3.6 mmol m−2 day−1 from an estuarine system (Adyar) in India. Maher et al. (2015) found CH4 fluxes in a subtropical Australian estuary to be 0.57 mmol m−2 day−1, and Linto et al. (2014) and Call et al. (2015) reported CH4 fluxes from tidal mangrove estuaries to be 0.35 and 0.21 mmol m−2 day−1, respectively.
Average CO2 atmospheric fluxes were 1.8 times higher in wet (1128 mmol m−2 day−1) than the dry seasons (620 mmol m−2 day−1) (Table 3). This difference may be related to higher temperatures in the wet season (summer) and subsequent higher rates of in situ respiration, and/or nitrification which has a net effect of decreasing alkalinity and pH and therefore increasing pCO2 (Frankignoulle et al. 1996; Gazeau et al. 2005; Borges and Abril 2011; Maher and Eyre 2012). Interestingly, water to air CH4 fluxes where higher in the dry (43 mmol m−2 day−1) than the wet season (17 mmol m−2 day−1), driven by higher concentrations (Fig. 2) rather than higher transfer velocities (Table 3). This is in spite of higher groundwater inputs in the wet season (Table 2). This may be due to seasonal differences in methane oxidation rate (Abril and Iversen 2002) or alternative sources or production rates of CH4 within the estuary during the two seasons. Further studies would be required to assess the factors controlling seasonal variability in surface water CH4 dynamics.
While CO2 emissions may dominate carbon gaseous fluxes, CH4 emissions could have a greater impact on global warming potential of the system (Gatland et al. 2014). Although CH4 losses to the atmosphere were smaller than CO2, CH4 is a more potent greenhouse gas compared to CO2, therefore, accounting only for CO2 evasion in systems where there may be high CH4 emissions, could result in an underestimation, in terms of global warming potential of the system (Gatland et al. 2014; Panneer Selvam et al. 2014; Neubauer and Megonigal 2015). Here, after CH4 fluxes were converted into CO2-equivalent emission estimates assuming a 100 year CH4 sustained-flux global warming potential [i.e., 1 kg CH4 = 45 kg of CO2; Neubauer and Megonigal (2015)], CH4 accounted for ~50 % of CO2-equivalent emissions from the estuary for both seasons, and therefore was significant in terms of greenhouse gas emissions.
Carbon Surface Water Exports
Table 5 presents estimates of DIC and DOC export to coastal waters from small estuarine and large riverine systems. In a review paper, Cai (2011) reported the global riverine DOC export rate to be 246 Tg year−1. Bauer and Bianchi (2011) also reported a similar global oceanic DOC export rate (250 Tg year−1). Based on the world wide surface areas of estuaries which is 1.05 × 1012 m2 (Cai 2011), global DOC export from estuarine systems is estimated to be 0.64 g C m−2 day−1. Hat Head estuary exported 15.5 g C m−2 of estuary day−1 of DOC, which is 24 times higher than the global estuarine DOC export estimate. DOC export per unit area from Hat Head estuary was also higher than much larger riverine systems (Striegl et al. 2007; Cai et al. 2008). Based on Table 5, DOC exports from systems of a similar small size to Hat Head estuary are higher; however, it should be noted that exports reported in Adame and Lovelock (2011), Bergamaschi et al. (2012), Maher et al. (2013a), Wang and Cai (2004), and Winter et al. (1996) are from mangrove or salt marsh systems which essentially have a minimal catchment area to water area ratio, thereby inflating the mmol C m−2 catchment day−1 (i.e., essentially all the catchment is intertidal). Moreover, DIC exports from the small Hat Head estuary were much higher than larger riverine systems on a catchment area basis (Table 5). Hat Head estuary DIC yield (i.e., export per unit of catchment area) were four times higher than the Gulf of Trieste catchment (Tamše et al. 2014), 11–40 times higher than the Yukon River (Striegl et al. 2007), 16–55 times higher than the six largest Arctic Rivers (Tank et al. 2012) and two orders of magnitude higher than DIC exports reported for the Chena River in Alaska (Cai et al. 2008) and the Guadalquivir Estuary, Spain (De La Paz et al. 2007). In comparison with smaller estuaries, Hat Head DIC yield was two orders of magnitude higher than the York River estuary (Raymond et al. 2000) and comparable with intertidal mangrove and saltmarsh systems (Wang and Cai 2004; Bouillon et al. 2008; Maher et al. 2013a; Winter et al. 1996).
The source of DIC is possibly from the surrounding mangroves, as Hat Heat estuary has an extensive mangrove environment throughout the estuary (Fig. 1). Mangrove environments tend to have high DIC export rates (Bouillon et al. 2008; Miyajima et al. 2009; Maher et al. 2013a). Moreover, a DIC versus salinity scatter graph (Fig. 5) showed a slight concave upward trend (at least in the dry season) which suggests mid-estuary inputs of DIC (perhaps from mangrove groundwater). DOC, however, had a conservative or sink nature in relation to salinity (Fig. 5), which suggests an upstream source (likely the freshwater wetlands or groundwater, see also Sanders et al. 2015) with some loss during estuarine transport (respiration, photomineralization, or flocculation). Our mass balance approach suggests significant groundwater inputs of all dissolved carbon species, yet the traditional salinity mixing model approach does not indicate a clear source. Wang et al. (2015) found a significant groundwater source of DIC in the Jiulong estuary (China), yet salinity versus DIC indicated conservative mixing. The authors suggested that the diffuse nature of SGD-derived solute inputs may lead to no deviation from conservative mixing, which may also be the case in Hat Head estuary, and other estuarine systems that have large areas of diffuse groundwater input along the estuary.
Alkalinity export to the coastal ocean ranged from 19 to 27 mmol m−2 of catchment day−1 in dry and wet seasons, respectively (Table 4). Previous studies have reported alkalinity exports, from larger systems; however, studies with time series sampling for calculating alkalinity exports from tidal estuaries are still scarce. Faber et al. (2014) identified that DIC export was mostly alkalinity in a mangrove- and seagrass-dominated tidal creek in southeast Australia. They reported export rates ranging from 140 to 460 mmol m−2 of water area day−1, which was an order of magnitude lower than alkalinity export estimates from Hat Head estuary (3564 mmol m−2 of water area day−1). Santos et al. (2015) reported groundwater-derived alkalinity exports 4.7 times higher than Hat Head from tidal flats in the Wadden Sea (Germany) where porewater alkalinity concentrations are extremely high at 20 mM. Alkalinity production has a significant influence on the global carbon budgets by affecting the oceanic carbonate system. In the case of alkalinity production, carbon is not lost to the atmosphere as CO2 and is exported to the ocean and acts as a buffer which facilitates the uptake of extra CO2 (Faber et al. 2014).
Table 5 shows that a general negative correlation may exist between dissolved carbon yield and catchment area and that small estuarine systems have the ability to deliver more dissolved organic carbon to the coastal ocean compared to larger riverine systems on a catchment area basis. This is mainly due to the shorter residence time of estuaries which reduces the potential for biogeochemical processes to modify the quantity and composition of organic matter. This highlights the importance of studying small systems with a short residence time, such as Hat Head, to obtain a better quantitative understating of global carbon exports to the ocean.
Groundwater-Derived Carbon Fluxes
We could not collect deep groundwater samples during the wet season, and shallow samples were only collected from the most downstream station during the wet season field campaign. We acknowledge the limitations with this approach. However, shallow samples collected at the downstream station were similar during both seasons (averages within 10 %), and previous studies have found that deep groundwater has relatively stable composition (Dhar et al. 2008; Chapagain et al. 2010). Further, shallow groundwater only dominates inputs during the dry season (Sadat-Noori et al. 2015), when we have adequate sampling coverage throughout the estuary to constrain the shallow groundwater endmember. Considering the uncertainty in shallow groundwater composition during the wet season (i.e., we have used the dry season data to estimate this), we have assigned a 100 % uncertainty to this term in our calculations (Table 4). The relative contributions of deep and shallow groundwater carbon inputs during wet and dry seasons basically follows the groundwater discharge rates with deep groundwater dominating carbon inputs in the wet season and shallow groundwater delivering more carbon in the dry season. Groundwater fluxes of each carbon species could not be calculated for each individual section during each season due to the lack of groundwater and surface water samples in the upper reaches of the estuary. Surface water samples were only collected at the downstream station for carbon parameters other than pCO2.
Another limitation to our groundwater fluxes is that the average flux presented here only considers the differences between the wet and dry season while other factors such as differences in spring-neap tide cycles and annual temperature variability may influence the groundwater discharge flux (de Sieyes et al. 2008; Constantz et al. 1994) and estuarine carbon fluxes. Moreover, tidal variability can also influence groundwater discharge rates and consequently carbon fluxes, as tidal pumping releases shallow saline groundwater into the estuary (Call et al. 2015, Maher et al. 2015; Santos et al. 2009). Tidal pumping was the dominant source of groundwater discharge in the dry season making the shallow saline groundwater contribution much higher than deep fresh groundwater. Therefore, some of the differences that were observed may be due to differences in tidal pumping.
Most of the carbon input to surface waters via groundwater was in the form of DOC and DIC and the smallest portion was contributed by CH4 (1 %) in both seasons (Fig. 6). The total groundwater-derived DIC flux entering surface waters was 4.6 times higher in the wet season compared to dry. While the total (deep + shallow) average groundwater-derived DIC fluxes from both seasons (687 ± 117 mmol m−2 of estuary day−1) were comparable to previous studies by Santos et al. (2012a), Dorsett et al. (2011), and Cai (2003) (see Table 6), higher DIC fluxes have been reported in salt marshes/estuaries (Moore et al. 2006) and freshwater tidal creeks (Atkins et al. 2013) (Table 6). The total (deep + shallow) average (wet and dry) groundwater-derived DOC fluxes found here (540 ± 731 mmol m−2 day−1) are high, being at least 3-fold higher than previous studies which have reported groundwater-derived DOC fluxes ranging from 21 to 170 mmol m−2 day−1 (Santos et al. 2012a; Maher et al. 2013a; Porubsky et al. 2014) (Table 6). These high fluxes are related to the high DOC concentrations in groundwater (Table 1).
pCO2 in the water column was positively correlated with radon (Fig. 5), indicating that groundwater is a source of free CO2 and/or [H+]. The strong relationship between pCO2, CH4, and radon (groundwater tracer) during both wet and dry season field campaigns (Fig. 5), in addition to the high groundwater pCO2 and CH4 concentrations, implies groundwater was a major driver of surface water pCO2 and CH4 in Hat Head estuary. This influence could either be directly by discharging pCO2- and CH4-enriched groundwater into the estuary or indirectly by groundwater delivering DOC, which can boost ecosystem respiration and increase pCO2 (Maher et al. 2015). The later process is further supported by the DOC versus radon plot (Fig. 5) which shows a positive correlation. The salinity mixing plots (Fig. 5) show that pCO2 and CH4 have a concave trend with salinity indicating an upstream input likely being groundwater discharge from the upper reaches as this area was found to be a groundwater hotspot in a concurrent study by Sadat-Noori et al. (2015).
Flux calculations offer stronger evidence that groundwater plays a major role in delivering greenhouse gasses to surface waters. The fluxes obtained from the groundwater mass balance approach show that groundwater-derived free CO2 and CH4 inputs can account for a large proportion (54 % for CO2 and 46 % for CH4) of the observed atmospheric fluxes (Table 3). Previous studies have also found groundwater to be a major driver of surface water pCO2 and CH4 (Faber et al. 2014; Macklin et al. 2014; Call et al. 2015). Atkins et al. (2013) reported that groundwater-derived CO2 fluxes into a flood plain creek estuary, NSW, Australia, averaged 1622 mmol m−2 day−1, a value twice as high as atmospheric CO2 evasion in the area, and 1.5 times larger than CO2 fluxes in our case. Conversely, Porubsky et al. (2014) stated groundwater-derived CH4 fluxes were 0.8 mmol m−2 day−1 in Okatee estuary in the USA, 15 times smaller than fluxes from Hat Head estuary.
Radon also had a positive correlation with DOC especially in the wet season, but not as clear as pCO2 and CH4, likely due to multiple processes driving surface water DOC dynamics in this system. For example, radon and DOC may have the same source (groundwater) but have different loss pathways as radon is a gas and its major loss pathway is atmospheric evasion, while DOC maybe lost through respiration, flocculation, and photomineralization. This creates a decoupling between radon and DOC which may not be so apparent as in the radon vs CO2 and CH4 plots (Fig. 5) and explains the stronger correlation between radon and the greenhouse gases.
Estuarine CO2 is driven by biological (productivity/respiration), hydrological (groundwater inputs and mixing of riverine and oceanic waters), and physical (temperature-driven groundwater convection and wind-driven evasion) process (Borges and Abril 2011; Maher et al. 2015). Here, the contribution of groundwater to CO2 loss (evasion and export (Tables 3 and 4)) from the estuary was on average 31 % over both seasons. The groundwater contribution to CH4 loss (evasion and export) was around 46 %, with significant differences in wet and dry seasons. This can be explained through the difference in the groundwater discharge rate between wet and dry seasons (Table 2), where the groundwater contribution is a function of groundwater discharge (which varied significantly in different hydrological conditions at our site) and groundwater end-member concentration (which was assumed to be the same during both seasons).
Dissolved carbon is transported from groundwater to the estuary and then the atmosphere (CO2 and CH4) and the ocean (DIC and DOC). Figure 3 illustrates this for CO2, where oceanic waters enter the estuary with the flood tide, becomes enriched in dissolved carbon within the estuary due to mixing with groundwater (and upstream wetland surface water) and leaves the system via ebb tide flow and atmospheric evasion. By investigating the CO2 versus salinity plot for the 4 stations (Fig. 7), we found hysteresis occurring at some stations with different CO2 values at the same salinity during the ebb and flood tide. For instance, observations at station 3 in the wet season showed that as the salinity starts to decrease from flood tide, CO2 remains low for 8 h. This continues until brackish waters occupy the station (salinity ~8), implying that the initial fresh water entering the estuary is fresh surface water relatively low in CO2. A subsequent increase in CO2 values implies the input of groundwater during the ebb tide, indicating a delayed groundwater input to the estuary. This interpretation is supported by the CO2 versus radon scatter that shows no hysteresis (Fig. 5).
Total average groundwater-derived CH4 fluxes (12 ± 17 mmol m−2 day−1) (Table 2) were much higher than groundwater-derived CH4 fluxes from a small tidal river estuary, in Okatee, USA (0.9 mmol m−2 day−1, Porubsky et al. 2014). They were also three orders of magnitude higher than CH4 fluxes on the Florida Gulf Coast (Cable et al. 1996). Groundwater-derived CH4 fluxes were accountable for almost all the export from the estuary, and much of the free CO2 export could be attributed to groundwater inputs (Table 4) indicating the major role of groundwater in CH4 and dissolved CO2 transported between terrestrial and aquatic environments.
Average DIC and DOC export from the estuary (3081 ± 602 and 1317 ± 258 mmol m−2 day−1, respectively) were 4.4- and 2.4-fold higher than groundwater-derived DIC (687 ± 117 mmol m−2 day−1) and DOC (540 ± 731 mmol m−2 day−1), indicating that groundwater can account for almost half of the dissolved organic carbon export from the estuary. In other words, the average contribution of groundwater to DOC export in both seasons was ~41 % while groundwater contributed ~22 % to DIC export with considerable differences in wet and dry seasons (Table 4). Maher et al. (2013a) also found that groundwater advection was a dominant pathway for DOC export and was responsible for 90 % of DOC export in a mangrove tidal estuary. Faber et al. (2014) reported that 90 % of the carbon loss from an estuary system was from groundwater DIC advection while DOC only accounted for 5 %. Liu et al. (2014) reported that groundwater DIC fluxes were 11–71 times higher than the combined input of local rivers, suggesting that SGD was the dominant source of DIC to the southwest Florida Shelf, USA. Wang et al. (2015) found that SGD input of DIC to the Jiulong River estuary in China was the equivalent to between 25 and 110 % of riverine DIC exports.
Groundwater had a minor contribution to TAlk input into the estuary (~3 %) in both seasons (Table 4). Average alkalinity export from estuary was almost 28-fold higher than groundwater-derived alkalinity inputs, suggesting that processes other than groundwater input are driving alkalinity export from this system. This alkalinity is thought to come from sulfate reduction in shallow porewaters (Faber et al. 2014); however, our groundwater sampling resolution was not adequate to capture this process (alkalinity may be produced in the upper cm of sediments, while our groundwater samples were taken from areas deeper than 1 m).
To summarize the key findings of this study, we present a conceptual diagram (Fig. 8) that illustrates the (1) groundwater discharge rates, (2) flux estimates of groundwater-derived carbon into the estuary, (3) estuary carbon export, and (4) carbon atmospheric evasion in wet and dry conditions in the whole estuary system. The large variability observed in groundwater discharge and carbon loss rates over a relatively short time scale indicates the need for more frequent measurements to be carried to assess the influence of groundwater on carbon cycling. Nevertheless, it is clear that this small wetland-surrounded subtropical estuary has a high carbon yield (in terms of both oceanic export and air–water exchange), and groundwater carbon inputs play a major role in estuarine carbon cycling in this system. Combined with the recent literature, this investigation demonstrates that groundwater may play a major role in estuarine carbon dynamics.
Conceptual diagram of the study area summarizing flux estimates ± standard error of groundwater-derived, estuary export and atmospheric evasion of carbon from the mouth of the estuary in (a) wet and (b) dry seasons. Groundwater discharge rates are in m−3 s−1, and all other parameters are in units of 104 mol day−1 (estuary area = 116,160 m2 and catchment area = 18 km2)
Conclusions
The Hat Head estuary had a high area normalized export rate of DIC (3081 ± 602 mmol m−2 day−1), DOC (1317 ± 258 mmol m−2 day−1), and TAlk (3564 ± 705 mmol m−2 day−1) to the coastal ocean and groundwater-derived carbon inputs were a significant component of this carbon export. Groundwater contribution to carbon loss from the estuary for DIC, DOC, TAlk, free CO2 and CH4 was found to be approximately 22, 41, 3, 75, and 100 %, respectively. The average estuary-wide CO2 and CH4 evasion rates were 870 ± 174 and 26 ± 5 mmol m−2 day−1 (some of the highest estuarine fluxes reported yet), and groundwater discharge accounted for 54 and 46 % of these evasions, respectively. Our observations indicate that small estuarine systems with a short residence time can pump more carbon to the coastal ocean compared to some larger riverine systems on a catchment area basis, and that groundwater exchange may deliver large amounts of carbon to surface estuarine waters.
References
Abril, G., and N. Iversen. 2002. Methane dynamics in a shallow non-tidal estuary (Randers Fjord, Denmark). Marine Ecology: Progress Series 230: 171–181.
Abril, G., S. Bouillon, F. Darchambeau, C.R. Teodoru, T.R. Marwick, F. Tamooh, F. Ochieng Omengo, N. Geeraert, L. Deirmendjian, P. Polsenaere, and A.V. Borges. 2015. Technical note: large overestimation of pCO2 calculated from pH and alkalinity in acidic, organic-rich freshwaters. Biogeosciences 12(1): 67–78.
Adame, M.F., and C.E. Lovelock. 2011. Carbon and nutrient exchange of mangrove forests with the coastal ocean. Hydrobiologia 663: 23–50.
Atkins, M.L., I.R. Santos, S. Ruiz-Halpern, and D.T. Maher. 2013. Carbon dioxide dynamics driven by groundwater discharge in a coastal floodplain creek. Journal of Hydrology 493: 30–42.
Bauer, J., and T. Bianchi. 2011. Dissolved organic carbon cycling and transformation. Treatise on Estuarine and Coastal Science 5: 7–67.
Bergamaschi, B.A., D.P. Krabbenhoft, G.R. Aiken, E. Patino, D.G. Rumbold, and W.H. Orem. 2012. Tidally driven export of dissolved organic carbon, total mercury, and methylmercury from a mangrove-dominated estuary. Environmental Science and Technology 46: 1371–1378.
Bianchi, T.S. 2007. Biogeochemistry of estuaries. New York: Oxford University Press.
Borges, A.V., and G. Abril. 2011. Carbon dioxide and methane dynamics in estuaries. In Treatise on estuarine and coastal science, vol. 5, ed. E. Wolanski and D.S. McLusky, 119–161. Waltham: Academic.
Borges, A., B. Delille, L.-S. Schiettecatte, F. Gazeau, G. Abril, and M. Frankignoulle. 2004. Gas transfer velocities of CO2 in three European estuaries (Randers Fjord, Scheldt and Thames). Limnology and Oceanography 49: 1630–1641.
Bouillon, S., et al. 2007. Importance of intertidal sediment processes and porewater exchange on the water column biogeochemistry in a pristine mangrove creek (Ras Dege, Tanzania). Biogeosciences Discussions 4: 317–348.
Bouillon, S. et al. 2008. Mangrove production and carbon sinks: a revision of global budget estimates. Global Biogeochemical Cycles 22. doi: 10.1029/2007GB003052
Burnett, W.C., et al. 2006. Quantifying submarine groundwater discharge in the coastal zone via multiple methods. The Science of the Total Environment 367: 498–543.
Burnett, W.C., R.N. Peterson, I.R. Santos, and R.W. Hicks. 2010. Use of automated radon measurements for rapid assessment of groundwater flow into Florida streams. Journal of Hydrology 380: 298–304.
Cable, J.E., G.C. Bugna, W.C. Burnett, and J.P. Chanton. 1996. Application of 222Rn and CH4 for assessment of groundwater discharge to the coastal ocean. Limnology and Oceanography 41: 1347–1353.
Cai, W.J. 2003. The geochemistry of dissolved inorganic carbon in a surficial groundwater aquifer in North Inlet, South Carolina, and the carbon fluxes to the coastal ocean. Geochimica et Cosmochimica Acta 67: 631–637.
Cai, W.-J. 2011. Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Annual Review of Marine Science 3: 123–145.
Cai, Y., L. Guo, and T.A. Douglas. 2008. Temporal variations in organic carbon species and fluxes from the Chena River, Alaska. Limnology and Oceanography 53: 1408–1419.
Call, M., et al. 2015. Spatial and temporal variability of carbon dioxide and methane fluxes over semi-diurnal and spring-neap-spring timescales in a mangrove creek. Geochimica et Cosmochimica Acta 150: 211–225.
Chapagain, S.K., V.P. Pandey, S. Shrestha, T. Nakamura, and F. Kazama. 2010. Assessment of deep groundwater quality in Kathmandu valley using multivariate statistical techniques. Water, Air, and Soil Pollution 210: 277–288.
Charette, M.A., and M.C. Allen. 2006. Precision ground water sampling in coastal aquifers using a direct‐push, Shielded‐Screen Well‐Point System. Groundwater Monitoring & Remediation 26: 87–93.
Chen, C.-T.A., T.-H. Huang, Y.-C. Chen, Y. Bai, X. He, and Y. Kang. 2012. Air–sea exchanges of CO2 in the world’s coastal seas. Biogeosciences 10: 6509–6544.
Constantz, J., C.L. Thomas, and G. Zellweger. 1994. Influence of diurnal variations in stream temperature on streamflow loss and groundwater recharge. Water Resources Research 30: 3253–3264.
Cyronak, T., I. R. Santos, A. McMahon, and B. D. Eyre. 2013. Carbon cycling hysteresis in permeable carbonate sands over a diel cycle: Implications for ocean acidification. Limnology & Oceanography, 58(1): 131–143.
Cyronak, T., I.R. Santos, D.V. Erler, D.T. Maher, and B.D. Eyre. 2014. Drivers of pCO2 variability in two contrasting coral reef lagoons: the influence of submarine groundwater discharge. Global Biogeochemical Cycles 28: 398–414.
De La Paz, M., A. Gómez-Parra, and J. Forja. 2007. Inorganic carbon dynamic and air–water CO2 exchange in the Guadalquivir Estuary (SW Iberian Peninsula). Journal of Marine Systems 68: 265–277.
de Sieyes, N.R., K.M. Yamahara, B.A. Layton, E.H. Joyce, and A.B. Boehm. 2008. Submarine discharge of nutrient-enriched fresh groundwater at Stinson Beach, California is enhanced during neap tides. Limnology and Oceanography 53: 1434–1445.
Dhar, R.K., Y. Zhenga, A. van Geen, Z. Cheng, M. Shanewaz, M. Shamsudduha, M.A. Hoque, M.W. Rahman, and K.M. Ahmed. 2008. Temporal variability of groundwater chemistry in shallow and deep aquifers of Araihazar, Bangladesh. Journal of Contaminant Hydrology 99: 97–111.
Dickson, A.G. 1990. Standard potential of the reaction: AgCl (s)+ 12H2 (g) = Ag (s)+ HCl (aq), and the standard acidity constant of the ion HSO4 − in synthetic sea water from 273.15 to 318.15 K. Journal of Chemical Thermodynamics 22: 113–127.
Dixon, J.L., J.R. Helms, R.J. Kieber, and G.B. Avery. 2014. Biogeochemical alteration of dissolved organic material in the Cape Fear River Estuary as a function of freshwater discharge. Estuarine, Coastal and Shelf Science 149: 273–282.
Dorsett, A., A. Jennifer Cherrier, J.B. Martin, and J.E. Cable. 2011. Assessing hydrologic and biogeochemical controls on pore-water dissolved inorganic carbon cycling in a subterranean estuary: A 14C and 13C mass balance approach. Marine Chemistry 127: 76–89.
Dürr, H.H., G.G. Laruelle, C.M. Van Kempen, C.P. Slomp, M. Meybeck, and H. Middelkoop. 2011. Worldwide typology of nearshore coastal systems: defining the estuarine filter of river inputs to the oceans. Estuaries and Coasts 34: 441–458.
Faber, P.A., V. Evrard, R.J. Woodland, I.C. Cartwright, and P.L. Cook. 2014. Pore-water exchange driven by tidal pumping causes alkalinity export in two intertidal inlets. Limnology and Oceanography 59: 1749–1763.
Ferrón, S., T. Ortega, A. Gómez-Parra, and J. Forja. 2007. Seasonal study of dissolved CH4, CO2and N2O in a shallow tidal system of the bay of Cádiz (SW Spain). Journal of Marine Systems 66: 244–257.
Frankignoulle, M., I. Bourge, and R. Wollast. 1996. Atmospheric CO2 fluxes in a highly polluted estuary (the Scheldt). Limnology and Oceanography 41: 365–369.
Frankignoulle, M., et al. 1998. Carbon dioxide emission from European estuaries. Science 282: 434–436.
Gagan, M.K., L.K. Ayliffe, B.N. Opdyke, D. Hopley, H. Scott‐Gagan, and J. Cowley. 2002. Coral oxygen isotope evidence for recent groundwater fluxes to the Australian Great Barrier Reef. Geophysical Research Letters 29: 43-1–43-4.
Gatland, J.R., I.R. Santos, D.T. Maher, T. Duncan, and D.V. Erler. 2014. Carbon dioxide and methane emissions from an artificially drained coastal wetland during a flood: Implications for wetland global warming potential. Geophysical Research: Biogeosciences 119: 1698–1716.
Gazeau, F., et al. 2005. Planktonic and whole system metabolism in a nutrient-rich estuary (the Scheldt estuary). Estuaries and Coasts 28: 868–883.
Gleeson, J., I.R. Santos, D.T. Maher, and L. Golsby-Smith. 2013. Groundwater–surface water exchange in a mangrove tidal creek: evidence from natural geochemical tracers and implications for nutrient budgets. Marine Chemistry 156: 27–37.
Goñi, M.A., and I.R. Gardner. 2003. Seasonal dynamics in dissolved organic carbon concentrations in a coastal water-table aquifer at the forest-marsh interface. Aquatic Geochemistry 9: 209–232.
Hunt, C.W., J.E. Salisbury, and D. Vandermark. 2011. Contribution of non-carbonate anions to total alkalinity and overestimation of pCO2 in New England and New Brunswick rivers. Biogeosciences 8: 3069–3076.
Kim, G., J.-S. Kim, and D.-W. Hwang. 2011. Submarine groundwater discharge from oceanic islands standing in oligotrophic oceans: Implications for global biological production and organic carbon fluxes. Limnology and Oceanography 56: 673–682.
Lee, J.-M., and G. Kim. 2006. A simple and rapid method for analyzing radon in coastal and ground waters using a radon-in-air monitor. Journal of Environmental Radioactivity 89: 219–228.
Linto, N., J. Barnes, R. Ramachandran, J. Divia, P. Ramachandran, and R. Upstill-Goddard. 2014. Carbon dioxide and methane emissions from mangrove-associated waters of the Andaman Islands, Bay of Bengal. Estuaries and Coasts 37: 381–398.
Liu, Q., et al. 2012. How significant is submarine groundwater discharge and its associated dissolved inorganic carbon in a river-dominated shelf system? Biogeosciences 9: 1777–1795.
Liu, Q., M.A. Charette, P.B. Henderson, D.C. Mccorkle, W. Martin, and M. Dai. 2014. Effect of submarine groundwater discharge on the coastal ocean inorganic carbon cycle. Limnology and Oceanography 59: 1529–1554.
Macklin, P.A., D.T. Maher, and I.R. Santos. 2014. Estuarine canal estate waters: hotspots of CO2 outgassing driven by enhanced groundwater discharge? Marine Chemistry 167: 82–92.
Maher, D.T., and B.D. Eyre. 2010. Benthic fluxes of dissolved organic carbon in three temperate Australian estuaries: Implications for global estimates of benthic DOC fluxes. Journal of Geophysical Research: Biogeosciences (2005–2012) 115, G04039. doi:10.1029/2010JG001433.
Maher, D., and B.D. Eyre. 2011. Insights into estuarine benthic dissolved organic carbon (DOC) dynamics using δ13C-DOC values, phospholipid fatty acids and dissolved organic nutrient fluxes. Geochimica et Cosmochimica Acta 75: 1889–1902.
Maher, D and B.D. Eyre 2012. Carbon budgets for three autotrophic Australian estuaries: Implications for global estimates of the coastal air‐water CO2 flux. Global Biogeochemical Cycles 26. doi: 10.1029/2011GB004075.
Maher, D.T., I.R. Santos, L. Golsby-Smith, J. Gleeson, and B.D. Eyre. 2013a. Groundwater-derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: The missing mangrove carbon sink? Limnology and Oceanography 58: 475–488.
Maher, D.T., et al. 2013b. Novel use of cavity ring-down spectroscopy to investigate aquatic carbon cycling from microbial to ecosystem scales. Environmental Science and Technology 47: 12938–12945.
Maher, D.T., K. Cowley, I.R. Santos, P. Macklin, and B.D. Eyre. 2015. Methane and carbon dioxide dynamics in a subtropical estuary over a diel cycle: insights from automated in situ radioactive and stable isotope measurements. Marine Chemistry 168: 69–79.
Millero, F.J., T.B. Graham, F. Huang, H. Bustos-Serrano, and D. Pierrot. 2006. Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Marine Chemistry 100: 80–94.
Miyajima, T., Y. Tsuboi, Y. Tanaka, and I. Koike. 2009. Export of inorganic carbon from two Southeast Asian mangrove forests to adjacent estuaries as estimated by the stable isotope composition of dissolved inorganic carbon. Journal of Geophysical Research: Biogeosciences. (2005–2012) 114. doi: 10.1029/2008JG000861
Moore, W.S. 2010. The effect of submarine groundwater discharge on the ocean. Annual Review of Marine Science 2: 59–88.
Moore, W.S., and R. Arnold. 1996. Measurement of 223Ra and 224Ra in coastal waters using a delayed coincidence counter. Journal of Geophysical Research: Oceans (1978–2012) 101: 1321–1329.
Moore, W.S., Blanton J.O., and Joye S.B. 2006. Estimates of flushing times, submarine groundwater discharge, and nutrient fluxes to Okatee Estuary, South Carolina. Journal Geophysical Research: Oceans (1978–2012) 111. doi: 10.1029/2005JC003041.
Neubauer, S.C., and J.P. Megonigal. 2015. Moving beyond global warming potentials to quantify the climatic role of ecosystems. Ecosystems 18(6): 1000–1013.
Nirmal Rajkumar, A., J. Barnes, R. Ramesh, R. Purvaja, and R. Upstill-Goddard. 2008. Methane and nitrous oxide fluxes in the polluted Adyar River and estuary, SE India. Marine Pollution Bulletin 56: 2043–2051.
Noriega, C., and M. Araujo. 2014. Carbon dioxide emissions from estuaries of northern and northeastern Brazil. Scientific Reports 4: 6164.
O’Reilly, C., I.R. Santos, T. Cyronak, A. McMahon and D.T. Maher. 2015. Nitrous oxide and methane dynamics in a coral reef lagoon driven by porewater exchange: Insights from automated high frequency observations. Geophysical Research Letters. 42(8), doi: 10.1002/2015GL063126.
Panneer Selvam, B., S. Natchimuthu, L. Arunachalam, and D. Bastviken. 2014. Methane and carbon dioxide emissions from inland waters in India—implications for large scale greenhouse gas balances. Global Change Biology 20: 3397–3407.
Pelletier, G., E. Lewis, and D. Wallace. 2007. CO 2 Sys. xls: a calculator for the CO 2 system in seawater for Microsoft Excel/VBA. Olympia: Washington State Department of Ecology/Brookhaven National Laboratory.
Peterson, R.N., W.C. Burnett, N. Dimova, and I.R. Santos. 2009. Comparison of measurement methods for radium-226 on manganese-fiber. Limnology and Oceanography: Methods 7: 196–205.
Porubsky, W.P., N.B. Weston, W.S. Mooreb, C. Ruppel, and S.B. Joye. 2014. Dynamics of submarine groundwater discharge and associated fluxes of dissolved nutrients, carbon, and trace gases to the coastal zone (Okatee River estuary, South Carolina). Geochimica et Cosmochimica Acta 131: 81–97.
Raymond, P.A., J.E. Bauer, and J.J. Cole. 2000. Atmospheric CO2 evasion, dissolved inorganic carbon production, and net heterotrophy in the York River estuary. Limnology and Oceanography 45: 1707–1717.
Rodríguez-Murillo, J., J. Zobrist, and M. Filella. 2015. Temporal trends in organic carbon content in the main Swiss rivers, 1974–2010. The Science of the Total Environment 502: 206–217.
Ruprecht, J.E., and W.A. Timms. 2010. Hat Head Effluent Disposal Scheme – Ongoing Monitoring Results to September 2010. WRL Technical Report.
Sadat-Noori, M., I. Santos, D. Maher, C. Sanders, and L. Sanders. 2015. Groundwater discharge into an estuary using spatially distributed radon time series and radium isotopes. Journal of Hydrology 528: 703–719.
Sanders, C.J., I.R. Santos, D.T. Maher, M. Sadat-Noori, B. Schnetger, and H.-J. Brumsack. 2015. Dissolved iron exports from an estuary surrounded by coastal wetlands: can small estuaries be a significant source of Fe to the ocean? Marine Chemistry 176: 75–82.
Santos, I.R., W.C. Burnett, T. Dittmar, I.G.N.A. Suryaputra, and J. Chanton. 2009. Tidal pumping drives nutrient and dissolved organic matter dynamics in a Gulf of Mexico subterranean estuary. Geochimica et Cosmochimica Acta 73: 1325–1339.
Santos, I.R., D.T. Maher, and B.D. Eyre. 2012a. Coupling automated radon and carbon dioxide measurements in coastal waters. Environmental Science and Technology 46: 7685–7691.
Santos, I.R., P.L.M. Cook, L. Rogers, J. De Weys, and B.D. Eyre. 2012b. The “salt wedge pump”: convection-driven pore-water exchange as a source of dissolved organic and inorganic carbon and nitrogen to an estuary. Limnology and Oceanography 57: 1415–1426.
Santos, I.R., et al. 2015. Porewater exchange as a driver of carbon dynamics across a terrestrial-marine transect: insights from coupled 222Rn and pCO2 observations in the German Wadden Sea. Marine Chemistry 171: 10–20.
Seitzinger, S., J. Harrison, E. Dumont, A.H. Beusen, and A. Bouwman. 2005. Sources and delivery of carbon, nitrogen, and phosphorus to the coastal zone: An overview of Global Nutrient Export from Watersheds (NEWS) models and their application. Global Biogeochemical Cycles 19. doi: 10.1029/2005GB002606.
Stewart, B.T., I.R. Santos, D.R. Tait, P.A. Macklin, and D.T. Maher. 2015. Submarine groundwater discharge and associated fluxes of alkalinity and dissolved carbon into Moreton Bay (Australia) estimated via radium isotopes. Marine Chemistry 174(20): 1–12.
St‐Jean, G. 2003. Automated quantitative and isotopic (13C) analysis of dissolved inorganic carbon and dissolved organic carbon in continuous‐flow using a total organic carbon analyser. Rapid Communications in Mass Spectrometry 17: 419–428.
Striegl, R.G., M.M. Dornblaser, G.R. Aiken, K.P. Wickland, and P.A. Raymond. 2007. Carbon export and cycling by the Yukon, Tanana, and Porcupine rivers, Alaska, 2001–2005. Water Resources Research. 43. doi: 10.1029/2006WR005201.
Tamše, S., N. Ogrinc, L.M. Walter, D. Turk, and J. Faganeli. 2014. River sources of dissolved inorganic carbon in the gulf of Trieste (N Adriatic): stable carbon isotope evidence. Estuaries and Coasts 38(1): 1–14.
Tank, S.E. et al. 2012. A land-to-ocean perspective on the magnitude, source and implication of DIC flux from major Arctic rivers to the Arctic Ocean. Global Biogeochemical Cycles 26. doi: 10.1029/2011GB004192.
Wang, Z.A., and W.-J. Cai. 2004. Carbon dioxide degassing and inorganic carbon export from a marsh-dominated estuary (the Duplin River): a marsh CO2 pump. Limnology and Oceanography 49: 341–354.
Wang, G., et al. 2015. Net subterranean estuarine export fluxes of dissolved inorganic C, N, P, Si, and total alkalinity into the Jiulong River estuary, China. Geochimica et Cosmochimica Acta 149: 103–114.
Wanninkhof, R. 1992. Relationship between wind speed and gas exchange over the ocean. J. Geophys. Res.: Oceans (1978–2012) 97: 7373–7382.
Weinstein, Y., W. Burnett, P. Swarzenski, Y. Shalem, Y. Yechieli, and B. Herut. 2007. Role of aquifer heterogeneity in fresh groundwater discharge and seawater recycling: An example from the Carmel coast, Israel. Journal of Geophysical Research: Oceans (1978–2012) 112. DOI: 10.1029/2007JC004112.
Weiss, R.F. 1974. Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Marine Chemistry 2: 203–215.
Wiesenburg, D.a., and N.L. Guinasso. 1979. Equilibrium solubilities of methane, carbon monoxide, and hydrogen in water and sea water. Journal of Chemical and Engineering Data 24(4): 356–360.
Winter, P.E., T.A. Schlacherl, and D. Baird. 1996. Carbon flux between an estuary and the ocean: a case for outwelling. Hydrobiologia 337: 123–132.
Zhang, G., J. Zhang, S. Liu, J. Ren, J. Xu, and F. Zhang. 2008. Methane in the Changjiang (Yangtze River) Estuary and its adjacent marine area: riverine input, sediment release and atmospheric fluxes. Biogeochemistry 91: 71–84.
Acknowledgments
We would like to thank Christian Sanders, Luciana Sanders, Paul Macklin, Ashley McMahon, Benjamin Stewart, Jennifer Taylor, and Judith Rosentreter for their support during field campaigns. IRS and DTM are funded through Australian Research Council DECRA Fellowships (DE140101733 and DE150100581). We acknowledge support from the Australian Research Council (DP120101645 and LE120100156). We would also like to acknowledge the Associate Editor Alberto Borges and two anonymous reviewers for their constructive comments which helped strengthen our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Alberto Vieira Borges
Rights and permissions
About this article
Cite this article
Sadat-Noori, M., Maher, D.T. & Santos, I.R. Groundwater Discharge as a Source of Dissolved Carbon and Greenhouse Gases in a Subtropical Estuary. Estuaries and Coasts 39, 639–656 (2016). https://doi.org/10.1007/s12237-015-0042-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-015-0042-4