Abstract
The mouth dynamics of temporarily open/closed estuaries (TOCEs) play a key role in their overall functioning. In this study, the effect of the inlet state (closed vs. artificially breached) on spatial variability of macrobenthic invertebrates (composition, abundance, and biomass) was assessed in a temporarily open/closed lagoon of South Brazil (28°35′S/48°52′W). Samplings were carried out in two periods during closed (July and November) and open phases (July and November). Additionally, in order to evaluate possible transitory effects of breaching, data obtained during closed and open phases were compared with those samples taken 60 days after the end of mechanical opening of the mouth (January). The artificial breaching markedly changed the dynamic of the benthic environment. After the inlet dredging and bulldozing, total organic content and microphytobenthic biomass were significantly reduced. The disturbance also resulted in a population crash of the macrobenthic invertebrates, with a reduction of 50% in biomass and 90% in density. Following the shock produced by the artificial breaching, most of the macroinvertebrates descriptors recovered, as shown by the univariate and multivariate analysis. However, a benthic community with a significantly different structure emerged. During the study, the macroinvertebrates from inner portions of the lagoon were less variable than those in the middle or near the lagoon inlet. The results of this study showed that the macrobenthic associations of Camacho lagoon were primarily structured by salinity and microphytobenthic biomass, which in turn, were regulated by the state of the inlet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temporarily open/closed estuaries (TOCEs) are shallow water bodies (barrier lagoons or rivers) intermittently isolated from the sea by the formation of a sand berm across the estuary mouth. The state of estuary mouth is determined by the balance between scouring forces (primarily catchment run-off and tidal prism) and blocking forces (primarily onshore and longshore deposition of sediments; Whitfield and Bate 2007). TOCEs are characterized by a moderate to low river inflow relative to volume and/or high rates of longshore and onshore sediment transport (Cooper et al. 1999; Schallenberg et al. 2010). Once closed, depending on the freshwater inputs and the time of closure, the system may become gradually fresher or even more saline. Natural reestablishment of a link to the marine environment will occur either from rising lagoon levels overtopping or through erosion by ocean waves (Stretch and Parkison 2006).
The mouth dynamics of TOCEs play a key role in their overall functioning. Intermittent breaching of estuary mouth may lead to remarkable changes in its physico-chemical environment over short time periods, which in turn triggers major biological responses in both pelagic and benthic compartments (Niekerk et al. 2005; Anandraj et al. 2008; Lawrie et al. 2010). The natural breaching process can also cause significant morphological changes because the strong breach outflows can scour large quantities of accumulated sediments from an estuary (Whitfield and Bate 2007). The intensity of breaching impacts may, however, reflect differences in water levels and morphological characteristics of the estuary or lagoon, which dictate the degree of tidal flushing when the barrier is open (Schallenberg et al. 2010).
Human interventions in permanently open estuarine system are common worldwide, and TOCEs are not different. Contrary to those permanently open, however, the reduced opportunities for flushing, potentially exacerbates the susceptibility of TOCEs to a range of anthropogenic impacts. The poor understanding of TOCEs functioning, together with unplanned developments on the estuary floodplain, have led managers in many countries to intervene in the dynamics of these systems by artificially breaching the mouth to, allegedly, improve water quality and fishery conditions, and to prevent flooding of adjacent properties (Roy et al. 2001; Dye and Barros 2005; Gladstone et al. 2006). Nevertheless, the response of TOCEs to mouth breaching may vary depending on a myriad of TOCEs-specific factors (Schallenberg et al. 2010). Eventually, artificial breaching may even led to unintended effects, such as nutrient enrichment (Santos et al. 2006) and increased chlorophyll a concentrations (Twomey and Thompson 2001; Gobler et al. 2005). Moreover, in the long term, artificial breaching may cause a significant buildup of sediments in estuaries as it prevents a sufficient head of water from building-up behind the berm which prevents effective scouring of sediments from the estuary during a breaching event (Bate 2007).
In Brazil, although TOCEs occur in large numbers, information about these systems is scanty (Oliveira et al. 2004; Santos et al. 2006). The majority of the information regarding TOCEs was derived from research carried out in South Africa, New Zealand, and Australia, where such systems make up an important fraction of the estuaries (e.g., Allanson and Baird 1999; Roy et al. 2001; Hume et al. 2007; Schallenberg et al. 2010). With respect to macrobenthic invertebrates, most previous studies have so far reported on comparisons between TOCEs and those of permanently open and/or closed lagoons (e.g., Teske and Wooldridge 2001, 2003; Hirst 2004; Dye and Barros 2005; Hastie and Smith 2006). The response of macrobenthic communities to artificial breaching of TOCEs is still inadequately studied. Decker (1987) observed that the largest reductions in macroinvertebrates species' abundance and biomass after artificial opening of an estuary were related to the collapse of the macrophyte Ruppia maritima, which is intolerant of high salinities. Yet Gladstone et al. (2006) showed that the macrobenthic invertebrates in entrance barriers of TOCEs were not affected and appear to be resilient to the habitat disturbance caused by artificial openings.
In this study, we hypothesize that the inlet state (closed or artificially breached) of a subtropical temporarily open/closed lagoon affects the spatial variability of the macrobenthic invertebrates (composition, abundance, and biomass). It is expected that during open phases, the intrusion of saline marine waters will increase spatial heterogeneity and faunal diversity. The opening and closure of an inlet, however, are not the only factors that may introduce faunal variability in a coastal lagoon, although it could be an important one (Dye and Barros 2005). Temporal variation is one factor that may confound the effects of a breaching event. For example, benthic macrofauna of nearby (open) coastal lagoons are known to exhibit a marked seasonal variability (Fonseca and Netto 2006; Meurer and Netto 2007). In order to try to reduce such effects, spatial replicate sampling was carried out in the same periods (months) but during closed and open phases. Additionally, in order to evaluate possible temporary effects of breaching on the macroinvertebrates, data obtained during closed and open phases were compared to samples taken 60 days after the artificial opening.
Material and Methods
Study Area
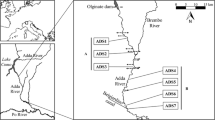
The study was conducted at the Camacho lagoon, a choked lagoon located in the south region of the Santa Catarina State, South Brazil (Fig. 1a). Camacho is one of a group of coastal lagoons that constitutes the South Santa Catarina Lagoon Complex and integral part of the Federal Environmental Protection Area (APA) of Southern Right Whale. The Camacho lagoon has an area of 24.17 km² with mean depth around 1.8 m. The lagoon receives the freshwater input of the Congonhas river and total annual mean rainfall is 1,260 mm, with no marked differences over the year; mean air temperatures are around 13°C in the winter and 22°C in the summer; water temperature may vary from 12°C in the winter to 25°C summer; NE and S–SE winds are the most frequent in the summer and winter, respectively (EPAGRI 2011). According to DeBlasis et al. (2007), this region presents high rates of sediment transport, coming both from the land and from the ocean, which is reflected on the presence of large dune fields.
The Camacho lagoon differs from the other local lagoons as it has an intermittently open inlet. This inlet, denominated Camacho Inlet, has a long described history of instability, back to 1927, and it is only open during extreme events of river discharge and/or meteorological tide (Oliveira et al. 2004). During open phases, the astronomical microtidal regime is classified as mixed, predominantly semidiurnal, and is under strong meteorological influence (INPH 1991). The instability of the Camacho inlet also determined an unsuccessful history of interventions in attempt to maintain the mouth permanently open to, supposedly, increase fishery production, especially shrimps, and improve water quality. The last intervention began in December 2007 (Fig. 1b), after more than 1 year of closure, by dredging and bulldozing the inlet. The Camacho lagoon was fully connected with the sea on January 3, 2008.
Sampling and Sample Processing
Samplings were carried out in the same months during closed and open periods (July 31 and November 23, 2007, closed; July 14 and November 5, 2008, open). Moreover, one additional sampling was conducted on March 1, 2008, in order to evaluate possible short term effects of breaching on the macrobenthic invertebrates.
In each sampling period, five shallow subtidal sites were established along the Camacho lagoon (Fig. 1a) from the inner area, near Congonhas river (site 1; Fig. 1a) to the outer lagoon (site 5). At each site, samples were taken for the microphytobenthic biomass, macroinvertebrates, granulometry, and total organic content.
For the analysis of the microphytobenthic biomass (chlorophyll a—chl a—and phaeopigments), four samples were taken at each site, where the first 5 cm were collected with a PVC corer of 4 cm in diameter. Pigments were extracted with a solution of acetone 90% v/v, using 20 ml of the solution to 10 ml of sediment and analyzed according to Strickland and Parsons (1972). Chl a and phaeopigment biomass were estimated using the equation of Lorenzen (1967).
Four samples were also taken in each site for the macroinvertebrates with a 15-cm diameter PVC core tube pushed into the sediment to a depth of 10 cm and fixed in 10% formalin. Fixed samples were sieved through a 0.5-mm mesh, preserved in 70% ethanol and sorted under a dissecting microscope. All invertebrates were identified to the lowest possible taxonomic level and counted. The total macrofauna biomass was measured as wet weight on ethanol preserved organisms, following suggestion of Wetzel et al. (2005) that showed that the choice of the preservative (ethanol or formalin) has little effect on biomass loss.
Samples for sediment granulometry and total organic content (also four samples for each site) were taken with a 10-cm diameter core tube to a depth of 5 cm. Sediment granulometry was determined by sieving and pipette analyses (Suguio 1973) and total organic content by combustion (550°C for 60 min; Dean 1974). Salinity and water temperature were measured in situ with an YSI multiparameter instrument (556MPS).
Data analysis
In order to test for differences between the closed and open phases along the sampling sites of the lagoon, univariate and nonparametric multivariate techniques were applied. As environmental descriptors, salinity, mean grain size, fine percentages (silt + clay), total organic content, chl a and phaeopigment concentrations were used. The chl a to phaeopigments ratio (chl a:phaeo) was also used as a measure of lability of organic matter in surface sediments (Garcia and Thomsen 2008). For the macrofauna, the descriptors were the number of species, total density, total biomass, Shannon–Wiener diversity index (loge), and the density of the most abundant species.
Two approaches were considered in order to describe the effects of the artificial breaching on the macroinvertebrates. First, the effect of the inlet state (closed and artificially open), spatial variability (sites 1 to 5) and the interaction between inlet state and spatial variability on the macrofauna and environmental descriptors were tested by a 2-way ANOVA. Homogeneity of variances were determined using Cochran's tests and, where appropriate, transformations log(x + 1) applied. Tukey's multiple comparison tests were used when significant differences were detected (p < 0.05; Quinn and Keough 2002). Procedures for adjusting significance levels to control type I error rates in multiple testing situations, such as the Bonferroni method, have been the subject of much debate (Bland and Altman 1995; Perneger 1998). Although this technique provides great control over type I errors, it is very conservative when there are many comparisons and may miss real differences (i.e., increase of type II errors; Quinn and Keough 2002). Therefore, the results of both corrected and uncorrected p values were used. Similarity matrices were then constructed using Bray–Curtis similarity measure and fauna data were ordinated by non-metric multidimensional scaling (MDS). The significance of the differences in the multivariate community structured was tested, with the same design as ANOVA, by non-parametric permutational multivariate analysis of variance (PERMANOVA; Anderson 2005).
Additionally, in order to evaluate possible short-term effects of the breaching event, sampling sites data were pooled and all sampling periods were compared (July and November 2007 closed, March 2008 breached, July and November 2008 open) by one-way ANOVA. Ordination was also by non-metric multidimensional scaling (MDS) and formal significance tests for differences among periods were performed using the ANOSIM permutation test (Clarke and Green 1988). The variability amongst samples in each sampling period and amongst sites in all periods was analyzed using the multivariate relative dispersion measure (MVDISP; Warwick and Clarke 1993).
Similarity percentage analysis (SIMPER; Clarke 1993) was employed to assess compositional similarity and identify the main macrofaunal species contributing to dissimilarities between closed and open phases. The relationships between multivariate community structure and combinations of environmental variables were analyzed using the Bio-Env procedure (Clarke and Ainsworth 1993) to define suites of variables that best explain the faunal structure. Finally, a correlation-based principal component analysis (PCA) was applied to all biotic and abiotic descriptors.
Results
Environmental Variables
Salinity values were significantly lower and spatially less variable during closed phases (0.2 to 1.6). Conversely, a salinity gradient, with mean values ranging from 7.59 (site 1) to 20.38 (site 5), was evident during the open phases (Fig. 2). Sediments were characterized by moderately well-sorted fine sands during both closed and open phases. The differences in the mean grain size, sand, and fine percentages (silt + clay) between phases were significant only at site 1 (Fig. 2; Table 1; Tukey HSD p < 0.05). The sediment total organic content, always lower than 2%, was significantly higher at the middle and inner reaches and during closed phases of the lagoon (Table 1; Fig. 2). The chl a and phaeopigments concentrations in the surface of the sediment were significantly higher during closed than open phases, independently of sample site (Table 1). Similarly, the chl a to phaeopigment ratio, an indicator of organic matter lability, was also higher during the periods of closed inlet (Table 1).
The results of one-way ANOVA comparing the closed (July and November 2007) breached (March 2008) and open (July and November 2008) phases (with site data pooled), showed that values of salinity increased significantly after the artificial breaching (F = 82.8; p < 0.0001), with a maximum mean value of 18.7 in July 2008. Sediment mean grain size and fine percentages did not varied significantly among the different sampling periods (all p > 0.05). Yet the total organic content of the sediment decreased significantly after breaching and remained lower compared with closed phases (F = 3.3; p = 0.01).
Chl a concentration and the ratio chl a:phaeopigment decreased sharply just after the artificial breaching (Fig. 3), increasing subsequently during open phase. Phaeopigments mean concentrations also decreased after breaching, but not significantly (Tukey's HSD, p > 0.05). Compared to closed phases, lower values of phaeopigments were observed in July 2008 (open inlet; Fig. 3).
Macrofauna
A total of 36 benthic macroinvertebrates taxa were recorded in this study (72% of these occurring in both closed and open phases; ESM Table 1). During the closed periods, 31 taxa were recorded with total densities ranging between 9,941 and 102,176 inds/m2. The surface crawler gastropod Heleobia australis largely dominated these samples, accounting for 81.8% of the fauna, and with densities of up to 99,058 inds/m2. An unidentified species of Chironomidae was the second most abundant species (3% of the total macrofauna collected). Five species occurred exclusively during closed phases, but they represented only 0.5% of the total macrofauna collected in this period. During the open phase, 30 taxa were recorded and total densities varied between 8,882 and 105,705 inds/m2. Again, the gastropod H. australis was numerically most abundant species (53% of the fauna), but polychetes, such as the capitelid Heteromastus similis and the nereidid Laeonereis culveri increased their abundances, accounting for 16% and 13% of the fauna, respectively. Seven species were recorded exclusively during open phase (mainly polychetes) and accounted for 2% of the total fauna.
Independent of the sampling site, the number of macrobenthic species and the densities of the polychete H. similis were significantly higher during open phase, while the densities of chironomids were significantly higher during closed phases (no significant interaction between inlet state and sites; Table 2). For all other univariate descriptors or abundant species, variations between open and closed periods were dependent on the sampling site (Table 2; Fig. 4). Overall, Tukey's HSD multiple comparison tests showed that inner sampling sites were less variable between phases than those in the middle or near the lagoon inlet (Fig. 4).
The macrofauna data obtained just after the inlet was artificially breached (March) were then analyzed together with those from closed and open phases (2007/closed; 2008/open). The inclusion of these data showed that almost all descriptors of the macrofauna crash just after the inlet opening (Fig. 5). The number of species, density, and biomass significantly dropped just after breaching and then increased (number of species) or kept a similar value compared to closed periods (density and biomass). The exception was the macrofauna diversity (H′) which values did not change significantly just after breaching, but increased during the last two sampling occasions when the inlet was open (Fig. 5). All the most abundant macrobenthic species also showed a collapse in their densities just after breaching, except for H. similis (Fig. 5). H. australis, L. culveri, and H. similis increased subsequently during open phase, while the unidentified morphotype of chironomid did not (Fig. 5).
MDS ordination derived from the averaged macrofauna data (Fig. 6a) mirrored changes observed in the univariate descriptors and showed a clear distinction between closed and open periods. Moreover, it can be observed in the plot that, only during open phase, the sampling sites showed a distribution along a gradient (from inner site 1 to lagoon mouth 5, Fig. 6a). The results of the PERMANOVA tests confirmed the significance of this difference and also showed a significant interaction between inlet state and site, indicating that the variations on benthic macrofauna between sampling sites were dependent on the inlet state (Table 3). The results of the SIMPER analysis showed that the species that contributed most for the distinction between open and closed phases were the polychete H. similis and the unidentified morphotype of chironomid.
When the macrofauna data derived from samples taken just after the inlet was breached were included, the MDS ordination also showed a clear distinction among periods (Fig. 6b), which was confirmed by the ANOSIM tests (all R > 0.677 and p = 0.001). The SIMPER analysis revealed that the (low) macrofauna densities and species number were the main features that characterized the samples taken just after the inlet was breached.
The variability among the macrofauna samples, as demonstrated by the multivariate relative dispersion index (IMD; Fig. 7a), was slightly higher during closed than open phase. However, just after the artificial breaching, macrofaunal samples variability increased (Fig. 7a). Changes in the inlet state (closed, breached, and open) also determined a gradient in the macrofauna samples variability along the lagoon during the study. This was demonstrated by the values of standard deviation (SD) of the relative dispersion index determined for all sampling periods at each sampling site (Fig. 7b). The standard deviation of the multivariate relative dispersion index was lower in the inner site (1) and increased towards the inlet (site 5).
Macrofauna and Environmental Variables Interactions
A correlation-based principal component analysis derived from sediment data, salinity, microphytobenthic concentration, macrofauna univariate descriptors, and dominant species showed a clear distinction among sites over different inlet states (Fig. 8a). It was also observed that during open phase, sampling sites (1 to 5) were distributed along a gradient, while during breached and closed phases, they were not. Moreover, samples taken at inner reaches (site 1) at different inlet phases were more or less grouped, suggesting low variability over the time. The principal component 1, where the distinction between open and closed/breached was evident, was responsible for 35.5% of the total variance. Yet the component 2, where differences between the samples taken just after breached and closed period was more clear, was responsible for 20.2% of the total variability. The projection of the variables on these components (Fig. 8b) showed that during closed phases, samples were associated with higher microphytobenthos biomass (chl a and ratio chl a:phaeopigments) and densities of the unidentified morphotype of chironomid. During open phases, samples were mainly related to higher salinity, densities of the polychetes H. similis and L. culveri, as well as higher macroinvertebrates diversity.
Ordination of macrofauna and environmental data derived from the close (dark symbols), breached (gray symbols), and open (white symbols) phases (a) and the projection of the variables on the principal component 1 and 2(b). Sal salinity; H′ Shannon diversity; S number of species; N macrofauna total density; Sort sorting; Fine fine percentages; Chl a chlorophyll a; Phaeo phaeopigments; TOC total organic content; Chiron chironomidae
The results of the Bio-Env analysis showed that salinity was the single variable that best explained the macrofauna multivariate structure (rho = 0.4) when closed vs. open periods were compared. When the macrofauna data obtained just after the inlet was breached were included, the relationship between macrofauna and environmental variables was weaker (rho = 0.32) and the explanatory variables were salinity and chl a concentration.
Discussion
The artificial mouth breaching of the Camacho lagoon dramatically changed the dynamic of the benthic environment. After the inlet dredging and bulldozing, the total organic content and microphytobenthic biomass were significantly reduced. The disturbance also resulted in a population crash of the macrobenthic invertebrates, with a reduction of 50% in biomass and 90% in density. Following the shock produced by the artificial breaching, the macrofauna recovered but, as shown by the univariate and multivariate analysis, a benthic community with a significantly different structure emerged.
The main obvious and expected change after breaching an intermittently open/closed lagoon is the intrusion of saline marine waters. A horizontal gradient in salinity is expected to occur, where the value of salinity along the lagoon will be dependent on size of the river catchment, flow, and tides. During closed phases, however, salinity would be less variable and a continued river input may result in systems gradually becoming fresher, while the absence of river inflow may result in the system gradually becoming more saline or, even hypersaline (Whitfield and Bate 2007). Indeed, salinity during the closed phases in the Camacho lagoon was homogeneous and low (0.2 to 1.6) due to constant Congonhas river inflow, as well as the long period of closure (more than 1 year). Breaching resulted in an increase of salinity in middle and lower reaches and, the constant intrusion of marine waters during open phases increased salinity even further (1.68 to 20.38).
Natural breaching of estuaries results in strong advection, flushing, and more water flowing into the sea (Niekerk et al. 2005). The sediment scouring from the estuary, therefore, is probably more effective during natural than artificial breaching as the latter prevents a sufficient head of water from building up behind the berm (Bate 2007). During the closed phases, the bottoms of the Camacho lagoon were already composed of sandy sediments (mean of 97%), and the artificial breaching did not change that composition. The exception was the inner site (site 1), where the fine percentages increased from 4% to 14% after breaching. This result probably indicated the increase of flow along the lagoon which, in turn, also increased the ability of the river to transport sediments into the lagoon, particularly in the upper reaches. Sediment scouring and flushing produced by breaching were also important to the decrease in the microphytobenthic biomass by 96% compared to pre-breaching levels. This rate of reduction in chl a concentrations in the sediment is similar to those recently described by Anandraj et al. (2008) after a natural breaching in an open/closed estuary from South African (94–99%).
During closed phases, estuarine water levels increase and sediment disturbance from wind and water generated turbulence are minimal, thus offering a fairly stable environment for micro- and macroalgal growth. Extensive growth of the filamentous green algae Ulva clathrata was also clearly observed. Besides, it is known that deposited pelagic algae can lead to benthic production of about 30–45% of total primary production in open systems (Riaux-Gobin and Bourgoin 2004). According to Gama (2007), when estuarine water levels decline, the sediment surface may become prone to destabilization by wind turbulence and associated water currents.
Once Camacho lagoon was breached, flushing and sediment scouring probably contributed to destabilize the microphytobenthic layer by dislodging and removing algal cells away from the sediment, washing then out to the sea. Accumulations of filamentous algae were not visible anymore. Following the breaching disturbance, benthic microalgal biomass increased again during open periods, attaining values similar to permanently open lagoons with sand bottoms nearby (Netto and Pereira 2009). Still, the mean values of microphytonbenthic biomass were lower compared to closed periods, probably due to prevailing mixing effects, as a result of tidal and riverine flows (Perissinotto et al. 2006).
One of the key factors that structures macrobenthic associations in lagoons and estuaries is the degree of connection with the sea, which affects recruitment of individuals and persistence of species that require a marine dispersal phase (Wooldridge 1999; Colling et al. 2007). At the Camacho lagoon, the macrobenthic associations were composed by typical estuarine endemic species which are known to inhabit the southwest Atlantic coastal lagoons (e.g., Bemvenuti 1998a; Fonseca and Netto 2006; Giménez et al. 2005). This was the case during closed, breached, and open phases. Species collected exclusively in one or another phase represented only a small fraction of the total macrofauna collected (less than 0.5% during closed phases and 2% during open). Despite this, differences were still consistently observed among the macrobenthic associations of closed, breached, and closed phases of the lagoon.
The macrofauna during closed phases of Camacho lagoon was distinguished by numerical dominance of the hydrobid surface crawler H. australis (85% of the total macrofauna). Hydrobiid snails occur widely in estuaries and coastal lagoons and are often among the most abundant species (Fenchel 1975; Ponder et al. 1991; Vieira et al. 2010). H. australis is short-lived, exhibits high growth and mortality rates, and occurs in small-scale aggregations within larger homogeneous patches (Gonçalves et al. 1998). The marked dominance during closed phases probably reflected their strong association with submerged vegetation and chlorophyll-enriched sediment particles, as this species can withstand a wide range of salinity (Bemvenuti 1998a). H. australis feeds mainly on benthic diatoms (Bianchi and Levinton 1984) but also ingest sediments particles, as well as its own fecal pellets when submitted to strong intraspecific competition (López-Figueroa and Niell 1988).
Another evident feature of macrobenthic associations during closed phases were the relatively high abundance of chironomid larvae. As with H. australis, the larvae of chironomid are common benthic component of open coastal lagoons but more often associated with oligohaline environments. Teske and Wooldridge (2003), studying the macrofauna of different types South African estuaries, observed that this taxon was more abundant in those areas that experience less variation in salinity (upper and lower reaches), suggesting that chironomid larvae were able to acclimatize to salinities approaching that of seawater if given sufficient time. In fact, once breached, chironomid larvae were virtually absent in all sites, increasing their abundance during open phase when the highest abundances were detected at the lower reaches of the lagoon.
Crash of epibenthic invertebrates after breaching temporarily closed/open estuaries due to river flooding has been reported elsewhere (Morant and Quinn 1999; Henninger et al. 2008). According to those authors, negative effects on the epibenthos were due to an increase of sediment instability and loss of submerged vegetation. In this study, the artificial breaching of the lagoon produced a similarly severe effect—a reduction of 97% in the densities of epibenthic fauna. However, the results of this study also showed that macroinfaunal organisms were affected by the artificial breaching, with a reduction in 56% of their densities. Sediment instability and loss of phytobenthic biomass were also the likely responsible factors for the increase in the environmental stress under breaching, as shown in multivariate dispersion index and for the macrofauna crash.
Estuarine species, by definition, are able to cope with salinity variations. Water circulation in coastal choked lagoons is usually wind driven and salinity varies in a more unpredicted fashion than other types of estuaries (Kjerfve 1994). The species present in such systems are therefore able to cope with this unpredictability. Indeed our results showed that many of the macroinvertebrates species were present during both open and closed phases. Besides, estuarine macrobenthic invertebrates have a myriad of strategies to avoid unfavorable salinities (e.g., horizontal and vertically migration, borrowing, closure of shells). However, it is very likely that the salinity shock due to the intrusion of marine waters in the lagoon after more than a year of closure may have also been a key factor for the macrofauna crash. The lagoon breaching, particularly after a long closed periods, results in large and very fast change in salinity and gives the animals very little time to adapt. In this study, the only organism which did not decrease in abundance during breaching was the polychete H. similis. A head-down deposit feeder, H. similis, may burrow and ingest sediment up to 15 cm below the sediment–water interface (Bemvenuti 1998b). Thus, this burrowing capacity would allow H. similis to avoid the salinity shock and sediment instability as well.
The recovery of macrofauna in temporarily open/closed estuaries could occur by different ways, depending on the adaptive strategy of the macrobenthic species. Henninger et al. (2008) pointed out that this could happen through larvae intrusion from the marine environment, by rafting from near estuaries and refugia within the estuary which will prevent the outwash into the marine environment. At the Camacho lagoon, the open phase after crash were characterized by the recovery of the macrofauna. Both density and biomass returned to levels similar to those prior to breaching. The decrease in abundance of H. australis and the occurrence of some more saline-related species in the lower reaches, such as the spionid Paraprionospio sp., resulted in higher diversity values of the macrobenthic associations during open phase. Besides, similar to the results of Dye and Barros (2005), and as showed by the multivariate analysis, the macrobenthic associations were then less variable and distributed along the new salinity gradient.
The results of this study showed that the macrobenthic associations of Camacho lagoon were primarily structured by salinity and to a lesser extent by microphytobenthic biomass, which were in turn, were regulated by the state of the inlet. The dynamics of opening and closure of the Camacho mouth is still poorly understood—the average frequency and length of closure are not known—and this knowledge is fundamental for management practices. TOCEs typically have small river catchments, which make them sensitive to changing inflow conditions (Whitfield 1992; Bollmohr et al. 2009). The number of TOCEs along the Brazilian coasts is largely unknown. In the last 10 years, regional irrigated rice fields, where tremendous amount of water is used, increased from 7.8 to 20.6 thousands ha (CEPA 2010). Besides, fast and unplanned occupation practices within the Camacho estuarine floodplain can result in potential pollution from precarious sewage systems during closed mouth conditions. Interventions, however, had solely focused on dredging and bulldozing to artificially breach the lagoon mouth, though management of a TOCE should not end at the defined boundary of the estuary (Bate 2007).
References
Allanson, B.R., and D. Baird. 1999. Estuaries of South Africa. Cambridge: Cambridge University Press.
Anandraj, A., R. Perissinotto, C. Nozais, and D. Stretch. 2008. The recovery of microalgal production and biomass in a South African temporarily open/closed estuary, following mouth breaching. Estuarine, Coastal and Shelf Science 79: 599–606. doi:10.1016/j.ecss.2008.05.015.
Anderson, M.J. 2005. PERMANOVA: a Fortran Computer Program for Permutational Multivariate Analysis of Variance. Auckland: Department of Statistics, University of Auckland.
Bate, G. 2007. Estuary management. In A review of information on temporarily open/closed estuaries in the warm and cool temperate biogeographic regions of South Africa, with particular emphasis on the influence of river flow on these systems, eds. A. Whitfield and G. Bate, 192- 214. Pretoria: Water Research Commission Report No. 1581/1/07.
Bemvenuti, C.E. 1998a. Trophic structure. In Subtropical convergence environments. The coast and sea in the Southwestern Atlantic, ed. U. Seeliger, C. Odebrecht, and J.P. Castello, 79–82. Berlin: Springer.
Bemvenuti, C.E. 1998b. Benthic invertebrates. In Subtropical convergence environments. The coast and sea in the Southwestern Atlantic, ed. U. Seeliger, C. Odebrecht, and J.P. Castello, 43–46. Berlin: Springer.
Bianchi, T.S., and J.S. Levinton. 1984. The importance of microalgae bacteria and particulate organic matter in the somatic growth of Hydrobia totteni. Journal of Marine Research 42: 431–443. doi:10.1357/002224084788502747.
Bland, J.M., and D.G. Altman. 1995. Multiple significance tests: the Bonferroni method. British Medical Journal, Statistics Notes 310: 170.
Bollmohr, S., P.J. van den Brink, P.W. Wade, J.A. Day, and R. Schulz. 2009. Spatial and temporal variability in particle-bound pesticide exposure and their effects on benthic community structure in a temporarily open estuary. Estuarine, Coastal and Shelf Science 82: 50–60. doi:10.1016/j.ecss.2008.12.008.
CEPA. 2010. Centro de Socio-economia e Planejamento Agrícola—Epagri/Cepa. http://cepa.epagri.sc.gov.br/Publicacoes/Sintese_2010/sintese%202010_inteira.pdf. Accessed 10 March 2011.
Clarke, K.R. 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18: 117–143. doi:10.1111/j.1442-9993.1993.tb00438.x.
Clarke, K.R., and M. Ainsworth. 1993. A method of linking multivariate community structure to environmental variables. Marine Ecology Progress Series 92: 205–219. doi:10.3354/meps092205.
Clarke, K.R., and R.H. Green. 1988. Statistical design and analysis for a ‘biological effects’ study. Marine Ecology Progress Series 46: 213–226.
Colling, L.A., C.E. Bemvenuti, and M.S. Gandra. 2007. Seasonal variability on the structure of sublittoral macrozoobenthic association in the Patos Lagoon estuary, southern Brazil. Iheringia, Série Zoologia 97(3): 257–262. doi:10.1590/S0073-47212007000300007.
Cooper, A., I. Wright, and T. Mason. 1999. Geomorpholy and sedimentology. In Estuaries of South Africa, ed. B.R. Allason and D. Baird, 2–25. Cambridge: Cambridge University Press.
Dean, W.E. 1974. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods. Journal of Sedimentology and Petrology 44: 242–248. doi:10.1306/74D729D2-2B21-11D7-8648000102C1865D.
DeBlasis, P., A. Kneip, R. Scheel-Ybert, P.C. Giannini, and M.D. Gaspar. 2007. Dinâmica natural e arqueologia regional no litoral sul do Brasil. Arqueologia Sul-Americana 3: 26–61.
Decker, H.P. 1987. Breaching the mouth of the bot river estuary, South Africa: impact on its benthic macrofaunal communities. Transactions of the Royal Society of South Africa 46(3): 231–250.
Dye, A., and F. Barros. 2005. Spatial patterns in macrofauna assemblages in intermittently open/closed coastal lakes in New South Wales, Australia. Estuarine, Coastal and Shelf Science 62: 357–371. doi:10.1016/j.ecss.2005.02.029.
EPAGRI. 2011. Empresa de Pesquisa Agropecuária e Extensão Rural de Santa Catarina (Epagri). http://ciram.epagri.sc.gov.br/portal/website/. Accessed 20 September 2011.
Fenchel, T. 1975. Factors determining the distribution patterns of mud snails (Hydrobiidae). Oecologia 20: 1–17.
Fonseca, G., and S.A. Netto. 2006. Shallow sublittoral benthic communities of the Laguna Estuarine System, South Brazil. Brazilian Journal of Oceanography 54: 41–54. doi:10.1590/S1679-87592006000100004.
Gama, P. 2007. Microalgae. In A review of information on temporarily open/closed estuaries in the warm and cool temperate biogeographic regions of South Africa, with particular emphasis on the influence of river flow on these systems, eds A. Whitfield and G. Bate, 68- 82. Pretoria: Water Research Commission Report No. 1581/1/07.
Garcia, R., and L. Thomsen. 2008. Bioavailable organic matter in surface sediments of the Nazaré canyon and adjacent slope (Western Iberian Margin). Journal of Marine Systems 74: 44–59. doi:10.1016/j.jmarsys.2007.11.004.
Giménez, L., A.I. Borthagaray, M. Rodríguez, A. Brazeiro, and C. Dimitriadis. 2005. Scale-dependent patterns of macrofaunal distribution in soft-sediment intertidal habitats along a large-scale estuarine gradient. Helgoland Marine Research 59: 224–236. doi:10.1016/j.jmarsys.2007.11.004.
Gladstone, W., N. Hacking, and V. Owen. 2006. Effects of artificial openings of intermittently opening estuaries on macroinvertebrate assemblages of the entrance barrier. Estuarine, Coastal and Shelf Science 67: 708–720. doi:10.1016/j.ecss.2006.01.008.
Gobler, C.J., L.A. Cullison, F. Koch, T.M. Harder, and W.K. Jerey. 2005. Influence of freshwater flow, ocean exchange, and seasonal cycles on phytoplankton and nutrient dynamics in a temporarily open estuary. Estuarine, Coastal and Shelf Science 65: 275–288. doi:10.1016/j.ecss.2005.05.016.
Gonçalves, J.E., J.J.I. Fonseca, and M.F.P. Callisto. 1998. Population dynamics of Heleobia australis (Gastropoda) in a coastal lagoon (Rio de Janeiro, Brazil). Verhandlungen der Internationalen Vereinigung fur Limnologie 26: 2056–2057.
Hastie, B.F., and S.D.A. Smith. 2006. Benthic macrofaunal communities in intermittent estuaries during a drought: comparisons with permanently open estuaries. Journal of Experimental Marine Biology and Ecology 330: 356–367. doi:10.1016/j.jembe.2005.12.039.
Henninger, T.O., P.W. Froneman, and A.N. Hodgson. 2008. The population dynamics of the estuarine isopod Exosphaeroma hylocoetes (Barnard, 1940) within three temporarily open/closed southern African estuaries. African Zoology 43(2): 202–217. doi:10.3377/1562-7020-43.2.202.
Hirst, A.J. 2004. Broad-scale environmental gradients among estuarine benthic macrofaunal assemblages of south-eastern Australia: implications for monitoring estuaries. Marine and Freshwater Research 55: 79–92. doi:10.1071/MF03011.
Hume, T.M., T. Snlder, M. Weatherhead, and R. Liefting. 2007. A controlling factor approach to estuary classification. Ocean & Coastal Management 50: 905–929. doi:10.1016/j.ocecoaman.2007.05.009.
INPH. 1991. Parecer técnico quanto aos aspectos hidráulicos-sedimentológicos relativos à obra de fixação da barra do Camacho. Rio de Janeiro: Instituto Nacional de Pesquisas Hidroviárias.
Kjerfve, B. 1994. Coastal lagoons. In Coastal lagoon processes, ed. B. Kjerfve, 1–8. Amsterdam: Elsevier.
Lawrie, R.A., D.D. Stretch, and R. Perissinotto. 2010. The effects of wastewater discharges on the functioning of a small temporarily open/closed estuary. Estuarine, Coastal and Shelf Science 87: 237–245. doi:10.1016/j.ecss.2010.01.020.
López-Figueroa, F., and F.X. Niell. 1988. Feeding behaviour of Hydrobia ulvae (Pennant) in microcosms. Journal of Experimental Marine Biology and Ecology 114: 153–167. doi:10.1016/0022-0981(88)90135-9.
Lorenzen, C.J. 1967. Determination of clorophyll and pheopigments: spectrophotometric equations. Limnology and Oceanography 12: 343–346.
Meurer, A.Z., and S.A. Netto. 2007. Seasonal dynamics of benthic communities in a shallow sublitoral site of Laguna Estuarine System (South, Brazil). Brazilian Journal of Aquatic Science and Technology 11(2): 53–62.
Morant, P., and N. Quinn. 1999. Influence of man and management of South African estuaries. In Estuaries of South Africa, ed. B.R. Allanson and D. Baird, 289–321. Cambridge: Cambridge University Press.
Netto, S.A., and T.J. Pereira. 2009. Benthic community response to an estuarine passive fishing gear in a coastal lagoon (South Brazil). Aquatic Ecology 43: 521–538. doi:10.1007/s10452-008-9177-8.
Niekerk, L., J.H. van der Merwe, and P. Huizinga. 2005. The hydrodynamics of the Bot River Estuary revisited. Water SA 31(1): 73–85.
Oliveira, D.B., J.L.B. Carvalho, and A.F. Klein. 2004. The stability of the Camacho Inlet, Santa Catarina, Brazil. Journal of Coastal Research, Special Issue 39: 561–564.
Perissinotto, R., K. Iyer, and C. Nozais. 2006. Response of microphytobenthos to flow and trophic variation in two South African temporarily open/closed estuaries. Botanica Marina 49: 10–22. doi:10.1515/BOT.2006.002.
Perneger, T.V. 1998. What's wrong with Bonferroni adjustments. British Medical Journal 316: 1236–1238.
Ponder, W.F., D.J. Colgan, and G.A. Clark. 1991. The morphology, taxonomy and genetic-structure of Tatea (Mollusca, Gastropoda, Hydrobiidae), estuarine snails from temperate Australia. Australian Journal of Zoology 39(4): 447–497.
Quinn, G.P., and M.J. Keough. 2002. Experimental design and data analysis for biologists. Cambridge: Cambridge University Press.
Riaux-Gobin, C., and P. Bourgoin. 2004. Microphytic standing stocks at Kerguelen Islands (Subantarctic, Indian Ocean), annual variations in relation to environmental factors: II, intertidal and subtidal microphytobenthos. Polar Biology 27: 735–747. doi:10.1007/s00300-004-0658-5.
Roy, P.S., R.J. Williams, A.R. Jones, I. Yassini, P.J. Gibbs, B. Coastes, R.J. West, P.R. Scanes, J.P. Hudson, and S. Nicol. 2001. Structure and function of south-east Australian estuaries. Estuarine, Coastal and Shelf Science 53: 351–384. doi:10.1006/ecss.2001.0796.
Santos, A.M., A.M. Amado, M. Minello, V.F. Farjalla, and F.A. Esteves. 2006. Effects of the sand bar breaching on Typha domingensis (PERS.) in a tropical coastal lagoon. Hydrobiologia 556(1): 61–68. doi:10.1007/s10750-005-1084-6.
Schallenberg, M., S.T. Larned, S. Hayward, and C. Arbuckle. 2010. Contrasting effects of managed opening regimes on water quality in two intermittently closed and open coastal lakes. Estuarine, Coastal and Shelf Science 86: 587–597. doi:10.1016/j.ecss.2009.11.001.
Stretch, D., and M. Parkison. 2006. The breaching of sand barriers at perched, temporary open/closed estuaries—a model study. Coastal Engineering Journal 48(1): 13–30. doi:10.1142/S0578563406001295.
Strickland, J.H.D., and T.R. Parsons. 1972. A practical handbook of seawater analysis. Ottawa: Fisheries Research Board of Canada.
Suguio, K. 1973. Introdução à sedimentologia. São Paulo: Blücher/EDUSP.
Teske, P.R., and T.H. Wooldridge. 2001. A comparison of the macrobenthic faunas of permanently open and temporarily open/closed South African estuaries. Hydrobiologia 464: 227–243. doi:10.1023/A:1013995302300.
Teske, P.R., and T.H. Wooldridge. 2003. What limits the distribution of subtidal macrobenthos in permanently open and temporarily open/closed South African estuaries? Salinity vs. sediment particle size. Estuarine, Coastal and Shelf Science 57: 225–238. doi:10.1016/S0272-7714(02)00347-5.
Twomey, L., and P. Thompson. 2001. Nutrient limitation of phytoplankton in a seasonally open Bar-Built Estuary: Wilson Inlet, Western Australian. Journal of Phycology 37: 16–29. doi:10.1046/j.1529-8817.1999.014012016.x.
Vieira, S.H., R. Coelho, J. Nolasco, R.B. Serôdio, and H. Queiroga. 2010. The circatidal rhythm of the estuarine gastropod Hydrobia ulvae (Gastropoda: Hydrobiidae). Biological Journal of the Linnean Society 100(2): 439–450.
Warwick, R.M., and K.R. Clarke. 1993. Increased variability as a symptom of stress in marine communities. Journal of Experimental Marine Biology and Ecology 172: 215–226. doi:10.1016/0022-0981(93)90098-9.
Wetzel, M.A., H. Leuchs, and J.H.E. Koop. 2005. Preservation effects on wet weight, dry weight and ash-free dry weight biomass estimates of four common estuarine macro-invertebrates: no difference between ethanol and formalin. Helgoland Marine Research 59: 206–213.
Whitfield, A.K. 1992. A characterization of southern African estuarine systems. Southern African Journal of Aquatic Sciences 12: 89–103. doi:10.1080/10183469.1992.9631327.
Whitfield, A., and G. Bate. 2007. A Review of Information on Temporarily Open/Closed Estuaries in the Warm and Cool Temperate Biogeographic Regions of South Africa, with Particular Emphasis on the Influence of River Flow on These Systems. Pretoria: Water Research Commission Report No. 1581/1/07.
Wooldridge, T. 1999. Estuarine zooplankton community structure and dynamics. In Estuaries of South Africa, ed. B.R. Allanson and D. Baird, 141–166. Cambridge: Cambridge University Press.
Acknowledgments
This study was partially supported by FAMASC (Santa Catarina Aquaculture Federation), CPNq (Brazilian National Research Council) and the State of Santa Catarina Government. We are grateful to André Francisco for his help in field work and Alexandre do Farol for the assistance during all phases of the study. We also thank three anonymous reviewers for their suggestions that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
Mean densities (inds./0.017m2) of the macrobenthic invertebrate taxa found in Camacho lagoon, S Brazil, at each site and sampling period. (DOC 191 kb)
Rights and permissions
About this article
Cite this article
Netto, S.A., Domingos, A.M. & Kurtz, M.N. Effects of Artificial Breaching of a Temporarily Open/Closed Estuary on Benthic Macroinvertebrates (Camacho Lagoon, Southern Brazil). Estuaries and Coasts 35, 1069–1081 (2012). https://doi.org/10.1007/s12237-012-9488-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-012-9488-9