Abstract
We estimated CO2 and CH4 emissions from mangrove-associated waters of the Andaman Islands by sampling hourly over 24 h in two tidal mangrove creeks (Wright Myo; Kalighat) and during transects in contiguous shallow inshore waters, immediately following the northeast monsoons (dry season) and during the peak of the southwest monsoons (wet season) of 2005 and 2006. Tidal height correlated positively with dissolved O2 and negatively with pCO2, CH4, total alkalinity (TAlk) and dissolved inorganic carbon (DIC), and pCO2 and CH4 were always highly supersaturated (330–1,627 % CO2; 339–26,930 % CH4). These data are consistent with a tidal pumping response to hydrostatic pressure change. There were no seasonal trends in dissolved CH4 but pCO2 was around twice as high during the 2005 wet season than at other times, in both the tidal surveys and the inshore transects. Fourfold higher turbidity during the wet season is consistent with elevated net benthic and/or water column heterotrophy via enhanced organic matter inputs from adjacent mangrove forest and/or the flushing of CO2-enriched soil waters, which may explain these CO2 data. TAlk/DIC relationships in the tidally pumped waters were most consistent with a diagenetic origin of CO2 primarily via sulphate reduction, with additional inputs via aerobic respiration. A decrease with salinity for pCO2, CH4, TAlk and DIC during the inshore transects reflected offshore transport of tidally pumped waters. Estimated mean tidal creek emissions were ∼23–173 mmol m−2 day−1 CO2 and ∼0.11–0.47 mmol m−2 day−1 CH4. The CO2 emissions are typical of mangrove-associated waters globally, while the CH4 emissions fall at the low end of the published range. Scaling to the creek open water area (2,700 km2) gave total annual creek water emissions ∼3.6–9.2 × 1010 mol CO2 and 3.7–34 × 107 mol CH4. We estimated emissions from contiguous inshore waters at ∼1.5 × 1011 mol CO2 year−1 and 2.6 × 108 mol CH4 year−1, giving total emissions of ∼1.9 × 1011 mol CO2 year−1 and ∼3.0 × 108 mol CH4 year−1 from a total area of mangrove-influenced water of ∼3 × 104 km2. Evaluating such emissions in a range of mangrove environments is important to resolving the greenhouse gas balance of mangrove ecosystems globally. Future such studies should be integral to wider quantitative process studies of the mangrove carbon balance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Continental shelves cover only 8 % of the ocean surface but they play a major role in marine biogeochemistry. Large nutrient fluxes from rivers and upwelling and high rates of organic matter remineralisation arising from benthic–pelagic coupling lead to much higher primary productivity than in the open ocean (Wollast 1998; Muller-Karger et al. 2005). Consequently, they are considered overall net sinks for tropospheric CO2, equivalent to perhaps as much as 25–60 % of the open ocean CO2 sink (Borges et al. 2005). Conversely, near-shore upwelling regions, estuaries, coral reefs and salt marsh and mangrove waters together constitute a net source to the atmosphere, not only of CO2 but also of CH4 (Borges et al. 2005; Bange 2006). The emissions are both high and uncertain, the uncertainty reflecting a regional data bias favouring studies in northern temperate locations.
Mangrove ecosystems cover ∼1.4 × 105 km2 of the global intertidal area, equivalent to ∼0.7 % of the total area of tropical forest (Giri et al. 2011). Nevertheless, they are among the world’s most productive biomes, accounting for ∼11 % of the total terrestrial carbon flux and ∼10 % of the terrestrial dissolved organic carbon flux, to the oceans (Jennerjahn and Ittekot 2002; Dittmar et al. 2006). Their overall status is net autotrophic (Alongi 2002), mangrove plant biomass both above and belowground accounting for around 15 % of organic carbon sequestration by contemporary marine sediments (Jennerjahn and Ittekot 2002). Even so, mangrove sediments and their overlying waters are widely considered to be net heterotrophic (Gattuso et al. 1998), reflecting large and variable organic carbon inputs from diverse sources such as mangrove and terrestrial detritus, microphytobenthos, phytoplankton and sea grasses (Bouillon and Boschker 2006). Consequently, mangrove surrounding waters have been shown to emit large amounts of CO2 and CH4 to the atmosphere (e.g., Borges et al. 2003; Barnes et al. 2006; Ramesh et al. 2007; Bouillon et al. 2007b; Bouillon et al. 2008; Koné and Borges 2008). Indeed, Chen and Borges (2009) estimate mangrove surrounding waters to emit around 0.05 Pg C annually as CO2, about 9–18 % of the total coastal source (estuaries, salt marshes and mangroves) of 0.28–0.57 Pg C year−1 and Barnes et al. (2006) suggest that mangrove surrounding waters could be the dominant coastal CH4 source. Given a net shelf CO2 sink of ∼0.33–0.36 Pg C year−1 (Chen and Borges 2009) quantifying mangrove emissions of gaseous carbon is important for better constraining the carbon budgets of tropical continental margins.
This paper analyses the seasonal (southwest and northeast monsoons) distributions of pCO2, dissolved CH4 and O2, total alkalinity (TAlk) and dissolved inorganic carbon (DIC) over 24-h cycles in two pristine mangrove creeks and in adjacent inshore surface water transects in the Andaman Islands, Bay of Bengal. In addition, annual CO2 and CH4 emissions were derived from these mangrove waters and using these and other data, the global carbon balance of mangrove ecosystems was briefly re-examined.
Materials and Methods
Study Area
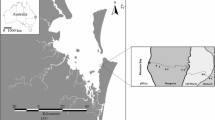
The Andaman Islands are an archipelago of more than 550 islands (524 uninhabited) covering 6,408 km2 of the southeastern Bay of Bengal and extending north-south for 360 km between latitudes 6–14° N and 92–94° E (Fig. 1). Subsoils derive mainly from argillaceous and algal limestone and climate is humid tropical with a relative humidity of 70–90 %. Temperature is rather constant with minima of 24–25 °C and maxima of 27–30 °C during all months; there is no discernable winter season (Singh et al. 1987). Mean annual precipitation is ∼314 cm and monthly precipitation is maximal (∼60 cm) at the peak of the south west monsoon (June–September) and minimal (<5 cm) between January and April (Singh et al. 1987). There is only one river, the Kalpong, which discharges to north-west North Andaman Island (Selvam 2003). Around 86 % of the total land area is covered by rainforest, some of which has been cleared for commercial plantation and agricultural use, leading to localised decreases in soil pH and organic content (Dagar et al. 1995).
Mangrove forest, which covers 892 km2 (∼0.6 % of the global total) and fringes around one quarter of the 1,900-km coastline, is among the world’s most pristine. Mangrove species diversity and biomass is high, reflecting the high rainfall amount and frequency. Some 24 mangrove species are present, of which Rhizophora apiculata, Rhizophora mucronata and Ceriops tagal dominate (Selvam 2003). Around 255 km2 of the mangrove forest is described as “dense” with the remainder listed as “moderately dense” or “open” (Forest Survey of India 2005). Mangrove clearance for agriculture, aquaculture and domestic and industrial development is significant and is increasing, causing soil erosion and the loss of organic matter and nutrients from some locations (Singh et al. 1986). Mangrove-associated open water totals at ∼2,700 km2; this is around three times the area occupied by mangrove forest and is 2 % of the global estimate of 0.15 × 106 km2 for open mangrove waters (Borges et al. 2005). The tidal range in Andaman open mangrove waters is 1.90 m (Selvam 2003), and the diurnal range in salinity is around 5–25 at the peak of the wet season (July–August) and 10–34 at the end of the dry season (March–April). Local times of sunrise and sunset are around 05.10 and 17.30 hours respectively, throughout the year.
Sampling and Analytical Techniques
Surveys of 24-h duration were conducted, commencing at midday (around 7 h after sunrise) with hourly surface water sampling for pH, TAlk, dissolved CH4 and O2, turbidity, salinity and temperature, at fixed locations in two tidal mangrove creeks: Wright Myo (11°47′27.7″ N, 92°42′24.3″ E), South Andaman, which is 8 km long and 20–40 m wide dependant on tidal state and Kalighat (13°07′30.7″ N; 92°56′48.2″ E), North Andaman, which is 10 km long and has a mean width of ∼100 m (Fig. 1). Wright Myo was surveyed at the beginning of the inter-monsoon period immediately following the northeast monsoon, on 14–15 April 2005 and 21–22 April 2006 (“dry season”) and during the peak of the southwest monsoon, on 22–23 August 2005 and 24–25 August 2006 (“wet season”). During 2004, this site was surveyed similarly for CH4 and N2O, as reported elsewhere (Barnes et al. 2006). Kalighat was surveyed on 11–12 April 2005 (inter-monsoon: “dry season”) and on 17–18 August 2005 (southwest monsoon: “wet season”). Additionally, wet and dry season surface water transects were carried out for pH, TAlk and CH4 across ambient salinity ranges between mangroves and adjacent shallow near shore waters: TI and TII during April and August 2005 and TIII and TIV during both April and August 2005 and April and August 2006 (Fig. 1). TI covered ∼18 km between Kalighat Creek (water depth ∼1 m) and Mayabander (water depth ∼10 m), TII covered ∼28 km from Mayabander to Interview Island (maximum water depths ∼5–10 m) via Austin creek (coral reef; maximum water depth ∼20 m), TIII was a 9-km survey along and just offshore of Wright Myo (maximum water depth ∼3-5 m) and TIV covered ∼47 km from Wandoor National Park to Chipiyatapu (open water coral reef and fringing mangroves; maximum water depth ∼15 m), through a channel connecting the Bay of Bengal to the Andaman Sea (Fig. 1).
Water samples were collected manually from 0.2 m depth (Richter & Wiese 2.5-L water sampler). Subsamples for dissolved CH4 analysis were transferred into 100 mL glass bottles via silicon tubing with the careful exclusion of air bubbles, poisoned with 0.1 mL saturated HgCl2 and sealed to leave no headspace. Analysis of CH4 was by single-phase equilibration gas chromatography (GC) with a routine analytical precision (1 σ) of ±1 % (Upstill-Goddard et al. 1996). Method calibration was with certified gas standards of 6.74 and 12.5 ppmv CH4 in N2 (National Physical Laboratory, New Delhi, India). Considering logistical constraints, pH was not determined in situ. Instead, pH and TAlk subsamples were decanted into 100 mL pre-combusted glass bottles, poisoned with 0.1 mL of saturated HgCl2 solution and sealed. Following transfer to the laboratory, they were stored in a refrigerator at 4 °C and subsequently allowed to equilibrate to room temperature (25 °C) prior to analysis. Corrections to in situ temperature were made as appropriate. The total time between sampling and analysis was always less than 4 days. TAlk was determined by electro-titration (Gran 1952), and pH was measured with a combination electrode (ORION) calibrated with NBS standard pH buffers. Typical reproducibility (1 σ) of TAlk and pH measurements was, respectively, ±4 μeq kg-1 and ±0.005. CO2Sys software (Pierrot et al. 2006) was used to calculate pCO2 from pH, TAlk, temperature and salinity, adopting a protocol for waters of varying salinity (Frankignoulle and Borges 2001) and using the dissociation constants of Millero et al. (2006). On samples from three of the tidal surveys (Kalighat and Wright Myo, August 2005; Wright Myo, April 2006), cross-checking was made on these pCO2 estimates by incorporating a methaniser (nickel catalyst at 250 °C in H2) in the GC system to convert CO2 to CH4. Linear correlation of the data yielded: pCO2 (GC) = 0.936 pCO2 (pH–TAlk) + 397.8 (r 2 = 0.941, n = 72). The offset is primarily a result of progressive divergence in the liquid junction potential between buffer and sample towards higher salinities (Frankignoulle and Borges 2001). Consequently, its effect is minimal at high pCO2 and low salinity, typically being around 0.25 % at pCO2 > 6,000 μatm but progressively increasing to around 3.6 % at pCO2 = 3,000 μatm and around 13 % at pCO2 < 2,000 μatm. Nevertheless, the likely range in pCO2 was considered to be sufficiently wide so as to preclude these inherent errors from materially affecting our subsequent data interpretation. For consistency, only those survey pCO2 data derived from pH and TAlk are discussed in this paper as these are the only complete pCO2 data set for our samples. Salinity, temperature, turbidity and dissolved O2 were determined in situ with a pre-calibrated multi-parameter probe (Horiba W-22.23). Precisions (1 σ) were ±0.01 salinity, ±0.2 °C, ±3 % turbidity and ±0.01 mg O2 L−1. Probe measurements of dissolved O2 were routinely cross-checked by collecting two samples in every five for standard Winkler titration. The two methods gave satisfactory agreement: O2 (probe) = 0.992 O2 (Winkler) − 0.02 (r 2 = 0.959, n = 61). Wind speed was recorded with a hand-held cup anemometer at 1 m height (Lutron LM8000; resolution ± 0.1 m s−1); 1 min means were subsequently converted to equivalent 10 m values (Amorocho and DeVries 1980). Water depths and mid-depth water current velocities were measured hourly (open channel flow meter, Valeport, UK) for all tidal surveys. In addition, gas bubbles effusing from Wright Myo creek sediments on 21 April 2006 were collected. Samples were captured over a 2-h period spanning the first low tide (1700–1900 hours), in a gas-tight syringe with an integral luer needle connected to an inverted glass funnel with a septum at the base of its stem. Samples were transferred to gas-tight vacutainers for subsequent gas chromatographic analysis of CO2 and CH4 as described above.
Sea-to-air emission fluxes (F) of CO2 and CH4 were estimated using F = k w L Δp, where k w is the gas transfer velocity (in centimetres per hour), L is the Ostwald solubility coefficient and Δp is the partial pressure difference (in atmospheres) across the air–water interface. Values of L were from Weiss (1974) for CO2 and Weisenburg and Guinasso (1979) for CH4. k w was estimated from the 10-m-derived wind speeds using the empirical k w—wind speed relations of Clark et al. (1995) and Borges et al. (2004) for CO2. Both were derived for use in shallow coastal waters where tidal current interaction with the seabed generates turbulence additional to that due to wind speed alone; the latter formally includes expressions for tidal current velocities and water depth, which is considered to be more applicable here than some other more commonly used relations (e.g., Liss and Merlivat 1986; Wanninkhof 1992). k w for CO2 was scaled to k w for CH4 using Schmidt numbers given in Wanninkhof (1992).
Results and Discussion
Creek Water Carbon During Tidal Surveys
Means and ranges of hourly pCO2, CH4, TAlk, DIC, O2, salinity and turbidity are given in Table 1. Mean CH4 did not differ significantly between dry and subsequent wet season surveys or in the case of Wright Myo, between consecutive years (mean of all samples, 349 ± 125 nmol L−1; 15,960 ± 5,930 % saturation). Similarly, there was no significant seasonal variation in pCO2 at Wright Myo (mean of all samples, 3,409 ± 919 μatm; 903 ± 243 % saturation). Previously, no seasonal variability in either CH4 or N2O at Wright Myo (Barnes et al. 2006) was found to be consistent with a negligible thermal effect on methanogenesis or nitrification/denitrification rates. Mean water temperatures were: dry season, 28.0 ± 1.0 °C (range, 25.5–30.2 °C); wet season, 27.6 ± 0.9 °C (range, 26.0–29.4 °C). By contrast, pCO2 at Kalighat was around twice as high in the wet season than in the dry season and dissolved O2 saturations were correspondingly lower (Table 1). These data are consistent with a wet season increase in net benthic and/or water column heterotrophy at Kalighat, perhaps reflecting enhanced organic matter inputs from adjacent mangrove forest and/or the flushing of soil waters enriched in CO2 by respiration, as inferred previously for mangrove surrounding waters (Koné and Borges 2008). The observed three- to fourfold higher turbidity during the wet seasons (Table 1) is consistent with an enhanced organic matter flux and would also tend to suppress photosynthesis, consistent with low dissolved O2 during the wet seasons (Table 1). Zhai et al. (2005) similarly found lowest dissolved O2 during the Pearl Estuary wet season, which was ascribed to elevated heterotrophy, both in contiguous mangrove sediments and their surrounding waters. The lack of seasonal contrast at Wright Myo implies that the effects of any such processes on CO2 dynamics are insignificant there.
Figures 2, 3 and 4 show temporal trends in carbon system components, dissolved O2 saturation, salinity and tidal height at Kalighat and Wright Myo. In all surveys tidal height was correlated positively with dissolved O2 and negatively with pCO2, CH4, TAlk and DIC, notwithstanding the correlations for TAlk and DIC being comparatively weak during the Kalighat wet season (Fig. 2). For most surveys the coincidence of maximal pCO2 and minimal O2 only broadly matched light-dark periods of photosynthesis-respiration. Most notably, during the Kalighat dry season the data were around 12 h out of phase, maximal O2 and minimal pCO2 occurring just prior to midnight (Fig. 2). Overall, the data are consistent with a dominance of tidal pumping, in which mangrove sediment pore waters depleted in O2 and enriched in the by-products of organic matter oxidation (CO2, CH4 and TAlk), seep into creek waters in response to the cyclic rise and fall in hydrostatic pressure (Ovalle et al. 1990), thereby obscuring quasi-coincident cycles in pCO2 and O2 driven by photosynthesis-respiration. We previously observed tidal pumping dominance of the diel cycles of CH4 and N2O at Wright Myo (Barnes et al. 2006) and Lara and Dittmar (1999) observed tidal pumping of nutrients in Brazilian mangrove waters. Tidal pumping of DIC is also well established in mangrove creeks (Borges et al. 2003; Kristensen et al. 2008; Zablocki et al. 2011; Maher et al. 2013) and has been shown to dominate resulting pCO2 distributions and CO2 emissions to air (Borges et al. 2003; Bouillon et al. 2007c; Koné and Borges 2008). The dominance of tidal pumping in the mangrove CO2 budget was recently demonstrated unequivocally through correlations of pCO2 with 222Rn, a natural tracer of dissolved pore water inputs (Santos et al. 2012; Maher et al. 2013). Hydrostatic pressure control of mangrove creek biogeochemical variables is thus now firmly established.
Tidal variation of water height (filled circles), salinity (open triangles), pCO2 (open squares), dissolved CH4 (open diamonds), percent O2 saturation (open circles), DIC (open squares) and TAlk (open circles) at Kalighat Creek during: a April 2005 (dry season) and b August 2005 (wet season). Shaded areas correspond to hours of darkness. Tidal heights are all relative to the mean of the lowest and highest tidal elevations observed during the survey
Tidal variation of water height (filled circles), salinity (open triangles), pCO2 (open squares), dissolved CH4 (open diamonds), percent O2 saturation (open circles), DIC (open squares) and TAlk (open circles) at Wright Myo creek during: a April 2005 (dry season) and b August 2005 (wet season). Shaded areas correspond to hours of darkness. Tidal heights are all relative to the mean of the lowest and highest tidal elevations observed during the survey
Tidal variation of water height (filled circles), salinity (open triangles), pCO2 (open squares), dissolved CH4 (open diamonds), percent O2 saturation (open circles), DIC (open squares) and TAlk (open circles) at Wright Myo creek during: a April 2006 (dry season) and b August 2006 (wet season). Shaded areas correspond to hours of darkness. Tidal heights are all relative to the mean of the lowest and highest tidal elevations observed during the survey
At Kalighat, the inverse relationship between CH4 and tidal height was partially disrupted by heavy rainfall during the first 4 hours of the wet season survey; dissolved CH4 was suppressed at low water (Fig. 2). This effect was also observed in an earlier study at Wright Myo and ascribed to the suppression of methanogenesis by O2 penetration of the sediment in the rain drops and by additional dilution with this freshwater flux (Barnes et al. 2006). This mechanism could also enhance the potential for aerobic respiration, consistent with the higher pCO2 observed. At Kalighat pCO2 was also higher during nighttime tidal minima than during daytime tidal minima, by ∼20 % during the dry season survey and ∼14 % during the wet season survey (Fig. 2). Based on this a crude estimate of the maximum photosynthetic removal of the pore water CO2 flux to the overlying creek water is ∼20 %.
Porewater production of CO2 and CH4
Net pore water production of CO2 and CH4 reflects several competing diagenetic reactions. Organic matter oxidation via aerobic respiration, denitrification and the reduction of nitrate, sulphate and iron and manganese oxides produces CO2. Methanogenesis can occur via CO2 reduction and from the fermentation of acetate and other low molecular weight compounds that also produces CO2, and CH4 is oxidised by sulphate (Berner 1980). Carbonate dissolution and precipitation lead to CO2 removal and addition respectively, and benthic primary producers are a CO2 sink (Berner 1980). Despite the complexity, creek water TAlk vs. DIC relationships (Fig. 5) contain important information on the likely dominant diagenetic pathways in creek pore waters. During individual seasons, TAlk and DIC were highly correlated with essentially identical relationships at both sites, but there was a significant seasonal TAlk: DIC offset (wet season, TAlk = 0.90 DIC + 0.10, r 2 = 0.99; dry season, TAlk = 0.92 DIC + 0.20, r 2 = 0.97; both, n = 72). An important detail is that TAlk and DIC concentrations at Wright Myo were lower overall than at Kalighat during both seasons, which could reflect the relative intensities of tidal pumping at the two sites. During the wet season when these differences were greatest (Fig. 5), tidal amplitudes at Kalighat were approximately twice those at Wright Myo (Figs. 2, 3 and 4). An additional possibility is some inter-site variability in organic matter decomposition rates. Alongi et al. (2001) recognise aerobic respiration and sulphate reduction as the major mechanisms of organic matter oxidation in mangrove sediments. However, the first step in organic matter diagenesis is hydrolysis of particulate organic carbon (POC), which yields TAlk but no other DIC (Krumins et al. 2013). While this could account for the observed TAlk intercepts (Fig. 5) and would imply a higher supply rate of reactive POC to mangrove sediments during the dry season (larger TAlk intercept), such an interpretation conflicts with our observations of higher turbidity during the wet season (Table 1), which was interpreted above to reflect higher wet season fluxes of organic carbon, consistent with increased pCO2 and decreased O2 deriving from sediment pore waters and a suppression of photosynthesis. Clearly, some remineralisation of POC also could additionally have occurred in the overlying water but our data do not allow any evaluation of this. Interestingly, other work strongly implies such seasonal variability to be insignificant (Bouillon et al. 2007b; Maher et al. 2013). An alternative explanation is that the observed TAlk vs. DIC seasonality (Fig. 5) reflects at least partly, seasonal changes in aerobic respiration and denitrification, the only primary or secondary diagenetic reactions for which ΔDIC exceeds ΔTAlk (Krumins et al. 2013). Higher aerobic respiration during the wet season is consistent with the correspondingly higher pCO2 and lower O2 observed (Fig. 2). There is evidence for sediment denitrification at some mangrove sites (Alongi et al 1998, 2000) and a ratio of 0.8 for ΔTAlk/ΔDIC during denitrification (Krumins et al. 2013) is close to that observed in this study (Fig. 5). Even so, denitrification is thought to be a relatively minor contributor to organic matter degradation in most mangrove ecosystems (Alongi et al. 2000; Koné and Borges 2008). Consistent with this, although a substantial pore water source of N2O in Wright Myo creek waters was previously found, based on concurrent observations of NO3 − and NO2 -, this study considered it more likely that this was a consequence of nitrification rather than denitrification (Barnes et al. 2006). Nitrification is a net consumer of TAlk (Krumins et al. 2013) but otherwise leaves DIC unaltered. Iron and manganese reduction have also been found to be important in some Asian mangroves (Alongi et al. 1998; Kristensen et al. 2000) but for both ΔTAlk/ΔDIC is far larger than we observed at Wright Myo or Kalighat. Carbonate dissolution can be a large contributor of TAlk but has a ΔTAlk/ΔDIC ratio of 2 (Krumins et al. 2013). The major diagenetic pathway most consistent with the TAlk and DIC data is sulphate reduction, for which ΔTAlk/ΔDIC is 1 (Krumins et al. 2013). Similar conclusions were reached by Koné and Borges (2008) based on Talk/DIC stoichiometry in waters surrounding Vietnamese mangroves. Having considered all the evidence, it can thus be concluded that the most likely major diagenetic pathway of organic matter oxidation in Andaman mangrove sediments is sulphate reduction, with a variable secondary role for aerobic respiration, broadly in line with previous conclusions for mangroves (Alongi et al. 2001; Koné and Borges 2008.
Carbon system components in surface water transects
TAlk vs. salinity or DIC vs. salinity relationships in surface water transects contain information on the potential lateral transport of “tidally pumped” creek waters. TAlk and DIC were linearly correlated in all transects (data not shown). TAlk vs. salinity is shown in Fig. 6. All dry season transects showed a small overall decrease in TAlk with increasing salinity, consistent with a weak but measurable influence of creek water TAlk in even the most offshore samples. By contrast, the wet season data were much more variable. Although in TI (Kalighat to Mayabander, Aug 2005) an overall decrease in TAlk with salinity was evident, all other wet season transects followed an opposing trend (Fig. 6). Notably, surface (10 m) TAlk concentrations in the Andaman Sea (November) are in the range 2.12–2.16 mmol kg–1 (Sarma and Narvikar 2001), essentially identical to much of the wet season data for salinities above 15 (Fig. 6). Evidently, the wet season creek water outflow signal of TAlk was largely masked by these values.
Overall pCO2 and CH4 decreased with salinity in all transects (Fig. 7). Means and ranges of pCO2 were generally highest during the wet seasons whereas means and ranges of CH4 showed no marked seasonality (Table 2). Highest pCO2 and CH4 was observed in TIII (Fig. 7; Table 2), which was in closest proximity to adjacent mangrove creek waters at Wright Myo (Fig. 1). Indeed, seasonality in these transects reflected that at Wright Myo, during 2005 pCO2 was overall lower during the dry season transect than at other times and CH4 showed little temporal variation (Fig. 7; Table 2), consistent with the results of the tidal surveys (Table 1). Whereas CH4 was highly supersaturated at all salinities, CO2 was consistently supersaturated only between Kalighat and Mayabander (TI) and along Wright Myo (TIII), during both the wet and dry seasons (Fig. 7; Table 2). Between Mayabander and Austin Creek (TII), mild CO2 undersaturation at high salinities was observed during April 2005 (dry season), and between Wandoor and Chipiyatapu (TIV), CO2 was always supersaturated only during the August 2006 (wet season) transect. Indeed, TIV had the overall lowest pCO2 and CH4 values of any transect (Table 2), consistent with its being the furthest removed from the influence of mangrove creeks. Undersaturation was observed in one TIV sample during April 2006 but during both the wet and dry season TIV transects of 2005 CO2 was predominantly undersaturated throughout.
Evidently, creek water tidal pumping can significantly influence the seasonal distributions of pCO2 and CH4 in mangrove-fringing near-shore waters, which like the creeks themselves, are year-round sources of tropospheric CH4. By contrast, the observed distributions of CO2 presumably reflect the balance of net photosynthesis versus creek water inputs. A significant imprint of tidally pumped CO2 and CH4 in mangrove fringing waters is not surprising given that offshore advection of DIC is a likely major export pathway for mangrove carbon (Bouillon et al. 2007c; Maher et al. 2013). Shelf regions are often considered to be net annual CO2 sinks (Chen and Borges 2009). For tropical shelves adjacent to extensive mangroves with year-round CO2 export via tidal pumping, this view may require further scrutiny.
Emission fluxes of CO2 and CH4
Considering the several potentially important physical and biogeochemical controls of air–sea gas exchange, selecting an appropriate k w parameterisation is problematic; those in most common use are based on wind speed alone and result in considerable uncertainty (Upstill-Goddard 2006). Two parameterisations specifically applicable in shallow coastal waters where tidal current–seabed interaction generates significant turbulence are Clark et al. (1995) and Borges et al. (2004). Although the latter formally quantifies tidal current velocities and water depth, neither parameterisation has proven universally applicable (Upstill-Goddard 2006). Both were therefore applied to the present data, resulting in an uncertainty range that may be considered typical of such estimates (Upstill-Goddard 2006).
Daily mangrove creek water emissions of CO2 and CH4 derived from the Clark et al. (1995) and Borges et al. (2004) relations (Table 3) are the sums of individual hourly estimates over the full 24-h tidal surveys (all, n = 24). Daily emissions derived from surface transect data (Table 4) are the means of individual daily estimates for each sampling station (all, n = 15); these emissions were only obtained using Clark et al. (1995) as no water depth or current data are available. Variability in wind speeds was low across all six creek surveys (Table 3); hence the values of k w derived from Clark et al. (1995) did not vary significantly. Given the almost identical Schmidt numbers of CO2 and CH4 (Wanninkhof 1992) k w derived from Clark et al. (1995) was also essentially identical for both gases in all six surveys: CO2, 1.31 ± 0.08 cm h−1; CH4, 1.31 ± 0.07 cm h−1 (n = 120). By contrast, k w estimates based on Borges et al. (2004) were somewhat more variable: CO2, 4.08 ± 1.56 cm h−1; CH4, 4.13 ± 1.63 cm h−1, the difference reflecting changes in water depths and current velocities over tidal cycles. Variations in k w evidently exert only weak control of Andaman creek CO2 and CH4 emissions, which over tidal cycles are primarily functions of changing hydrostatic pressure and porewater concentration gradients. At Kalighat wet season CO2 emissions derived using Clark et al (1995) were more than twice as high as dry season CO2 emissions whereas at Wright Myo the seasonal differences were much smaller. By contrast, wet season CO2 emissions derived using Borges et al (2004) were more than twice as high as dry season emissions at both sites (Table 3). Koné and Borges (2008) reported a similar but larger (fivefold) seasonal trend in CO2 emissions from Kiên Vàng mangroves in Vietnam, which they attributed to higher net benthic and/or water column heterotrophy during the rainy season, consistent with our earlier explanation for the higher wet season pCO2 at Kalighat. That the CH4 data do not support this may be explained by our earlier observation of a significant suppression of mangrove creek CH4 emissions at Wright Myo immediately following rainfall (Barnes et al. 2006). Consistent with observed simultaneous changes in inorganic nitrogen speciation and turbidity, this behaviour was ascribed to CH4 oxidation during direct disturbance of the creek sediment surface by raindrops (Barnes et al. 2006). The seasonal variation in Andaman CH4 emissions was much smaller than for CO2 overall, irrespective of the k w formulation used (Table 3). No significant difference between wet and dry season CH4 emissions at Wright Myo (Barnes et al. 2006) was previously found.
Table 5 summarises previously published emissions of CO2 and CH4 from mangrove surrounding waters. In so far as the authors are aware, only three other studies have reported simultaneous such estimates for CO2 and CH4 (Ramesh et al. 2007; Bouillon et al. 2007b; Kristensen et al. 2008). Ramesh et al. (2007) summarise our earlier emissions estimates for Wright Myo and Kalighat and it is noteworthy that these are lower than our present estimates, by up to an order of magnitude in the case of CH4 (Tables 3 and 5). Based on these data, emissions of CO2 and CH4 from mangrove creek waters evidently exhibit high inter-annual variability. In other earlier work at Wright Myo we also estimated CH4 emissions over tidal cycles, in this case using static and floating chambers (Barnes et al. 2006). Our current estimates (Table 3) are at the lower end of this earlier range (Table 5), which is perhaps not surprising given that flux chambers can tend to give larger fluxes than those based on formulations of k w (Barnes et al. 2006).
The emission estimates for CO2, both for the tidal creeks and the surface water transects (Tables 3 and 4), appear typical of mangrove associated waters globally (Table 5) whereas the estimated CH4 emissions (Tables 3 and 4) are towards the low end of the published range (Table 5). However, it should be noted that the CH4 data in Table 5 span two orders of magnitude because of the inclusion of both anthropogenically impacted and pristine sites. Highest CH4 emissions, from for example the Jiulonjiang estuary (Alongi et al. 2005) and SE Indian (Purvaja and Ramesh 2001) and Adyar estuary mangroves (Ramesh et al. 2007), tend to reflect urbanisation and/or large inputs of domestic sewage. Kristensen et al. (2008) also found higher CH4 emissions from an anthropogenically impacted site (Mtoni) than from a pristine site (Ras Dege) in Tanzania although the difference was less pronounced than in these other studies (Table 5). Our new data for Kalightat and Wright Myo (Table 3) support our previous contention that mangrove ecosystems could dominate coastal CH4 emissions worldwide and that these may have been seriously underestimated in the past (Barnes et al. 2006; Upstill-Goddard et al. 2007), but they do not materially affect previous estimates of mangrove water emissions of either CO2 or CH4 globally (Table 5). Worth noting, however, are the observations of substantial ebullition at both Kalighat and Wright Myo during the tidal surveys. These imply that ebullition, which is not quantified in Table 5, may make an important contribution to total emissions, especially for CH4. This is because bubbles collected at Wright Myo (21 April 2006; dry season) were around 1 % by volume CO2 (26 times ambient air) and 5 % by volume CH4 (28000 times ambient air). Studies of CH4 ebullition in subtropical estuaries (Shalini et al. 2006; Rajkumar et al. 2008), a tropical wetland (Marani and Alvala 2007) and a hypertrophic temperate lake (Casper et al. 2000) estimated ebullition contributions ∼60–90 % to total CH4 emissions. While these results imply that the CH4 emissions presented in Table 5 could be rather conservative, accurately estimating ebullition fluxes is a substantial challenge (Upstill-Goddard 2006). Consequently, accurately quantifying the ebullition contribution to mangrove water CO2 and CH4 emissions requires more detailed study.
Scaling the emission fluxes listed in Table 3 to a creek open water area of∼2,700 km2 gives total Andaman creek water emissions of 3.6 × 1010 mol CO2 year−1 and 3.7 × 107 mol CH4 year−1 based on the k w formulation of Clark et al. (1995). Corresponding emissions derived using the k w formulation of Borges et al. (2004) are somewhat larger: 9.2 × 1010 mol CO2 year−1 and 3.4 × 108 mol CH4 year−1. Taking an additional account of the contribution from surrounding inshore waters (Table 4) is problematic because the full area extent of the CO2 and CH4 outflow plumes is not known, but based on the surveys conducted, these could be estimated to be around ten times the creek area. If so and taking account of their average lower emissions per unit area (around 42 and 71 % respectively, of the creek water values for CO2 and CH4; Tables 3 and 4), using k w derived from Clark et al (1995), the additional emissions could be as much as 1.5 × 1011 mol CO2 year−1 and 2.6 × 108 mol CH4 year−1, giving total emissions of ∼1.9 × 1011 mol CO2 year−1 and ∼3.0 × 108 mol CH4 year−1 from a total area of mangrove influenced water of ∼3 × 104 km2. Using the k w formulation of Borges et al. (2004) would undoubtedly yield larger total emissions; however, the lack of water depth and current data for the inshore water transects precludes this.
Mangrove Water Emissions and the Mangrove Carbon Budget
To consider the scale of gaseous carbon emissions from mangrove-associated waters in the context of other compartments of the mangrove carbon budget is instructive. Combining the emissions estimates (Tables 3 and 4) with those compiled in Table 5, an average emissions of ∼55 ± 40 mmol CO2 m−2 day−1 and ∼1.2 ± 3.0 mmol CH4 m−2 d−1 from mangrove-associated waters can be estimated globally. Our figure for CO2 is close to an earlier estimate of ∼59 ± 52 mmol CO2 m−2 day−1 derived from a slightly smaller dataset (Bouillon et al. 2008). Water column emissions of gaseous carbon are a subset of total sediment emissions and attributing fractional contributions to the total from inundated and exposed sediments individually is problematic because of the intertidal nature of the system. However, a compilation by Bouillon et al (2008) of CO2 emission estimates derived for exposed mangrove sediments using benthic chambers gave a global average of 61 ± 46 mmol CO2 m−2 day−1. Given the uncertainties that arise from spatio-temporal variability and measurement methodologies, this is identical to their figure for mangrove waters derived from pCO2 data, which prompted them to set total global mangrove CO2 emissions from sediments/water column at ∼60 ± 45 mmol m−2 day−1, equivalent to ∼42 ± 31 Tg C year−1. Our estimates for CO2 and CH4 do not materially affect this figure considering the inherent uncertainties arising from the high spatio-temporal variability of tidal and inter-tidal mangrove waters and given the above indication that more than 98 % of the total gaseous carbon flux is CO2.
Additional mangrove carbon sinks include burial and lateral export. Recent work in an Australian mangrove creek provides the first direct estimate of the relative carbon export contributions from DIC, DOC and POC (Maher et al. 2013). This showed DIC export to be at least an order of magnitude greater than DOC export and that POC was consistently imported, at rates comparable to or exceeding, those of DOC export. Given the associated uncertainties the DIC: DOC export ratio is consistent with an earlier synthesis (Bouillon et al. 2008) that set global mangrove carbon sinks (in teragrammes C per year) at: burial, 18.4; DIC export, 178 ± 165; DOC export, 24 ± 21; and POC export, 21 ± 22. Together, these sinks were considered sufficient to balance mangrove net primary production (Bouillon et al. 2008). Clearly, mangrove water emissions of gaseous carbon are a significant term in the mangrove carbon budget, apparently of the same order as DOC and POC fluxes. However, the available data are limited and the associated uncertainties are very large. Importantly, other potentially relevant mechanisms of CO2 and CH4 emission remain unaccounted for; for example, crab burrows, which are not routinely included in sediment efflux measurements, may be important conduits for enhanced CO2 loss (Kristensen et al. 2008), and there is evidence that plant pneumatophores could at least make a significant contribution to total CO2 emissions (Bouillon et al. 2008) while they may even dominate CH4 emissions (Purvaja et al. 2004). Moreover, there are no reliable estimates of global mangrove ebullition rates for either CO2 or CH4, even though these might well be quantitatively significant.
Conclusions
Our estimates of CO2 and CH4 emissions from Andaman mangrove tidal creeks and adjacent inshore waters are within previously published ranges for these systems, consistent with a significant contribution to the mangrove sink for global carbon. Evaluating such emissions in a range of mangrove environments is important to resolving the greenhouse gas balance of mangrove ecosystems globally; however, future such studies should be integral to much wider quantitative process studies of the overall mangrove carbon balance. Improving our current understanding of mangrove carbon dynamics is important, not only for reducing current uncertainties, but for predicting the system response to large-scale changes such as mangrove replanting and clearance, both of which are becoming increasingly important aspects of mangrove “management” and for which the response through gaseous carbon emissions may not be well anticipated.
References
Alongi, D.M. 2002. Present state and future of the world’s mangrove forests. Environmental Conservation 29: 331–349.
Alongi, D.M., A. Sasekumar, F. Tirendi, and P. Dixon. 1998. The influence of stand age on benthic decomposition and recycling of organic matter in managed mangrove forests of Malaysia. Journal of Experimental Marine Biology and Ecology 225: 197–218.
Alongi, D.M., F. Tirendi, L.A. Trott, and X.X. Xuan. 2000. Benthic decomposition rates and pathways in plantations of the mangrove, Rhizophora apiculata, in the Mekong delta, Vietnam. Marine Ecology Progress Series 194: 87–101.
Alongi, D.M., G. Wattayakorn, J. Pfitzner, F. Tirendi, I. Zagorskis, G.J. Brunskill, et al. 2001. Organic carbon accumulation and metabolic pathways in sediments of mangrove forests in southern Thailand. Marine Geology 179: 85–103.
Alongi, D.M., J. Pfitzner, L.A. Trott, P. Dixon, and D.W. Klumpp. 2005. Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang estuary, China. Estuarine, Coastal and Shelf Science 63: 605–618.
Amorocho, J., and J.J. DeVries. 1980. A new evaluation of the wind stress coefficient over water surfaces. Journal of Geophysical Research 85: 433–442.
Bange, H.W. 2006. Nitrous oxide and methane in European coastal waters. Estuarine, Coastal and Shelf Science 70: 361–374.
Barnes, J., R. Ramesh, R. Purvaja, A. Nirmal Rajkumar, B. Senthil Kumar, K. Krithika, et al. 2006. Tidal dynamics and rainfall control N2O and CH4 emissions from a pristine mangrove creek. Geophysical Research Letters 33, L15405. doi:10.1029/2006GL026829.
Berner, R.A., 1980. Early Diagenesis: a Theoretical Approach. Princeton: Princeton University Press
Biswas, H., S.K. Mukhopadhyay, and T.K. De. 2004. Biogenic controls on the air-water carbon dioxide exchange in the Sundarban mangrove environment, northeast coast of Bay of Bengal, India. Limnology and Oceanography 49: 95–101.
Biswas, H., S.K. Mukhopadhyay, S. Sen, and T.K. Jana. 2007. Spatial and temporal patterns of methane dynamics in the tropical mangrove dominated estuary, NE coast of Bay of Bengal, India. Journal of Marine Systems 10: 1–8.
Borges, A.V., S. Djenidi, G. Lacroix, J. Théate, B. Delille, and M. Frankignoulle. 2003. Atmospheric CO2 flux from mangrove surrounding waters. Geophysical Research Letters 30: 1558. doi:10.1029/2003GL017143.
Borges, A.V., J.P. Vanderborght, L.-S. Schiettecatte, F. Gazeau, S. Ferrón, B. Delille, et al. 2004. Variability of the gas transfer velocity of CO2 in a macrotidal estuary (the Scheldt). Estuaries 27: 593–603.
Borges, A.V., B. Delille, and M. Frankignoulle. 2005. Budgeting sinks and sources of CO2 in the coastal ocean: Diversity of ecosystems counts. Geophysical Research Letters 32: 1–5.
Bouillon, S., and H.T.S. Boschker. 2006. Bacterial carbon sources in coastal sediments: a cross-system analysis based on stable isotope data of biomarkers. Biogeosciences 3: 175–185.
Bouillon, S., M. Frankignoulle, F. Dehairs, B. Velimirov, A. Eiler, G. Abril, et al. 2003. Inorganic and organic carbon biogeochemistry in the Gautami Godavari estuary (Andhra Pradesh, India) during pre-monsoon: The local impact of extensive mangrove forests. Global Biogeochemical Cycles 17: 1114. doi:10.1029/2002GB002026.
Bouillon, S., F. Dehairs, L.-S. Schiettecatte, and A.V. Borges. 2007a. Biogeochemistry of the Tana estuary and delta (northern Kenya). Limnology and Oceanography 52: 45–59.
Bouillon, S., F. Dehairs, B. Velimirov, G. Abril, and A.V. Borges. 2007b. Dynamics of organic and inorganic carbon across contiguous mangrove and seagrass systems (Gazi bay, Kenya). Journal of Geophysical Research-Biogeosciences 112, G02018. doi:10.1029/2006JG000325.
Bouillon, S., J.J. Middelburg, F. Dehairs, A.V. Borges, G. Abril, M.R. Flindt, et al. 2007c. Importance of intertidal sediment processes and porewater exchange on the water column biogeochemistry in a pristine mangrove creek (Ras Dege, Tanzania). Biogeosciences 4: 311–322.
Bouillon, S., A.V. Borges, E. Castañeda-Moya, K. Diele, T. Dittmar, N.C. Duke, et al. 2008. Mangrove production and carbon sinks: a revision of global budget estimates. Global Biogeochemical Cycles 22, GB2013. doi:10.1029/2007gb003052.
Casper, P., S.C. Maberly, G.H. Hall, and B.J. Finlay. 2000. Fluxes of methane and carbon dioxide from a small productive lake to the atmosphere. Biogeochemistry 49: 1–19.
Chen, C.T.A., and A.V. Borges. 2009. Reconciling opposing views on carbon cycling in the coastal ocean: continental shelves as sinks and near-shore ecosystems as sources of atmospheric CO2. Deep Sea Research II 56: 578–590.
Clark, J.F., P. Schlosser, R. Wanninkhof, H.J. Simpson, W.S.F. Schuster, and D.T. Ho. 1995. Gas transfer velocities for SF6 and 3He in a small pond at low wind speeds. Geophysical Research Letters 22: 93–96.
Dagar, J.C., A.D. Mongia, and N.T. Singh. 1995. Degradation of tropical rain forest soils upon replacement with plantations and arable crops in Andaman and Nicobar Islands in India. Tropical Ecology 36: 89–101.
Dittmar, T., N. Hertkorn, G. Kattner, and R.J. Lara. 2006. Mangroves, a major source of dissolved organic carbon to the oceans. Global Biogeochemical Cycles 20(1), GB101210. doi:10.1029/2005GB002570.
Forest Survey of India. 2005. State of Forest Report, Ministry of Environment and Forests. India: Dehradun.
Frankignoulle, M., and A.V. Borges. 2001. Direct and indirect pCO2 measurements in a wide range of pCO2 and salinity values (the Scheldt estuary). Aquatic Geochemistry 7: 267–273.
Gattuso, J.P., M. Frankignoulle, and R. Wollast. 1998. Carbon and Carbonate Metabolism In Coastal Ecosystems. Annual Reviews of Ecological Systems 29: 405–434.
Ghosh, S., T.K. Jana, B.N. Singh, and A. Choudhury. 1987. Comparative study of carbon dioxide system in virgin and reclaimed mangrove waters of Sundarbans during freshet. Mahasagar Bulletin, National Institute of Oceanography 20: 155–161.
Giri, C., E. Ochieng, L.L. Tieszen, Z. Zhu, A. Singh, T. Loveland, et al. 2011. Status and distribution of mangrove forests of the world using earth observation satellite data. Global Ecology and Biogeography 20: 154–159.
Gran, G. 1952. Determination of the equivalence point in potentiometric titrations- Part II. Analyst 77: 661–671.
Harriss, R.C., E. Gorham, D.I. Sebacher, K.B. Bartlett, and P.A. Flebbe. 1998. Methane flux from northern peatlands. Nature 315: 652–654.
Jennerjahn, T.C., and V. Ittekot. 2002. Relevance of mangroves for the production and deposition of organic matter along tropical continental margins. Naturwissenschaften 89: 23–30.
Koné, Y.J.M., and A.V. Borges. 2008. Dissolved inorganic carbon dynamics in the waters surrounding forested mangroves of the Ca Mau Province (Vietnam). Estuarine, Coastal and Shelf Science 77: 409–421.
Kreuzwieser, J., J. Buchholz, and H. Rennenberg. 2003. Emission of methane and nitrous oxide by Australian mangrove ecosystems. Plant Biology 5: 423–431.
Kristensen, E., F.Ø. Andersen, N. Holmboe, M. Holmer, and N. Thongtham. 2000. Carbon and nitrogen mineralization in sediments of the Bangrong mangrove area, Phuket, Thailand. Aquatic Microbial Ecology 22: 199–213.
Kristensen, E., M.R. Flindt, S. Ulomi, A.V. Borges, G. Abril, and S. Bouillon. 2008. Emission of CO2 and CH4 to the atmosphere by sediments and open waters in two Tanzanian mangrove forests. Marine Ecology Progress Series 370: 53–67.
Krumins, V., M. Gehlen, S. Arndt, P. van Cappellen, and P. Regnier. 2013. Dissolved inorganic carbon and alkalinity fluxes from coastal marine sediments: model estimates for different shelf environments and sensitivity to global change. Biogeosciences 10: 371–398. doi:10.5194/bg-10-371-2013.
Lara, R.J., and T. Dittmar. 1999. Nutrient dynamics in a mangrove creek (North Brazil) during the dry season. Mangroves and Salt Marshes 3: 185–195.
Lekphet, S., S. Nitisoravut, and S. Adsavakulchai. 2005. Estimating methane emissions from mangrove area in Ranong Province, Thailand. Songklanakarin Journal of Science and Technology 27: 153–163.
Liss, P.S., and L. Merlivat. 1986. Air-sea gas exchange rates: Introduction and synthesis. In The Role of Air-Sea Exchange in Geochemical Cycling, ed. P. Buat-Menard. New York: Springer.
Maher, D., I.R. Santos, J. Gleeson, L. Golsby-Smith, and B.D. Eyre. 2013. Groundwater-derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: The missing mangrove carbon sink? Limnology and Oceanography 58: 475–488.
Marani, L., Alvala., P. C., 2007. Methane emissions from lakes and floodplains in Pantanal, Brazil. Atmospheric Environment 41,1627–1633
Millero, F.J., W.T. Hiscock, W.T., Huang, F., Roche, M., Zhang, J.Z., 2001. Seasonal variation of the carbonate system in Florida Bay. Bulletin of Marine Science, 68, 101–123
Millero, F.J., T.B. Graham, F. Huang, H. Bustos-Serrano, and D. Pierrot. 2006. Dissociation constants of carbonic acid in sea water as a function of salinity and temperature. Marine Chemistry 100: 80–94.
Muller-Karger, F. E., Varela, R., Thunell, R,. Luerssen,R., Hu, C., Walsh, J. J., 2005. The importance of continental margins in the global carbon cycle. Geophysical Research Letters 32, L01602, doi:10.1029/2004GL021346, 2005.
Ovalle, A.R.C., C.E. Rezende, L.D. Lacerda, and C.A.R. Silva. 1990. Factors affecting the hydrochemistry of a mangrove tidal creek, Sepetiba Bay, Brazil. Estuarine, Coastal and Shelf Science 31: 639–650.
Pierrot D., Lewis E., Wallace, D. W. R., 2006. MS Excel program developed for CO2 system calculations. ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee.
Purvaja, R., and R. Ramesh. 2001. Natural and anthropogenic methane emission from coastal wetlands of south India. Environmental Management 27: 547–557.
Purvaja, R., R. Ramesh, and P. Frenzel. 2004. Plant-mediated methane emission from an Indian mangrove. Global Change Biology 10: 1–10.
Rajkumar, A.N., J. Barnes, R. Ramesh, R. Purvaja, and R.C. Upstill-Goddard. 2008. Methane and nitrous oxide fluxes in the polluted Adyar River and estuary, SE India. Marine Pollution Bulletin 56: 2043–2051.
Ramesh, R., R. Purvaja, V. Neetha, J. Divia, J. Barnes, and R.C. Upstill-Goddard. 2007. CO2 and CH4 emissions from Indian mangroves and its surrounding waters. In Greenhouse Gas and Carbon Balances in Mangrove Coastal Ecosystems, ed. Y. Tateda, R.C. Upstill-Goddard, T. Goreau, D. Alongi, A. Nose, E. Kristensen, and G. Wattayakorn. Kanagawa, Japan: Gendai Tosho.
Santos, I.R., D. Maher, and B.D. Eyre. 2012. Coupling automated radon and carbon dioxide measurements in coastal waters. Environmental Science and Technology 46: 7685–7691.
Sarma, V.V.S.S., and P.V. Narvikar. 2001. A study on inorganic carbon components in the Andaman Sea during the post monsoon season. Oceanologica Acta 24: 125–134.
Selvam, V. 2003. Environmental classification of mangrove wetlands of India. Current Science 84: 759–765.
Shalini, A., R. Ramesh, R. Purvaja, P. Frenzel, and J. Barnes. 2006. Spatial and temporal distribution of methane in an extensive shallow estuary, South India. Journal of Earth System Science 115: 451–460.
Singh, V. P., Garge, A., Pathak, S. M., Mall, L. P., 1986. Mangrove forests of Andaman Islands in relation to human interference. Environmental Conservation. 13, 169172 doi:10.1017/S0376892900036821
Singh, V.P., A. Garge, S.M. Pathak, and L.P. Mall. 1987. Pattern and process in mangrove forests of the Andaman Islands. Vegetatio 71: 185–188.
Upstill-Goddard, R.C. 2006. Air-sea gas exchange in the coastal zone. Estuarine, Coastal and Shelf Science 70: 388–404.
Upstill-Goddard, R.C., A.P. Rees, and N.J.P. Owens. 1996. Simultaneous high-precision measurements of methane and nitrous oxide in water and seawater by single-phase equilibration gas chromatography. Deep-Sea Research 43: 1669–1682.
Upstill-Goddard, R.C., J. Barnes, and R. Ramesh. 2007. Are mangroves a source or a sink for greenhouse gases? In Greenhouse Gas and Carbon Balances in Mangrove Coastal Ecosystems, ed. Y. Tateda, R.C. Upstill-Goddard, T. Goreau, D. Alongi, A. Nose, E. Kristensen, and G. Wattayakorn. Kanagawa, Japan: Gendai Tosho.
Wanninkhof, R. 1992. Relationship between wind speed and gas exchange over the ocean. Journal of Geophysical Research 97: 7373–7382.
Weiss, R.F. 1974. Carbon dioxide in water and seawater: the solubility of a non-ideal gas Marine Chemistry 2: 203–215.
Wiesenburg, D.A., and N.L. Guinasso. 1979. Equilibrium solubilities of methane, carbon monoxide and hydrogen in water and seawater. Journal of Chemical Engineering Data 24: 354–360.
Wollast, R. 1998. Evaluation and comparison of the global carbon cycle in the coastal zone and in the open ocean. In The Sea, 10th ed, ed. K.H. Brink and A.R. Robinson. New York: John Wiley and Sons.
Zablocki, J.A., A.J. Andersson, and N.R. Bates. 2011. Diel aquatic CO2 system dynamics of a Bermudian mangrove environment. Aquatic Geochemistry 17: 841–859. doi:10.1007/s10498-011-9142-3.
Zhai, W.D., M.H. Dai, W.J. Cai, Y.C. Wang, and Z.H. Wang. 2005. High partial pressure of CO2 and its maintaining mechanism in a subtropical estuary: the Pearl River estuary, China. Mar + ine. Chemistry 93: 21–32.
Acknowledgments
The authors thank their colleagues at the Institute of Ocean Management (IOM) at Anna University and the School of Marine Science and Technology at Newcastle University for their assistance and support. The authors specifically thank The Leverhulme Trust (http://www.leverhulme.ac.uk/) for their financial support through Research Grant F/00 125/R and the Council of Scientific and Industrial Research (CSIR) University Grants Commission (UGC), Government of India, for facilitating a Junior Research Fellowship (PhD) in support of Neetha V.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Alberto Vieira Borges
Rights and permissions
About this article
Cite this article
Linto, N., Barnes, J., Ramachandran, R. et al. Carbon Dioxide and Methane Emissions from Mangrove-Associated Waters of the Andaman Islands, Bay of Bengal. Estuaries and Coasts 37, 381–398 (2014). https://doi.org/10.1007/s12237-013-9674-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-013-9674-4