Abstract
The influence of a passive shrimp fishing gear on benthic communities was studied at Laguna Estuarine System (South Brazil), a shallow choked coastal lagoon. The gear is composed by a group of fyke nets (25 mm mesh size) set in contact to the bottom, fixed with stakes forming a cage-like structure (around 30 m2). Samplings were conducted in the two main fishery areas of the estuarine system, Mirim (sand bottoms) and Imaruí (muddy bottoms) lagoon, in May 2005. In each area, 10 fyke net enclosures and 10 nearby sites without nets (control) were sampled. Microphytobenthos biomass (chlorophyll a and phaeopigments), number of taxa/species, density, Hill’s number N 1 and N 2, and estimated number of species (ES100) were used as community attributes. For the nematodes, values of the maturity index and abundance of Wieser’s feeding type were used as well. The effects of the small-scale passive shrimp fishing gear on the coastal lagoon bottoms were dependent on the benthic component analyzed and the type of sediment. Whereas macrofauna was not affected by the net enclosures, meiofauna and nematodes, particularly from the mud sites were. At the sand site, the fyke net enclosures caused a decrease in the microphytobenthos biomass and changed the relative abundances of non-selective deposit feeding and epigrowth-feeding nematodes. The results indicated that small-scale static nets, such as the studied fyke enclosures, produced low intensity levels of disturbance. However, the enclosed area by nets at Laguna had already reached around 25,000 m2. Given the large proportion of the coastal population involved and the area closed by nets, management policies should consider site-specific differences within the same estuarine system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bottom tending gear may be either active (bottom trawls and dredges) or passive (traps, sink gillnet and stake net) during fishing operations. Most of the concern about fishing gear impacts has been directed at mobile bottom gear, because the area of sea floor swept and the potential disturbance are much larger than with fixed gear. Research on the effects of many different active fishing gears has been conducted on a wide variety of sea floors, differing in depth, substrate type and benthic component (e.g. Jones 1992; Dayton et al. 1995; Schratzberger et al. 2002; Kaiser et al. 2002). Recently, Kaiser et al. (2006) identified the types of active fishing gear with the greatest impact on the seabed as well as the most vulnerable groups of organisms to fishing activities. All these studies were only focused on industrial fishery.
Small-scale fisheries, often also referred to as artisanal fisheries, are difficult to define unambiguously, as the term tends to apply to different circumstances in different countries. In general, they are traditional fisheries involving fishing households (contrary to commercial companies), using relatively small amounts of capital and energy. The small-scale fisheries are often characterized by a low degree of mechanization, production capacity and catch per unit effort. Artisanal fisheries can be very specialized but, in general, target a wide range of species, using a broad variety of gears, generating diverse fishing strategies and flexibly adapting to seasonal or inter-annual natural variability (FAO 2007). Still, according to the FAO fishery statistics (2007), artisanal fisheries produce about 50% of the world’s capture fisheries harvest.
The small-scale estuarine fishery has experienced profound changes in the last decades. In many countries, particularly in the developing ones, access to small-scale fisheries is most often neither limited nor controlled by fisheries management authorities. Some of the gears used by artisanal fishers are unselective, such as small-meshed beach-seines and some types of trap, lift and cast nets (Vieira et al. 1996; Stergiou et al. 1996; McClanahan and Mangi 2004). Overall, the number of small-scale fishers in area without control has been constantly growing.
Small-scale estuarine fishery, particularly those associated with passive or static fishing gears, are believed to have minor effect on benthic communities (Jawad 2006). However, regardless of its importance, information on the effects of small-scale passive fishing gear on benthic communities is, as far as we are aware, limited to one study in a lagoon of the Ivory Coast, where Guiral et al. (1995) observed that brush park fisheries had a significant impact on benthic fauna diversity.
The aim of this study was to assess the response of benthic communities—microphytobenthos, meiofauna and macrofauna—to a passive shrimp fishing gear largely used by the small-scale fishers. It was also investigated whether the effects on the benthos would be similar on the communities of shallow sublittoral mud and sand bottoms of the Laguna Estuarine System, South Brazil.
Materials and methods
Study area and the fishing gear
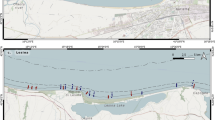
The Laguna Estuarine System, located at the southern coast of Brazil (S 28°24′ E 48°52′, Fig. 1), is a choked lagoon (sensu Kjerfve 1994) with an area of 184 km². The estuarine system consists of three main lagoons lying parallel to the shore line and connected one to another and with the adjacent Atlantic Ocean by narrow channels (Fig. 1). Mean air temperatures are around 13°C in the winter and 22°C in the summer; total mean annual rainfall is 1,260 mm with no marked seasonal differences (EPAGRI 2007). Information on the composition and seasonal variability of benthic communities of the lagoons were provided by Fonseca and Netto (2006).
The shrimp fishery has experienced marked changes in fishing technologies and practice in the last few decades. The number of small-scale fishers at Laguna increased around 800%, since the 70s (Sunye 2006). Statistics on the small-scale shrimp fishery production are poor and there is no official estimate of the number of nets along the lagoon. The aviãozinho, fyke nets (FAO 2007) used in groups, is now largely dominant gear used for shrimp fishery. Pereira and Netto (2005) estimated that the total catch by fyke nets in the 2004/2005 fishing season were 145 ton, of which the target species, Farfantepenaeus paulensis, contributed with 37% (55 ton); the accessory (non-target species but marketable), mainly the blue crab Callinectes sapidus, represented 36% (52 ton) of the catches; the discarded by-catches was composed by 32 fish species (80% of them were juveniles) and accounted for with 27% (38 ton) of the total catch. According to Nedelec and Prado (1990), fyke nets, used separately or in groups, are a common gear used in shallow waters for shrimp fishery. The arrangement of the nets by fishers, however, may vary between and within countries. At Laguna, the fyke nets are set in a group of 5–7 in contact to the bottom, fixed with stakes in shallow waters (1–2 m depth; Fig. 2a, b). Each group of nets forms a cage-like structure. In the centre of enclosure, a fluorescent lamp produced by a car battery is placed on a stake. The positively phototropic shrimp are attracted to the light and enter the net. The body and sleeves of the nets have a 25 mm mesh size. Shrimps are harvested daily by suspending the nets. The area closed by each group of nets is around 30 m2 and the estimated total area closed, determined by two surveys along the estuarine system, was approximately 25,000 m2. At the time this study was carried out, there was no close period for the shrimp fishery at Laguna since the 80s. The nets are always kept in place, except for occasional retrieval for cleaning. However, the stakes are kept in place as a way to mark the fishers’ area.
Benthic community samples collection and processing
As a result of lack of fishery management, there is no defined zone for the setting up the net enclosures along the Laguna Estuarine System. However, there are areas where the number of nets is concentrated. In order to evaluate the response of benthic communities to the fyke net enclosures, samplings were conducted in the two main fishery areas of the estuarine system, Mirim and Imaruí lagoon (Fig. 1). In each area, 10 fyke net enclosures and 10 nearby sites without nets (control) were sampled. The body of the nets is not fixed and small movements are constant. The daily shrimp harvest, when nets are suspended, is also likely to affect superficial sediments inside the enclosures. Samples inside the enclosures were randomly taken between the bodies of the nets (Fig. 2a). All sampled fyke net enclosures had been harvested in the night before the sampling. According to the fishers, the enclosures sampled were in the same place for at least 1 year, except for cleaning or repairing (not more than 2 days). The control samples were taken around 5 m from net enclosures (Fig 2a), taking care in avoiding sites marked with stakes but without nets, as they could have been previously disturbed. Apart from the fyke nets, no other fishing gear is used near the net enclosures. Samples were collected by diving at a depth of 1.2 ± 0.2 m.
Sampling inside and outside the fyke net enclosures were carried out on May 2005. For microphytobenthos, the first centimetre of the sediment was sampled with a 2 cm diameter core tube. Pigments were extracted with 90% v/v acetone and analyzed according to Strickland and Parsons (1972). Chlorophyll a (Chl a) and phaeopigment biomass were estimated using Lorenzen’s (1967) equation. Meiofauna samples were taken with a plastic syringe (2 cm in diameter to a depth of 5 cm) and sectioned into 0–2 cm (upper) and 2–5 cm (lower) strata. Samples were immediately fixed in 10% formalin. In the laboratory, samples were sieved through a 63 μm mesh and the fauna extracted by flotation with Ludox TM (specific gravity 1.15, De Jonge and Bouwman 1977). Samples were then evaporated with anhydrous glycerol and mounted on permanent slides (Somerfield and Warwick 1996). Macrofauna samples were taken with a 10 cm diameter core tube pushed into the sediment to a depth of 10 cm and immediately fixed in 10% formalin. Fixed samples were sieved through a 0.5 mm mesh, and sorted under a dissection microscope. All invertebrates were identified to the lowest possible taxonomic level.
Additionally, samples for sediment granulometry and total organic content were taken with a 10 cm diameter core tube to a depth of 5 cm. Sediment granulometry was determined by sieving and pipette analyses (Suguio 1973) and total organic content by combustion (550°C for 60 min, Dean 1974). Water salinity, temperature and depth were measured in situ.
Data analysis
In order to test for differences between treatments (inside and outside the nets), univariate and non-parametric multivariate techniques were used (Clarke and Warwick 1994). For the macrofauna, meiofauna and nematodes, total abundance and number of species (Hill number N 0) were calculated for each sample. Diversity and dominance were expressed as Hill numbers N 1 and N 2 (Hill 1973), respectively, which describe different aspects of the community and differ only in their tendency to include or ignore the relatively rare species. In addition, the Hurlbert Index (ESn), less dependent on sample size (Soetaert and Heip 1990), based on the rarefaction technique of Sanders (1968) and modified by Hurlbert (1971) was used. In this index the expected number of species (ES) is calculated among certain number of individuals, e.g. of 100 individuals; ES100 was used in the present study. For the nematodes, the maturity index (MI), derived from life history characteristics of nematode genera was calculated for each sample according to Bongers (1990) and Bongers et al. (1991; 1995). Nematodes were classified along a scale of 1–5, with colonisers (inter alia short life cycle, high reproduction rates, high colonisation ability and tolerant to disturbance) weighted as 1, and persisters (inter alia long life cycles, low colonisation ability, few offspring and sensitive to disturbance) weighted as 5. Nematode feeding types according to Wieser (1953) were also used. Chl a and phaeopigment concentration were used as descriptors for the microphytobenthos. Sediments were characterized by mean grain size, sand and silt percentages and total organic content.
Differences in univariate descriptors between treatments (inside and outside fyke nets), sites (mud and sand), and treatment and site interaction were tested by 2-way ANOVA. Cochran’s C tests were applied to test for homogeneity of variances, and data were log(x + 1) transformed wherever necessary. Tukey’s multiple comparison tests were used when significant differences were detected (P < 0.05, Sokal and Rholf 1997).
The influence of the fyke nets on vertical distribution of the meiofauna and nematode univariate descriptors of each sampling site was analyzed using a two-way analysis of variance (2-way ANOVA) with treatment (inside and outside fyke nets) and sediment strata (0–2 and 2–5 cm) as fixed factors. As sediment layers were not independent, a ‘split-plot’ design was constructed with replicates nested within ‘treatment’ but not within ‘sediment strata’. As described earlier, homogeneity of variances were tested, data were transformed wherever necessary, and Tukey’s multiple comparison tests were used when significant differences were detected.
Similarity matrices were constructed using Bray–Curtis similarity measure on faunistic data. Ordination was by non-metric multidimensional scaling (MDS) and formal significance tests for differences between treatment and site were performed using the ANOSIM permutation test (Clarke and Green 1988). Similarity percentage analysis (SIMPER; Clarke 1993) was employed to assess compositional similarity and identify the main species of taxa contributing to dissimilarities between mud and sand sites, and treatments assemblages.
Spearman rank correlation was performed to investigate the nature and the statistical significance of the relationship between environmental variables and univariate community attributes. The relationships between multivariate community structure and combinations of environmental variables were analyzed using the BIO-ENV procedure (Clarke and Ainsworth 1993) to define suites of variables that best explain the faunistic structure.
Procedures for adjusting significance levels to control type I error rates in multiple testing situations, such as the Bonferroni method, have been the subject of much debate (Bland and Altman 1995; Perneger 1998). Although this technique provides great control over type I errors, it is very conservative when there are many comparisons and may miss real differences (i.e. increase of type II errors; Quinn and Keough 2002). Therefore, the results of both corrected and uncorrected P-values were used.
Results
Sediments
Sediment properties such as mean grain size, sand, silt and clay percentages, as well as total organic content, did not vary significantly (P > 0.05) between treatments inside and outside the fyke nets. Significant differences were detected only between sites (Fig. 3). Imaruí (mud site) was characterized by muddy sediments with organic content around 7%; Mirim sediments (sand site) were composed by fine sand and total organic content of 2%. From now on, samples from Imaruí will be called “mud” and those from Mirim lagoon “sand”.
Microphytobenthic biomass
Total microphytobenthic biomass was significantly higher in the sandy (mean of 16 mg.cm−3) than in muddy sediments (5.7 mg cm−3). Both Chl a and phaeopigment concentrations in the surface sediment between treatments were dependent on the site (treatment vs. site interaction significant, P < 0.05). Whilst at the mud Chl a and phaeopigment biomass did not differ significantly between treatments, at the sand site their values were significantly lower inside than outside the nets enclosures (P < 0.05, Fig. 4).
Macrofauna
A total of 17 macrobenthic species were identified with densities raging from 2,571 to 107,571 inds m−2 . The gastropod Heleobia australis largely dominated the macrofauna at both sites, accounting for more than 71% of the total macrofauna collected in the mud and 64% in the sand. The bivalve Erodona mactroides was the second most abundant species (12.6% of the fauna) and the polychaete Laeonereis acuta represented 4.4% of the macrofauna. None of the macrofauna univariate descriptors, as well as the densities of H. australis, differed significantly between sites (Table 1; Fig. 5). However, E. mactroides was significantly more abundant in mud whilst L. acuta in sand (P < 0.05).
The results of the ANOVA tests evaluating the effects of the nets on the univariate descriptors of the macrofauna (Table 1; Fig. 5), and the numerically dominant species, did not show any significant differences. MDS ordination showed a clear distinction of the sand and mud macrofauna assemblages (Fig. 6a), and it was confirmed by the ANOSIM tests (Table 3). The similarities percentages analysis (SIMPER) derived from macrofauna data showed that variations in the relative abundance of E. mactroides and L. acuta contributed most to the breakdown of similarity between sites. However, both the MDS ordinations (Fig. 6b) and ANOSIM tests (Table 3) failed to detect any differences between treatments inside and outside the net enclosures.
Meiofauna
The number of meiofaunal groups and the estimated number of meiofaunal groups (ES100) did not vary significantly between mud and sand (Table 1; Fig. 7), with the same seven groups recorded at both sites. Nematodes largely dominate the all samples, accounting for 87% of the total number of organisms (65% in mud and 91% in sand). Copepods (including nauplii) had the second highest numerical importance in the samples and represented ∼14% of the meiofauna in mud and 5% in sand. Hill’s numbers N 1 and N 2 were significantly higher in mud (Table 1; Fig. 7), indicating a higher dominance of fewer meiofaunal groups in the sand site. The density of the meiofauna was also significantly higher in the sand (mean of 391 inds 10 cm−2) than in the mud site (mean of 188 inds 10 cm−2). The ANOSIM tests confirmed the significance of the differences between the mud and sand meiofauna assemblages (Table 3). The SIMPER analysis showed that densities of nematodes accounted most to the breakdown of similarity between sand and mud sites. Regarding vertical distribution within each site (Table 3), differences in the meiofauna univariate measures were more evident in the mud, where all descriptors showed significantly higher values in the upper than in the lower strata. In the sand, only the Hill’s numbers N 1 and N 2 were significantly higher in the upper than in the lower (Table 2).
The effects of fyke nets on meiofauna (5 cm integrated) were detected for the estimated number of groups (ES100) and Hill’s numbers N 1 and N 2, all showing significant higher values outside the nets (Table 1, Fig. 7). The number of groups and the density of the meiofauna, however, did not differ significantly between treatments (Table 1, Fig. 7). Concerning the effects of the nets on the vertical distribution of the meiofauna descriptors, the results of the ANOVA tests (Table 2) showed that, at the mud site, all values were significantly higher outside the nets in the upper sediment layer (no significant interaction between sediment strata and treatment). The exception was the meiofauna density that did not differ significantly between treatments (Table 2). Conversely, at the sand site, the ANOVA tests showed that none of the meiofauna univariate descriptors varied significantly between treatments (Table 2).
Nematode assemblages
A total of 31 genera belonging to 15 families were recorded. At the mud site, Daptonema and Sabatieria (Xyalidae and Comesomatidae, respectively) dominated numerically the nematode assemblages (32% and 29% of the collected organisms). These genera were also the most frequent genera in the mud site, where Sabatieria was present in all samples and Daptonema was recorded in 95% of the samples. Yet at the sand site, Terschellingia (Linhomoidae) and Desmodora (Desmodoridae) were the most abundant genera accounting for 50.1% of the nematodes collected (25.8% and 24.3%, respectively). Terschellingia and Theristus (Xyalidae) were also the most frequent genera in the sand site (present in all samples), though Desmodora, Daptonema, Parodontophora (Axonolaimidae), Leptolaimus (Leptolaimidae) and Viscosia (Oncholaimidae) were also frequent (present in more than 75% of the samples).
The results of the ANOVA tests evaluating the significance of the differences in the nematodes univariate descriptors between sand and mud sites (5 cm integrated) showed that all values were significantly higher in the sand than in the mud site (Table 1; Fig. 8); the exception was the relative abundances of non-selective deposit feeding nematodes, significantly higher in the mud (Table 1; Fig. 9). As the meiofauna, significant differences in univariate measures between sediment strata in the mud site were detected for most of the descriptors (Table 2). The exception was the number of genera, the estimated number of genera (ES100), and the relative abundances of epigrowth-feeders and omnivores/predators (Table 2). At the sand site, the values of nematode density, abundance of non-selective deposit feeders, and the maturity index were significantly higher in the upper than in the lower strata; abundance of selective deposit feeders were significantly higher in the lower strata (Table 2). The MDS ordination of log transformed abundance of nematode genera (5 cm integrated data) also showed a clear distinction between the sand and mud nematode assemblages (Fig. 10a); the ANOSIM tests confirmed the significance of the differences (Table 3). The results from the SIMPER analysis showed that variations in the abundance of Desmodora and Sabatieria contributed most to the breakdown of similarity between sand and mud sites.
Nematode feeding types (Wieser 1953) outside (white symbols) and inside (black symbols) of the net enclosures at the mud and sand sites (mean ± SE)
The Hill’s number N 1 and N 2, the estimated number of genera (ES100), and the maturity index derived from nematode data (5 cm integrated) were significantly higher outside than inside the net enclosures (Table 1; Fig. 8). The values of nematode number of genera and density did not show any effects of fyke nets (Table 1; Fig 8). The abundances of different nematode feeding types did not differ significantly between samples inside and outside the nets as well (Table 1; Fig. 9).
Regarding the effects of the net enclosures on the nematode vertical distribution at the mud site, the ANOVA tests (Table 2) showed that Hill’s number N 1 and N 2, and values of maturity index were significantly higher outside the nets in both sediment strata. Yet at the sand site, significant interactions between treatments and strata were detected for the abundances of non-selective feeders and epigrowth-feeders (Table 2): The non-selective feeding nematodes did not differ between sediment stratum in the control, but they were significantly more abundant in the upper layers within the net enclosures; the epigrowth-feeders abundances increased significantly in lower sediment stratum inside the net enclosures (Table 2).
For the mud site, the MDS ordinations derived from nematode data, both total (5 cm integrated) and upper sediment layer (0–2 cm), indicated a clear distinction between the assemblages inside and outside the net enclosure (Fig. 10b, c). The results of the ANOSIM tests confirmed the significance of the differences in nematode structure between control and fyke net enclosure for total and upper sediment layer (Table 3). The breakdown of similarities between control and fyke net for both total and upper layer nematode data, according to the results of the SIMPER analysis, was due to variations in abundance of the nematodes Terschellingia and Anonchus.
Relationship between environmental variables and benthic communities
Results from the correlation analyses revealed statistically significant relationships between faunal and the measured environmental variables (Table 4). Both the number of species and density of macrofauna were positively correlated with the percentages of fine sediments (0.47 and 0.48, respectively). The phaeopigment biomass was the only variable that did not showed any significant correlation with benthic fauna descriptors. Nematodes showed the highest values of correlations, particularly with fine percentages and total organic content (Table 4)
The interrelationships between multivariate community structure and combinations of environmental data were examined by the BIO-ENV procedure. The highest correlations (0.63) were also found for nematodes (5 cm integrated). Sediment grain size, total organic and chlorophyll biomass were the variables that best explained the variations in the structure of the fauna.
Discussion
Shallow sublittoral soft sediment benthic communities are subject to a range of natural disturbance regimes that are dominated by physical processes (Hall 1994). In such areas, sediment composition is largely controlled by hydrodynamic forces over the substratum (Snelgrove and Butman 1994) such that, clean, sandy bottoms predominate in high-energy environments, whereas silty or muddy sediments develop in very low-energy environments. The results showed that the passive shrimp fishing gear did not affect the measured parameters of the sediment. Guiral et al. (1995) showed that brush park fisheries (acadja) have turned an open-water environment into a confined area. The enormous quantity of branches interferes with local currents and causes changes to sedimentation. At the Laguna Estuarine System, during the summer 2004/2005, the total area closed by the nets was around 25,000 m2. Contrary to active gears (Blaber et al. 2000) and brush park fisheries, changes in sediment properties due to passive bottom nets, if occurs, would be restricted to the top sediment or at the sediment water interface. The standard granulometric methods may not be sensitive enough to detect such effects (Dernie et al. 2003). In addition, as the sediment cores were taken at minimum 5 cm depth and the granulometric analyses were carried out for the entire 5 cm, probably this can be masking change occurring in the superficial sediments.
The distribution of estuarine microphytobenthic biomass can be variable across space and time. Previous study at Laguna (Fonseca and Netto 2006) did not show clear relationships between sediment type and microphytobenthic biomass. In this study, total microphytobenthic biomass was significantly higher in sand than in muddy sublittoral sediments and similar results were also observed by McIntyre and Amspoker (1986) and Cahoon et al. (1999). Although sediment composition inside and outside the net enclosures within each site did not differ significantly, the total microphytobenthic biomass did. At the sand site, values of the Chl a biomass inside the net enclosures were almost half from those obtained outside the nets. A complex set of interacting factor could be responsible for the decrease in the microalgal biomass only in the fine sand bottoms. Decrease in the Chl a may be linked to the constant, albeit low, physical disturbance on the less cohesive superficial sand sediment promoted by the nets. The sand bottoms could also interact with local currents and may change the sediment–water interface. Besides, shading could also be an important factor affecting microalgal biomass (Kromkamp et al. 1995). Within estuaries, turbidity level is often associated with substrate type (Walsh et al. 1999). Areas where the substrate is sand, turbidities are often lower than in areas where the substrate is mud. As the 25 mm mesh nets are all made by dark polyamide, it is possible that shading effects would be more important at the sand site.
Differential response to physical disturbance between meiofauna and macrofauna had also been reported by other studies (e.g. Warwick et al. 1990; Gallucci and Netto 2004; Widdicombe and Austen 2005). Benthos may respond in several ways depending on the intensity of disturbance (e.g. trawling vs. passive nets). Low levels of physical disturbance may influence the meiobenthic community structure (Steyaert et al. 2003; Hendelberg and Jensen 1993) but not the macrofauna (Zajac 2003). Austen and Widdicombe (2006) suggested that at higher levels of physical disturbance, sediment disruption could counteract the positive oxygenating effects and the meiobenthos would be less sensitive than the macrofauna. Meiofauna was found to be resistant to disturbance by trawling (Schratzberger et al. 2002), but large body-size fauna has lower intrinsic rates of increase and a lower capacity to sustain elevated mortality, thus they are more vulnerable to active bottom-fishing (Kaiser et al. 2006). In the case of the net enclosures, static nets producing low intensity levels of disturbance and possibly acting in the sediment–water interface changed the meiofauna community structure but not the macrofauna.
Shallow coastal lagoons are characterized by a high variability and low predictability of their environmental conditions, which determines a low number of macrobenthic species that might colonize these ecosystems (Gray 1974; McLusky 1989). Low values of diversity, low numbers of species and strong dominance of a few species have been found often in lagoonal ecosystems (Arias and Drake 1994; Bemvenuti 1997; Bemvenuti and Netto 1998; Reizopoulou and Nicolaidou 2004). These studies had also pointed out that, for the macrofauna, univariate community descriptors such as number of species, number of individuals and diversity may fail in detecting significant spatial or temporal differences. On contrary multivariate analysis, which registered community changes in species dominance, would be more sensitive. Indeed, differences in macrofauna between sand and mud at Laguna were clearly detected by MDS ordination and ANOSIM, but not by the ANOVA tests.
The low intensity levels of disturbance produced by the net enclosures coupled with the presence of the deeper-burrowing (such as the polychaete Heteromastus similis) and high mobile macrobenthic organisms (as the surface crawler gastropod H. australis) resulted in the absence of any significant change in the macrofauna in this study. Recently, Angonesi (2005) studying the effects of different small-scale shrimp trawling on macrobenthic associations of Patos Lagoon also did not observed any significant effects. The author suggested that the high environmental variability of the coastal lagoon together with the low macrobenthic diversity probably result in a high resistance of these species against disturbances. Still, according to Fonseca and Netto (2006) and Meurer and Netto (2007), peaks of reproduction activity of most macrofauna species at Laguna occurs in summer and spring. As this study was carried out in the autumn, it is possible that different response of the macrofauna would occur in periods of reproduction.
Meiofauna communities found in sandy habitats generally is less sensitive to or perhaps more readily able to re-establish following physical disturbance than those found in less energetic muddy environments (Schratzberger and Warwick 1999). Indeed, the results of the univariate and multivariate analysis showed that the effects of the net enclosures were mainly observed at the mud, though the fauna associated with the sand substrate also exhibited some changes in the univariate descriptors. Static nets, such as the fyke net enclosures, were expected to produce low intensity levels of disturbance when compared to towed bottom-fishing gears. Besides, the net enclosures would act mainly in the upper layers of the sediment or in the water–sediment interface. Therefore, effects of the nets are probably associated with the faunal vertical distribution, and it was at the mud site where most of the community descriptors differed significantly between upper and lower strata. Whereas, the meiofauna and nematodes inside of the net enclosures showed a significant low value of most of univariate descriptors, it was not observed any significant effect on the density of a particular meiofauna group or nematode genus. Increase of dominance (lower N 1 and N 2 values) and decrease of estimated number of genera were due to combinations of generally small increases or decreases in abundances of common genera. This was the case of the numerically dominant nematode genera Daptonema—which relative density increased inside the nets and Terschellingia—which showed a decline inside the nets. Daptonema and Terschellingia are common nematodes genera in intertidal and subtidal sediments and can be abundant either in mud or sand habitats (e.g. Alongi 1990; Soetaert et al. 1995; Fonseca and Netto 2006). Schratzberger and Jennings (2002) studying the response of meiofauna to different levels of trawling disturbance, showed a significant increase of Daptonema in areas subjected to medium disturbances. On other hand, meso- and microcosms experiments carried out by Austen et al. (1998) and Schratzberger and Warwick (1999), respectively, showed that densities of Terschellingia may be negatively affected by low disturbance levels.
The fauna inside the net enclosures at the sand site showed a significant increase in the abundance of non-selective deposit feeding nematodes in the upper strata and an increase of epigrowth-feeders in the lower sediment strata. The increase of deposit feeding nematodes inside the enclosures was not a surprise, as the nets could increase detritus deposition. The slightly increase in fine sediments (mean of 3% in control and 9% inside the net enclosures) and the total organic content (mean of 1% in control and 2% inside the net enclosures) were also observed, indicating a small increase in the sedimentation inside the nets at the sand site. At the mud site, mean abundance of deposit feeding nematodes also increased, but differences were not significant with the control.
It was surprisingly, however, that the abundances of epigrowth-feeder at the sand site did not change in upper sediment layers inside the net enclosures, as the total microphytobenthos biomass significantly decreased. Instead, abundances of epigrowth-feeder increased in deeper sediment layers. Although epigrowth-feeders do not feed exclusively on microalgae, benthic diatoms are an important food item for these nematodes (Moens and Vincx 1997). Unfortunately, we had not analyzed the vertical distribution of microphytobenthos biomass and we can only hypothesize on their relationships. The physical and chemical conditions within the sediment reflect the type of microphytobenthos found and the nature of the vertical distribution of microphytobenthos biomass as well (Paterson and Hagerthey 2001). In sandy sediments, the microphytobenthos is normally more evenly distributed with depth, presenting a less migrational nature (Kromkamp et al. 1995). It is possible that the fyke net enclosures, besides changing total microphytobenthos biomass in the superficial sediments, could also affect vertical biomass distribution or promote a different downward migration pattern than control samples, due to shading for example. Recently, Wasmund (2007) observed that both Chl a and total microphytobenthic biomass minima were found at the sediment surface on several occasions due to physical disturbance. This would explain the higher abundance of epigrowth-feeder in deeper sediment within the net enclosures at the sand site.
Nematode assemblages inside net enclosures at both mud and sand sites showed significant lower values of maturity index, suggesting a shift towards r-selected nematode assemblages. However, though significant, differences in the index values between the assemblages inside and outside net enclosures were smaller than 1. Thus this result also suggested that net enclosures produced low intensity levels of disturbance. The maturity index assumed that if stable habitats are characterized by a range of species with narrow ecological niches, disturbance influences the most sensitive species and the niches of species adversely affected will remain vacant or will be filled by less specialized species or with by those with a higher reproductive potential, leading to a decrease in the index value (Bongers et al. 1991). The maturity index has been successfully used in assessing different sources of disturbance in marine and brackish water sediments (e.g. Bongers et al. 1991; Essink and Keidel 1998; Vanhove et al. 1999), showing a decrease in their values with increased levels of disturbance.
Conclusion
Our results showed that small-scale static nets, such as the studied fyke enclosures, produced low intensity levels of disturbance when compared with seasonal changes in the same area (Fonseca and Netto 2006; Meurer and Netto 2007) or effects of towed bottom-fishing gears (e.g. Kaiser et al. 2006, and references herein). The effects of the small-scale passive shrimp fishing gear on the coastal lagoon bottoms were dependent on the benthic component analyzed and the type of sediment. Whereas macrofauna was not affected by the net enclosures, meiofauna and nematodes, particularly from the mud site were. At the sand site, the fyke net enclosures caused a decrease in the microphytobenthos biomass and changed the relative abundances of nematode feeding types. Although each net enclosure is small, the total enclosed area by nets at Laguna had already reached around 25,000 m2. Given the large proportion of the coastal population involved and the variety of gears used by small-scale fishery, we think that, firstly, more studies are needed to understand the role of small-scale fisheries in coastal lagoons ecology. Secondly, management policies should consider site-specific differences within the same system.
References
Alongi DM (1990) The ecology of tropical soft-bottom benthic ecosystems. Oceanogr Mar Biol Ann Rev 28:381–496
Angonesi LG (2005) Dinâmica de curto prazo da macrofauna bentônica em uma enseada estuarna da Lagoa dos Patos: efeitos antrópicos e mecanismos de persistêncai e resistência. PhD thesis Universidade do Rio Grande, Rio Grande
Arias M, Drake P (1994) Structure and production of the benthic macroinvertebrate community in a shallow lagoon in the Bay of Cadiz. Mar Ecol Prog Ser 115:151–167
Austen MC, Widdicombe S (2006) Comparison of the response of meio- and macrobenthos to disturbance and organic enrichment. J Exp Mar Biol Ecol 330:96–104
Austen MC, Widdicombe S, Villano-Pitacco N (1998) Effects of biological disturbance on diversity and structure of meiobenthic nematode communities. Mar Ecol Prog Ser 174:233–246
Bemvenuti CE (1997) Benthic invertebrates. In: Seeliger U, Odebrecht C, Castello JP (eds) Subtropical convergence environments. the coast and sea in the southwestern Atlantic. Springer-Verlag, Berlin, pp 43–46
Bemvenuti CE, Netto SA (1998) Distribution and seasonal patterns of the sublittoral benthic macrofauna of Patos Lagoon (south Brazil). Rev Bras Biol 58:211–221
Blaber SJM, Cyrus DP, Albaret JJ, Ching CV, Day JW, Elliott M, Fonseca MS, Hoss DE, Orensanz J, Potter IC, Silvert W (2000) Effects of fishing on the structure and functioning of estuarine and nearshore ecosystems. ICES J Mar Sci 57:590–602
Bland JM, Altman DG (1995) Multiple significance tests: the Bonferroni method. BMJ Stat Note 310:170
Bongers T (1990) The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologia 83:14–19
Bongers T, Alkemade R, Yeates GW (1991) Interpretation of disturbance-induced maturity decrease in marine nematode assemblages by means of the Maturity Index. Mar Ecol Prog Ser 76:135–142
Bongers T, de Goede RGM, Korthals GW, Yeates GW (1995) Proposed changes of c-p classification for nematodes. Russ J Nematol 3:61–62
Cahoon LB, Nearhoof JE, Tilton CL (1999) Sediment grain size effect on benthic microalgal biomass in shallow aquatic ecosystems. Estuaries 22:735–741
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables. Mar Ecol Prog Ser 92:205–219
Clarke KR, Green RH (1988) Statistical design and analysis for a ‘biological effects’ study. Mar Ecol Prog Ser 46:226–231
Clarke KR, Warwick RM (1994) Changes in marine communities: an approach to statistical analyses and interpretation. Natural Environment Research Council, United Kingdom
Dayton PK, Thrush SF, Agardy MT, Hofman RJ (1995) Environmental effects of marine fishing. Aquat Conserv Mar Freshwat Ecosyst 5:205–232
De Jonge VN, Bouwman LA (1977) A simple density separation technique for quantitative isolation of meiobenthos using the colloidal silica Ludox-TM. Mar Biol 42:143–148
Dean WE (1974) Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods. J Sed Petrol 44:242–248
Dernie KM, Kaiser MJ, Richardson EA, Warwick RM (2003) Recovery of soft sediment communities and habitats following physical disturbance. J Exp Mar Biol Ecol 285:415–434
EPAGRI (2007) Empresa de Pesquisa Agronômica e Extensão Rural de Santa Catarina. Centro Integrado de Meteorologia e Recursos Hídricos de Santa Catarina. http://www.epagri.rct-sc.br
Essink K, Keidel H (1998) Changes in estuarine nematode communities following a decrease of organic pollution. Aquat Ecol 32:195–202
FAO (2007) Food and Agriculture Organization of the United Nations: essential documents, statistics, maps and multimedia resources. http://www.fao.org
Fonseca G, Netto SA (2006) Shallow sublittoral benthic communities of the Laguna Estuarine System, South Brazil. Braz J Oceanogr 54:41–54
Gallucci F, Netto SA (2004) Effects of the passage of cold fronts over a coastal site: an ecosystem approach. Mar Ecol Prog Ser 281:79–92
Guiral D, Gourbault N, Helleouet MN (1995) Etude sédimentologique et méiobenthos d’un écosystème lagunaire modifié par un récif artificiel à vocation aquacole: l’acadja. Oceanol Acta 18:543–555
Gray JS (1974) Animal-sediment relationships. Oceanogr Mar Biol Ann Rev 12:223–261
Hall SJ (1994) Physical disturbance and marine benthic communities: life in unconsolidated sediments. Oceanogr Mar Biol Ann Rev 32:179–239
Hendelberg M, Jensen P (1993) Vertical distribution of the nematode fauna in coastal sediment influenced by seasonal hypoxia in the bottom water. Ophelia 37:83–94
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432
Hurlbert SH (1971) The nonconcept of species diversity: a critique and alternative parameters. Ecology 52:577–586
Jawad LA (2006) Fishing gear and methods of the lower Mesopotamian plain with reference to fishing management. Mar Mesop Online 1:1–37
Jones JB (1992) Environmental impact of trawling on the seabed: a review. NZ J Mar Freshwat Res 26:59–67
Kaiser MJ, Collie JS, Hall SJ, Jennings S, Poiner IR (2002) Modification of marine habitats by trawling activities: prognosis and solutions. Fish Fisher 3:114–136
Kaiser MJ, Clarke KR, Hinz H, Austen MCV, Somerfield PJ, Karakassis I (2006) Global analysis of response and recovery of benthic biota to fishing. Mar Ecol Prog Ser 311:1–14
Kjerfve B (1994) Coastal Lagoons. In: Kjerfve B (ed) Coastal lagoon processes. Elsevier-Oceanographic Series 60, New York, pp 1–8
Kromkamp J, Peene J, Van Rijswijk P, Sandee A, Goosen N (1995) Nutrients, light and primary production by phytoplankton and microphytobenthos in eutrophic, turbid Westerschelde estuary (The Netherlands). Hydrobiology 311:9–19
Lorenzen CJ (1967) Determination of chlorophyll and phaeopigments: spectrometric equations. Limnol Oceanogr 12:343–346
McClanahan TR, Mangi SC (2004) Gear-based management of a tropical artisanal fishery based on species selectivity and capture size. Fish Manage Ecol 11:51–60
McIntyre CD, Amspoker MC (1986) Effects of sediment properties on benthic primary production in the Columbia River estuary. Aquat Bot 24:249–267
McLusky DS (1989) The estuarine ecosystem, 2nd edn. Chapman and Hall, New York
Meurer AZ, Netto SA (2007) Seasonal dynamics of benthic communities in a shallow sublitoral site of Laguna Estuarine System (South, Brazil). Braz J Aquat Sci Tech 11(2):53–62
Moens T, Vincx M (1997) Observations on the feeding ecology of estuarine nematodes. J Mar Biol Ass UK 77:211–227
Nedelec C, Prado J (1990) Definition and classification of fishing gear categories. FAO, Roma
Paterson DM, Hagerthey SE (2001) Microphytobenthos in contrasting coastal ecosystems: biology and dynamics. In: Reise K (ed) Ecological comparisons of sedimentary shores. Ecological Studies 151 Springer, 105–126 pp
Pereira TJ, Netto SA (2005) Rejeitos da pesca de camarão no Sistema Estuarino de Laguna (Shrimp fishery bycatch at the Laguna Estuarine System). In: II Brazilian Conference of Oceanography, Espirito Santo Federal University, Vitória, 9–12 October 2005
Perneger TV (1998) What’s wrong with Bonferroni adjustments. BMJ 316:1236–1238
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge, UK
Reizopoul S, Nicolaidou A (2004) Benthic diversity of coastal brackish-water lagoons in western Greece. Aquat Conserv: Mar Freshwat Ecosyst 14:93–1024
Sanders HL (1968) Marine benthic diversity: A comparative study. Am Nat 102:243–282
Schratzberger M, Jennings S (2002) Impacts of chronic trawling disturbance on meiofaunal communities. Mar Biol 141:991–1000
Schratzberger M, Warwick RM (1999) Effects of physical disturbance on nematode communities in sand and mud: a microcosm experiment. Mar Biol 130:643–650
Schratzberger M, Dinmore TA, Jennings S (2002) Impacts of trawling on the diversity, biomass and structure of meiofauna assemblages. Mar Biol 140:83–93
Snelgrove PVR, Butman CA (1994) Animal–sediment relationships revisited: cause versus effect. Oceanogr Mar Biol Annu Rev 32:111–177
Soetaert K, Heip C (1990) Nematode assemblages of deep-sea and shelf break sites in the North Atlantic and Mediterranean Sea. Mar Ecol Prog Ser 125:171–83
Soetaert K, Vincx M, Wittoeck J, Tulkens M (1995) Meiobenthic distribution and nematode community structure in five European estuaries. Hydrobiologia 311:185–206
Sokal RR, Rohlf FJ (1997) Biometry. WH Freeman and Company, New York
Somerfield PJ, Warwick RM (1996) Meiofauna in marine pollution programmes. A laboratory manual. MAFF Directorate of Fisheries Research, Lowestoft
Stergiou KI, Petrakis G, Politou CY (1996) Small-scale fisheries in the South Euboikos Gulf (Greece): species composition and gear competition. Fish Res 26:325–336
Steyaert M, Vanaverbeke J, Vanreusel A, Barranguet C, Lucas C, Vincx M, (2003) The importance of fine-scale, vertical profiles in characterising nematode community structure. Estuar Coast Shelf Sci 58:353–366
Strickland JHD, Parsons TR (1972) A practical handbook of seawater analysis, 2nd edn. Fisheries Research Board of Canada, Ottawa
Suguio K (1973) Introdução à sedimentologia. Editora. Blücher, São Paulo
Sunye PS (2006) Diagnóstico da pesca no litoral do estado de Santa Catarina. In: Isaac VJ, Martins AS, Haimovici M, Andriguetto JM (eds) A Pesca Marinha e Estuarina do Brasil no Início do Século XXI: Recursos, Tecnologias, Aspectos Socioeconômicos e Institucionais. Editora Universitária UFPA, Belém, pp 141–156
Vanhove S, Arntz W, Vincx M (1999) Comparative study of nematode communities of southeastern Weddel Sea shelf and slope (Antarctica). Mar Ecol Prog Ser 181:237–256
Vieira JP, Vasconcellos MC, Silva RE, Fischer LG (1996) A rejeição da pesca do camarão rosa (Penaeus paulensis) no estuário da Lagoa dos Patos, RS, Brasil. Atlântica 18:123–142
Walsh HJ, Peters DS, Cyrus DP (1999) Habitat utilization by small flatfishes in a North Carolina estuary. Estuaries 22:803–813
Warwick RM, Platt HM, Clarke KR, Agard J, Gobin J (1990) Analysis of macrobenthic and meiobenthic community structure in relation to pollution and disturbance in Hamilton Harbour, Bermuda. J Exp Mar Biol Ecol 138:119–142
Wasmund N (2007) Production and distribution of the microphytobenthos in sediment of Lake Mikolajskie. Int Reveu ges Hydrobiol 69:215–229
Wieser W (1953) Die Beziehung zwishen Mundhöhlengestalt, Ernährungsweise und Vorkommen bei freilebenden marine Nematoden—eine ökologischmorphologische Studie. Ark Zool 4:439–483
Widdicombe S, Austen MC (2005) Setting diversity and community structure in subtidal sediments: The importance of biological disturbance. In: Kostka J, Haese R, Kristensen E (eds) Interactions between macro- and microorganisms in marine sediments, AGU, New York, pp 217–231
Zajac R (2003) Macrofaunal responses to pit–mound patch dynamics in an intertidal mudflat: local versus patch-type effects. J Exp Mar Biol Ecol 313:2297–315
Acknowledgements
The authors are grateful to all fishermen who allowed us to onboard a scientific observer, despite the great restrictions imposed by the boat size. We also thank Gustavo Fonseca and Fabiane Gallucci for their help in the field. Dr Patricia Sunye (EPAGRI), Dr. Axayácatl Rocha-Olivares and Mc. Ruth Gingold (CISESE) are thanked for commenting the manuscript. This study was founded by the Fundação de Apoio à Pesquisa do Estado de Santa Catarina (FAPESC). Finally, we thank three anonymous referees for their contribution in improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Netto, S.A., Pereira, T.J. Benthic community response to a passive fishing gear in a coastal lagoon (South Brazil). Aquat Ecol 43, 521–538 (2009). https://doi.org/10.1007/s10452-008-9177-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-008-9177-8