Abstract
The St. Lucie Estuary, located on the southeast coast of Florida, provides an example of a subtropical ecosystem where seasonal changes in temperature are modest, but summer storms alter rainfall regimes and external inputs to the estuary from the watershed and Atlantic Ocean. The focus of this study was the response of the phytoplankton community to spatial and temporal shifts in salinity, nutrient concentration, watershed discharges, and water residence times, within the context of temporal patterns in rainfall. From a temporal perspective, both drought and flood conditions negatively impacted phytoplankton biomass potential. Prolonged drought periods were associated with reduced nutrient loads and phytoplankton inputs from the watershed and increased influence of water exchange with the Atlantic Ocean, all of which restrict biomass potential. Conversely, under flood conditions, nutrient loads were elevated, but high freshwater flushing rates in the estuary diminished water residence times and increase salinity variation, thereby restricting the buildup of phytoplankton biomass. An exception to the latter pattern was a large incursion of a cyanobacteria bloom from Lake Okeechobee via the St. Lucie Canal observed in the summer of 2005. From a spatial perspective, regional differences in water residence times, sources of watershed inputs, and the proximity to the Atlantic Ocean influenced the composition and biomass of the phytoplankton community. Long water residence times in the North Fork region of the St. Lucie Estuary provided an environment conducive to the development of blooms of autochthonous origin. Conversely, shorter residence times in the mid-estuary limit autochthonous increases in biomass, but allochthonous sources of biomass can result in bloom concentrations of phytoplankton.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The structure and function of many estuaries around the world are being impacted by human development, both directly and through alterations of their watersheds (Nixon 1995; Cloern 2001), and there is growing evidence that cultural eutrophication is increasing the frequency, intensity, and distribution of algal blooms (Glibert and Burkholder 2006; Anderson et al. 2008; Heisler et al. 2008). The type and magnitude of anthropogenic impacts on bloom dynamics vary according to the specific characteristics of individual estuaries. The potential for blooms in estuaries is related to a wide range of features, such as geographic location, size, depth, hydrologic conditions (e.g., water residence times, freshwater inflows), character of the watershed, and the type of biological communities present (Cloern 2001; Smayda 2008; Cloern and Jassby 2010). Phytoplankton abundance in flow-restricted or microtidal estuaries is often more sensitive to increases in nutrient loads than in well-flushed ecosystems (Knoppers et al. 1991; Monbet 1992; Oliviera and Kjerfve 1993; Bledsoe et al. 2004; Phlips et al. 2004), creating a greater potential for algal blooms (Paerl 1988; Phlips et al. 1999, 2011). The responses of estuaries to anthropogenic influences are also affected by variability in climatic conditions (Smetacek and Cloern 2008; Brinceño and Boyer 2010; Cloern and Jassby 2010; Hallegraeff 2010; Paerl et al. 2010; Phlips et al. 2010; Zingone et al. 2010), which in turn can be altered by human activities (e.g., greenhouse gas emissions and global warming). The effects of climatic variability can be complex and multifaceted (Winder and Cloern 2010). Increases in rainfall levels are often associated with increased nutrient loads from watersheds to estuaries, which can enhance primary production, but increases in rainfall can also increase freshwater flushing rates, which restrict the buildup of biomass. An understanding of the interactions between nutrient availability, hydrology, and climatic conditions is an important component of defining phytoplankton structure, abundance, and dynamics.
The St. Lucie Estuary (SLE), located on the southeast coast of Florida, provides an example of a subtropical ecosystem where seasonal changes in temperature are modest, but summer storms and El Niño/La Niña cycles alter rainfall regimes and external inputs to the estuary from the watershed and Atlantic Ocean. The impacts of changes in rainfall are accentuated by the microtidal character of the SLE and its connection to sources of nutrient-rich freshwater, including Lake Okeechobee (Phlips et al. 1997; Havens et al. 1996), the Indian River agricultural district, and rapidly expanding urban development (Parmer et al. 2008). The connection of the estuary to Lake Okeechobee, a large eutrophic lake prone to cyanobacterial blooms (Havens et al. 1996; Phlips et al. 1997), poses challenges to the stability of the estuary in terms of hydrology, nutrient loads, salinity variation, sedimentation of organic matter, and influxes of freshwater algae blooms. The hydrologic and watershed characteristics of the SLE result in large shifts in salinity and nutrient concentration (Chamberlain and Hayward 1996; Doering 1996; Millie et al. 2004; Ji et al. 2007; Parmer et al. 2008). The eutrophic character of external freshwater inputs to the estuary enhances the potential for algae blooms, which can be expressed in the form of allochthonous inputs of algal biomass, or as autochthonous production of estuarine phytoplankton within the SLE.
The focus of this study was the response of the phytoplankton community to spatial and temporal shifts in salinity, nutrient concentration, watershed discharges, and water residence times. The responses were evaluated within the context of temporal patterns in rainfall, which is a key driver in the variability of the latter parameters. To evaluate the potential responsiveness of the phytoplankton community to changes in nitrogen and phosphorus availability, nutrient limitation bioassay experiments were carried out using natural plankton populations collected in the SLE. In addition, allochthonous inputs of fresh water phytoplankton to the estuary were examined as a potential source of freshwater algae blooms. The results of the study demonstrate the key role that climate-driven changes in hydrologic conditions play in controlling the abundance and structure of phytoplankton in the SLE.
Methods
Site Description
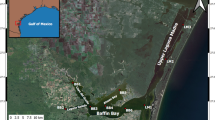
The SLE extends over an area of 29 km2 and is shallow throughout (i.e., mean depth 2.4 m). Until the late nineteenth century, the system was freshwater, but in 1892, the St. Lucie Inlet was opened for navigation (Fig. 1). Since 1892, the SLE has been predominantly saline, except during high rainfall periods when freshwater discharges from the major inflows to the SLE can drive salinities in large portions of the estuary below 2 (Doering 1996). In addition to two natural inflows, Ten-mile Creek and Old South Fork, three major manmade canals (C-23, C-24, and C-44) were added to the system in the first half of the twentieth century to provide water supply and drainage for agricultural, industrial, urban, and suburban land use, as well as water releases from Lake Okeechobee into the St. Lucie Canal (C-44) for flood control (Fig. 1). The canals provide an average of 75% of the freshwater discharge into the estuary, and all the inflows contain water control, such as locks, dams, and water pumping stations (Doering 1996).
The hydrology of the estuary is strongly influenced by freshwater discharge from the five major inflows (Ten-Mile Creek, Old South Fork, C-23, C-24, C-44) and tidal water exchange with the Atlantic Ocean. The shallow depth and relatively small size of the SLE result in rapid and spatially extensive responses to changes in discharge (Ji et al. 2007). Salinity isoclines can move substantial distances up and down the estuary on time scales of days to weeks, and vertical stratification is generally short-lived. The estuary is microtidal (amplitude <0.5m) (Ji et al. 2007). Hydrologic characteristics of the SLE result in a dynamic environment in terms of salinity, nutrient levels, and phytoplankton biomass (Chamberlain and Hayward 1996; Doering 1996, Millie et al. 2004; Yang et al. 2008).
Monthly rainfall totals for the meteorological station at Stuart (US National Climatic Data Center, www.ncdc.noaa.gov) was used for correlation analyses. The subtropical region of Florida, where the SLE is located, is characterized by a wet season from May through October, which coincides with the tropical storm season, and a dry season from November through April.
Sampling Regime
Five sites were sampled on weekly basis from May 2005 through April 2008 (Fig. 1). Site 1 was located in the North Fork of the inner estuary, near the inflow of Ten-Mile Creek, a tributary that is characterized by a watershed with agricultural and urban land uses (Parmer et al. 2008). Site 2 was located in the South Fork of the Inner estuary, near the inflow of the St. Lucie Canal, a manmade structure which receives outflow water from Lake Okeechobee. Site 3 was located in the mid-estuary, near the confluence of North and South Fork. Site 4 was located several kilometers from the St. Lucie Inlet and subject to regular tidal water exchange with Atlantic Ocean water. Site 5 was located just inside the St. Lucie Inlet. Mean depths ranged to 2–3 m at all five sites.
A more intensive sampling regime was used to generate GIS images of chlorophyll a and bottom oxygen concentrations on August 12, 2005, during an incursion of a cyanobacteria bloom from the St. Lucie Canal. Nineteen sites distributed evenly through the estuary were sampled on August 12.
Temperature, salinity, and oxygen concentration were measured at the surface and near the bottom at each site with a Hydrolab Quanta environmental multi-probe. Light attenuation was determined using a Secchi disk. Water samples for chemical and phytoplankton analysis were collected with a vertical integrating sampling tube that captured water from the surface to within 0.1 m of the bottom, to avoid sample bias resulting from vertical stratification of phytoplankton. Water samples were subdivided on site into aliquots for chlorophyll a, phytoplankton composition, and water chemistry analysis. Samples for chlorophyll a analysis were filtered on site onto 0.7-μm glass fiber filters and stored frozen in the dark for subsequent analysis. Filtrate from the chlorophyll a sample preparation was stored on ice for subsequent analysis of nitrate, nitrite, ammonium, and soluble reactive phosphorus.
Water Residence Time Estimates
Water residence times are expressed as E 60 values (i.e., time in days for 60% water exchange), otherwise referred to as the e-folding time.
Water residence times in the SLE are strongly influenced by tidal water exchange rates as well as freshwater flushing rates; therefore, both factors are incorporated into the model formulations of water residence time (Sun 2009, 2011). Water residence time estimates for the study period were based on linear regression relationships developed between historical E 60 values derived from a hydrodynamic model for 1997–1999 (Sun 2009, 2011) and rainfall levels integrated over a period of 2 weeks prior to the date of the E 60 model estimate. Regression for North Fork was E 60 = −0.2534X + 17.028, R 2 = 0.77. Regression for South Fork was E 60 = −0.0257X + 2.9239, R 2 = 0.21. The slope of the regression relationship for South Fork was shallow, reflecting the strong influences of tidal mixing and freshwater discharge from the St. Lucie Canal on E 60 in that region of the SLE.
Analytical Methods
Chlorophyll a and pheophytin concentrations were determined using spectrophotometric methods (Sartory and Grobbelaar 1984; APHA 1998).
Colored dissolved organic matter (CDOM) was analyzed spectrophotometrically using filtered water against a platinum cobalt standard (APHA 1998) and reported as platinum cobalt units (PCU).
Total nitrogen and total phosphorus were determined from unfiltered samples using the persulfate digestion method (Parsons et al. 1984; APHA 1998). Additional analyses were performed on samples collected on the dates of the nutrient limitation bioassays. Nitrate, nitrite, and ammonium were determined using the oxidation method on a Bran-Luebbe 3 Autoanalyzer (Parsons et al. 1984; APHA 1998). Dissolved inorganic nitrogen (DIN) was calculated by summing nitrate, nitrite, and ammonium concentrations. Soluble reactive phosphorus was determined with the ascorbic acid method using a spectrophotometer (Parsons et al. 1984; APHA 1998). Silica was determined from unfiltered samples using the molybdosilicate method (Parsons et al. 1984; APHA 1998).
Specific methods for water collection, preservation, holding time, and analysis followed the guidelines set forth in our Florida Department of Health/DEP-approved NELAP Quality Assurance/Quality Control Plan (E72883).
Microcystin levels were determined using an ELISA kit (Envirologix, Portland, ME). Five milliliters of whole water samples collected from each site was transferred into glass tubes and kept at −20°C until analysis. Microcystin extraction was performed by boiling the tubes at 100°C for 1 min, followed by cooling at 4°C, and centrifugation at 3,000×g for 30 min (Metcalf and Codd 2000). Supernatants were used in the ELISA according to the manufacturer’s instructions. Dilutions were performed when necessary.
Bioassay Experiments
Nutrient limitation/growth bioassay experiments for phytoplankton were performed on a monthly basis for three sampling sites, Sites 1, 2, and 4. Whole water samples for the experiments were collected on May 2, May 29, June 25, July 23, August 27, September 25, October 29, November 18, and December 11, 2007, and January 29, February 24, and March 30, 2008. The experimental design was based on methods described by Aldridge et al. (1995) and Phlips et al. (1997). Assays were carried out under laboratory conditions in 500-ml Erlenmeyer flasks with 400 ml of whole water. Treatments (in triplicate) consisted of a control (no additions), N addition in the form of potassium nitrate (400 μg N l−1), 2N addition (800 μg N l−1), P addition in the form of potassium phosphate (40 μg P l−1), N + P addition (400 μg N l−1 + 40 μg P l−1), and N + P + Si (in the form of sodium meta silicate) addition (400 μg N l−1 + 40 μg P l−1 + 400 μg Si l−1).
Incubations were done in temperature-controlled water baths with bottom illumination. Incubation temperatures were set near ambient temperatures recorded on each sampling date. Light flux was fixed at 80 μE m−2 s−1. Photoperiods were adjusted by season. Algal biomass was estimated using in vivo fluorescence of Chl-a using a Turner Designs fluorometer at fixed time intervals over a 14–21-day incubation period. Fluorescence values were compared to extracted chlorophyll values at selected intervals to allow for conversion of fluorometric data to chlorophyll a concentration.
A nutrient was considered limiting when the addition of that nutrient, or combination of nutrients, resulted in significantly (p ≤ 0.05) greater growth than the control. When algal biomass (expressed as chlorophyll a) in the control treatment increased significantly over the incubation period, it was concluded that surplus bioavailable nutrients were present at the time of sampling.
Increases in phytoplankton biomass during the bioassay were estimated from changes in chlorophyll a concentration using several assumptions: (1) chlorophyll a concentration is roughly equivalent to phosphorus concentration in phytoplankton, (2) phosphorus concentration is roughly equivalent to 1% of cell dry weight, (3) dry weight is roughly 25% of wet weight, and (4) 1 g of cell wet weight is roughly equivalent to a milliliter of cell volume (Reynolds 2006).
The potential biomass increase represented by the concentrations of soluble reactive phosphorus and dissolved inorganic nitrogen (nitrite + nitrate + ammonium) present in the water at the beginning of the bioassay was estimated using the Redfield ratio (Redfield et al. 1963) and the aforementioned assumptions.
Phytoplankton Analyses
Phytoplankton composition was determined microscopically using the Utermohl settling method (Utermohl 1958). Lugols preserved samples were settled in 19-mm diameter cylindrical glass chambers. Cells were identified and counted at ×400 and ×100 with a Nikon phase contrast inverted microscope. At ×400, a minimum of 100 cells of a single taxa and at least 30 grids were counted. At ×100, a total bottom count was completed for taxa greater than 30 μm. Picoplanktonic cyanobacteria were counted using epifluorescence microscopy at 1000x magnification following methods described by Phlips et al. (1999). Counts for individual taxa were converted to cell volumes using the closest geometric shape method (Smayda 1978). Mean volume was calculated for each species from specific phytoplankton dimensions measured for a minimum of 30 randomly selected cells or as many as possible for rare species. Species which vary significantly in size, due to life history or seasonal variability, were subdivided into size classes to provide more accurate estimates of biovolume.
Statistical Analyses
Statistical analyses were performed using SAS Statistics for PCs Version 9.2.
Results
Physical–Chemical Parameters
In 2005, the Ft. Pierce and Stuart meteorological stations recorded well above average rainfall in March, June, October, and November, in association with series of major storms (Fig. 2). By contrast, 2006 was a low rainfall year, with below average rainfall in most months. Low rainfall conditions continued through the spring of 2007, after which rainfall levels increased through much of the last year of the study period.
Monthly rainfall totals (top) and departure from normal (bottom) at the Stuart meteorological station (US National Climatic Data Center, www.ncdc.noaa.gov)
Water residence times (E 60, time for 60% water turnover) were estimated for Sites 1 and 2 in the North and South Fork regions of the SLE (Fig. 3). Estimated E 60 values at Site 1 ranged from 2.5 to 16.5 days, while at Site 2, values ranged from 1.0 to 4.0 days. Values at both sites declined during the wet seasons, most prominently in 2005 and 2007.
Salinities at the five sampling sites reflected the distance from the inlet to the Atlantic Ocean (St. Lucie Inlet) and rainfall patterns in the region. Sites 1, 2, and 3, in the inner estuary, had the greatest range and variability in salinity (Fig. 4). During the strong tropical storm season of 2005, salinities at all five sites were exceptionally low. By contrast, during the drought period of 2006/2007, salinities in the inner estuary were substantially elevated, remaining between 10 and 30 psu. The return of higher rainfall levels in the summer of 2007 resulted in declines in salinity through much of the estuary. Differences between bottom and surface salinities were small, except at Sites 3 and 4 in the mid-estuary during high rainfall periods.
Water temperatures exceeded 20°C during most of the sampling period, except January of 2008 (Fig. 5). Water temperatures generally exceeded 25°C from April through October throughout the SLE. No significant vertical stratification of temperature was observed. Bottom and surface temperature were generally separated by less than 1°C.
CDOM levels reflected the proximity to watershed inflows and rainfall levels. Mean dry season CDOM values at Sites 1–5 were 50, 65, 46, 36, and 17 PCU, respectively. Mean wet season CDOM at Sites 1–5 were 82, 110, 79, 49, and 20 PCU.
Light transmission through the water column was on average higher in the dry season than in the wet season. Mean dry season Secchi depth values at Sites 1–5 were 1.1, 0.7, 1.0, 1.2, and 1.6 m, respectively. Mean wet season Secchi depth at Sites 1–5 were 0.8, 0.5, 0.6, 0.9, and 1.3 m.
Mean total nitrogen (TN) and total phosphorus (TP) values were generally higher in the wet than the dry season (Tables 1 and 2). Mean TN concentrations ranged from 35.1 μM at Site 5 in the wet season to 78 μM at Site 2 in the wet season (Table 1). Mean TP concentrations ranged from 1.6 μM at Site 5 in the dry season to 8.0 μM at Site 1 in the wet season (Table 2). The influence of freshwater inflows on nutrient concentrations was indicated by the significant negative correlations between salinity and both TN and TP (Table 3).
Mean DIN, soluble reactive phosphorus (SRP), and silica concentrations were determined on a monthly basis, for the 1-year period from April of 2007 through March of 2008. Mean DIN concentrations ranged from 6.1 μM at Site 5 in the wet season to 18.6 μM at Site 3 in the wet season (Table 1). Mean SRP and DIN concentrations were higher in the wet than dry season (Tables 1 and 2). Mean SRP concentrations ranged from 0.5 μM at Site 5 in the dry season to 5.5 μM at Site 1 in the wet season (Table 2). Mean Si concentrations ranged from 17.1 μM at Site 5 in the dry season to 83.5 μM at Site 2 in the wet season (Table 1). Si concentrations dropped below 17.9 μM at Site 5 on a number of dates, a concentration which is considered to be potentially limiting for the growth of some diatoms, although it is above the half-saturation constant for Si uptake by some species (Reynolds 2006).
Phytoplankton Abundance
Mean chlorophyll a concentrations over the study period were highest in the inner estuary (Sites 1 and 2) and lowest at Site 5 near the St. Lucie Inlet (Table 2). Mean values were higher in the wet season than the dry season at all five sampling sites (Table 2). Similar spatial and temporal trends were observed for phytoplankton biovolume (Fig. 6). The highest phytoplankton biovolumes were generally observed in the wet season, although significant peaks in biovolume were observed in both the dry and wet seasons (Fig. 6). All three wet seasons included in the study exhibited peaks in phytoplankton biovolume in the inner estuary; however, the composition of the blooms in 2005 involved freshwater taxa of allochthonous origin, while those in 2006 and 2007 were primarily dominated by marine taxa. Several of the largest biovolume peaks in 2006 and 2007 followed periods of increasing TN (Fig. 7), suggesting a link to rainfall events and the associated spikes of nutrients from the watershed. The regression relationship between the increase in phytoplankton biovolume during major bloom events and the corresponding change in TN concentration over the 2 weeks prior to the observed bloom peaks was positive with an r 2 of 0.43. However, prolonged high rainfall from the late summer through fall of 2007 (Fig. 2) was characterized by an extended period of low salinity (Fig. 4) and low phytoplankton biovolume (Fig. 6), despite elevated nutrient concentrations (Fig. 7). These periods were also associated with declining water residence times (Fig. 3).
Phytoplankton Community Structure and Environmental Interrelationships
The dominant phytoplankton species in the SLE varied temporally and spatially. At Site 1, in North Fork, freshwater diatoms dominated summer phytoplankton peaks in 2005, including Cyclotella spp. and Aulacoseira granulata (Fig. 6). In 2006 and 2007, marine dinoflagellates and diatoms were the major bloom formers, most prominently the dinoflagellates Akashiwo sanguinea, Prorocentrum minimum, and Protoperidinium sp., and the diatoms Skeletonema costatum, Odontella regia, and Rhizosolenia setigera.
At Site 2, in South Fork, bloom levels of the toxic freshwater cyanobacterium Microcystis aeruginosa, introduced from Lake Okeechobee, dominated peaks of phytoplankton biovolume in the summer of 2005 (Fig. 6). The next 2 years were characterized by peaks in marine taxa, predominantly the same dinoflagellate and diatom taxa observed at Site 1, plus the dinoflagellate Gyrodinium instriatum and the diatom Leptocylindrus minimus (Fig. 6).
At Site 3, in the mid-estuary, the phytoplankton community featured the same species observed in North and South Fork (Fig. 6). In the summer of 2005, residual portions of M. aeruginosa blooms from Lake Okeechobee were observed in the wet season. In 2006 and 2007, biovolume peaks of marine dinoflagellates and diatoms involved the same suite of species observed at Sites 1 and 2.
At Sites 4 and 5, in the outer estuary, marine diatoms and dinoflagellates dominated the phytoplankton community, except in August of 2005 when high concentrations of M. aeruginosa was observed at Site 4 and in September of 2005 when a freshwater diatom bloom originating from North Fork was observed at Site 4 (Fig. 6). For most of the study period, peaks in phytoplankton biomass at Site 4 were dominated by the dinoflagellate A. sanguinea or the diatom S. costatum. At Site 5 near the St. Lucie Inlet, the phytoplankton community was dominated by the marine diatoms S. costatum, Dactyliosolen fragilissimus, Leptocylindrus danicus, Coscinodiscus spp., or Pseudo-nitzschia calliantha, along with a substantial presence of picoplanktonic cyanobacteria.
The two most common bloom-forming marine taxa observed during the study period were A. sanguinea and S. costatum. Both species were observed over a wide range of temperatures and salinities (Fig. 8), but showed somewhat different salinity preferences in terms of bloom events. Major blooms of A. sanguinea were observed at salinities from seven to 23, while the largest S. costatum blooms were observed at salinities between 20 and 30 (Fig. 8). In terms of total phosphorus, the magnitude of A. sanguinea blooms increased with concentration, while most S. costatum blooms were observed at lower concentrations (Fig. 8). Both species showed the highest bloom intensities at intermediate total nitrogen concentrations (Fig. 8).
The 2005 Cyanobacteria Bloom Event
In the summer of 2005, exceptionally strong tropical storm activity impacted South Florida (Havens et al. 2011), precipitating a major discharge of water from Lake Okeechobee into the St. Lucie Canal for the purpose of flood control. The discharged water contained a bloom of the toxic cyanobacterium M. aeruginosa, which entered the South Fork region of the SLE (Fig. 9). Water column concentrations of chlorophyll a increased dramatically between the July 26 and August 10 water sampling dates, along with a decline in salinity (Table 4). Elevated chlorophyll a levels extended well out into the estuary (Fig. 10), and significant amounts of M. aeruginosa were observed in water samples out to Site 4 (Fig. 6). By contrast, Site 1 in North Fork had minimal concentrations of M. aeruginosa but high biovolumes of the diatom Cyclotella sp. (Fig. 6). By September 2, chlorophyll a concentrations returned to pre-event levels (Table 4). Salinities in the inner estuary remained low but increased in the mid- and outer regions (Table 4).
Oxygen concentrations in the bottom of the water column on August 12, 2005 were below 3.5 mg L−1 in the inner and mid-estuary (Fig. 10), although surface concentrations remained above 3.5 mg L−1 at all of the sampling sites. Low oxygen concentrations were observed in both South Fork/mid-estuary, where high concentrations of M. aeruginosa were observed, and in North Fork, where M. aeruginosa levels were low, likely due to inflows of low oxygen containing water from various tributaries, including Ten-Mile Creek.
The M. aeruginosa bloom event was also characterized by dense surface scums of cells (Fig. 9). Surface scum samples collected on August 12 near Sites 2, 3, and 4 had chlorophyll a concentrations ranging from 1,125 to 2,862 μg l−1 (Table 5). Concentrations of the toxin microcystin in the surface layer were estimated using ELISA analyses and ranged from 163 to 1,188 μg l−1 (Table 5). Integrated water column samples also contained microcystins at concentrations from 2.1 μg l−1 at Site 5 to 16.7 μg l−1 at Site 2 (Table 5). No microcystin was detected at Site 1 in North Fork. Contemporaneous collection of a surface scum in Lake Okeechobee, near the entrance of the St. Lucie Canal, on August 10 revealed a chlorophyll a concentration of 739 μg l−1 and microcystin level of 492 μg l−1.
Nutrient Limitation Bioassays
The results of the bioassays showed that nitrogen was the primary limiting nutrient for phytoplankton growth in the inner (Sites 1 and 2) and outer estuary (Site 4), except when the presence of surplus nitrogen, phosphorus, and silica was indicated by significant growth in the control groups (i.e., no added nutrients) (Table 6). Even in cases where the bioassays showed no initial nutrient limitation, the mesocosms eventually shifted to nitrogen or nitrogen and phosphorus co-limitation as biomass levels peaked. The high levels of SRP observed in the SLE help to explain the observed nitrogen limitation of phytoplankton growth shown in the bioassays. The potential for nitrogen limitation was further indicated by low DIN/SRP ratios relative to the Redfield Ratio (i.e., N/P of 16) (Reynolds 2006), i.e., mean ratios of 2.4, 3.5, 3.3, 5.4, and 13.3 for Sites 1–5, respectively. This relationship is further reflected in the estimates of supportable biovolume increase represented by ambient concentrations of SRP and DIN in the water used for the bioassay experiments (Table 6). In many cases, the estimated biovolume increase supportable by the observed DIN levels was similar to the increases in phytoplankton biovolume observed in the “control” group of the bioassay experiment, but estimates based on SRP concentrations were always substantially higher than the increases observed in the control group of bioassays. The former observation also suggests that the natural zooplankton community present in the bioassay mesocosms did not significantly impede the accumulation of phytoplankton biomass. In addition, the peak phytoplankton biovolumes observed in the St. Lucie a week after the bioassay experiment dates did not reflect the potential represented by the observed levels of DIN and SRP, indicating that factors other than nutrient availability or zooplankton grazing limited the buildup of biomass.
Discussion
The results of this study provide insight into how changes in climatic conditions, principally rainfall, influence spatial and temporal variability in hydrology, nutrient levels, and salinity, which in turn control phytoplankton biomass and composition in the SLE. The relatively modest seasonal variation in temperature and light flux in the subtropical environment of South Florida is reflected in less consistent seasonality in phytoplankton biomass than typically observed in temperate ecosystems (Kennish 1990; Winder and Cloern 2010). Over the 3-year study, three distinct rainfall periods were observed: (1) a well-above-average rainfall and freshwater discharge period in 2005, related to several major tropical storms in the summer and fall, (2) a generally below average rainfall and discharge period from 2006 through the spring of 2007, and (3) a period of variable rainfall conditions from the summer of 2007 through the early spring of 2008. Shifts in rainfall levels were associated with concomitant changes in water chemistry, hydrology, and phytoplankton biomass and composition. It is useful to view these temporal changes in terms of regional differences in hydrologic conditions, such as water residence time and the character of freshwater discharges from local watersheds, which impact nutrient loads and salinity.
In terms of water residence time, the SLE can be subdivided into two distinct regions, North Fork (represented by Site 1), where E 60 values (time for 60% water exchange) range from 2.5 to 18+ days, and the rest of the estuary from South Fork to the inlet (represented by Sites 2–5), where E 60 values range from <1 to 4 days (Ji et al. 2007; Sun 2011). The E 60 values in the latter region have the potential to restrict the phytoplankton biomass associated with autochthonous primary production, while the influence of residence time on biomass in North Fork varies according to rates of freshwater discharge and tidal water exchange with the outer estuary. Several researchers have noted the negative effect of very high tributary inflows on phytoplankton biomass in the SLE (Chamberlain and Hayward 1996; Doering 1996, Millie et al. 2004). High freshwater discharge is known to dampen biomass potential in estuaries subject to large shifts in water residence time (Knoppers et al. 1991; Pinckney et al. 1997; Eyre 2000; Bledsoe et al. 2004; Phlips et al. 2004; Murrell et al. 2007).
The relative influences of freshwater inputs from the watershed versus tidal water exchange with the Atlantic Ocean on the phytoplankton community in the SLE vary with distance from the St. Lucie Inlet. At Sites 4 and 5 near the St. Lucie Inlet, abundance and composition of phytoplankton are strongly influenced by water exchange with the Atlantic Ocean, as reflected by the dominance of marine diatoms and picoplanktonic cyanobacteria. In the inner (Sites 1 and 2) and mid-estuary (Site 3), freshwater discharge is a major factor in determining the potential for phytoplankton blooms of allochthonous or autochthonous origin. The major freshwater inputs to the SLE are eutrophic but have regionally different watershed and phytoplankton characteristics. The watersheds associated with Ten-Mile Creek and the other main sources of freshwater for North Fork (i.e., the C-23 and C-24 canals) include substantial surface water runoff from agricultural, urban, and suburban land-use areas, while South Fork is strongly impacted by inflows from the St. Lucie Canal (C-44), which periodically receives large discharges of freshwater from Lake Okeechobee (Parmer et al. 2008). All of the major sources of freshwater for the SLE have water-flow control structures; therefore, large releases of freshwater can occur on an episodic basis, strongly influencing nutrient concentrations, salinity, and hydrology (Parmer et al. 2008). Such releases can also result in allochthonous inputs of freshwater algal biomass, including toxic forms of cyanobacteria.
Allochthonous Phytoplankton Blooms
In the high rainfall year of 2005, North Fork had peaks of freshwater diatoms, similar in composition to those observed in Ten-Mile Creek (E. Phlips and S. Badylak, unpublished data), one of the major sources of freshwater and nutrients for North Fork (Parmer et al. 2008; Yang et al. 2008). By contrast, in South Fork, the phytoplankton bloom in the summer of 2005 was dominated by the toxic cyanobacterium M. aeruginosa, emanating from the St. Lucie Canal (C-44), which periodically receives significant discharges of water from Lake Okeechobee, a large eutrophic lake subject to frequent blooms of cyanobacteria (Havens et al. 1996; Phlips et al. 1997). During the 2005 bloom event, dense surface scums of M. aeruginosa extended out from South Fork to mid-estuary, with chlorophyll a concentrations on the water surface of up to 2,863 μg l−1 and concentrations of the toxin microcystin up to 1,188 μg L−1. The potential spatial extent and longevity of M. aeruginosa blooms within the estuary is exacerbated by the relatively high salinity tolerance of M. aeruginosa, which has been shown to survive at salinities from 7 to 17 psu (Sellner et al. 1988; Robson and Hamilton 2003; Orr et al. 2004; Tonk et al. 2007, Black et al. 2011). The spatial differences observed in the ratios between microcystin and chlorophyll a (Table 5) from surface scum samples might be due to several factors, including different genotypes of M. aeruginosa capable of producing different amounts of the toxin in the population or previous release of the toxins from dying cells (Kardinaal and Visser 2005). Concentrations of microcystin observed in the surface scum samples are high given that the World Health Organization cautions of possible adverse health effects during recreational exposure to concentrations above 20 μg l−1 microcystin (Chorus and Bartram 1999).
Incursions of toxic M. aeruginosa into estuaries have been identified as an issue of concern in a number of estuaries linked to eutrophied watersheds in the USA, such as the Neuse River estuary (Paerl 1988), San Francisco Bay (Lehman et al. 2005), the Potomac River estuary (Sellner et al. 1988), the St. Johns River estuary (Phlips et al. 2007), and several estuaries in South Carolina (Lewitus et al. 2008). Similar concerns have been raised for a number of estuaries around the world, such as the Swan River estuary in Australia (Atkins et al. 2001; Robson and Hamilton 2003), Patos Lagoon in Brazil (Yunes et al. 1996), Jacarepaguá Lagoon in Brazil (Magalhães et al. 2001), Sepetiba Bay in Brazil (Magalhães et al. 2003), the Guadiana estuary in Spain (Rocha et al. 2002), the Niva estuary in Finland (Nikulina 2003), and deltas in Holland (Verspagen et al. 2006). The potential impacts of toxic M. aeruginosa blooms in estuaries include bioaccumulation of toxins in aquatic food webs (including human food resources), direct threats to the health of aquatic organisms, and human recreational exposure to toxins (Williams et al. 1997; Magalhães et al. 2001, 2003; Landsberg 2002; Ibelings and Chorus 2007; Carmichael 2008; Ibelings and Havens 2008). The specific impacts of cyanobacterial toxins in the SLE remain to be investigated; however, recent evaluations of the health of benthic biological communities in the SLE, including seagrasses and fauna, reveal issues of concern, particularly in the inner estuary (Haunert and Startzman 1985; Sime 2005; Arnold et al. 2008; Parmer et al. 2008; Parker and Geiger 2009; Borja and Tunberg 2010).
Dense blooms of cyanobacteria can also represent a risk for localized anoxia or hypoxia, particularly since scum-forming taxa like M. aeruginosa are subject to wind-driven concentration of biomass (Paerl 1988). Bottom water hypoxia was periodically observed in this study and has been identified as an important management issue in the SLE (Chamberlain and Hayward 1996; Sime 2005; Millie et al. 2004; Parmer et al. 2008). In addition to effecting the health and distribution of aquatic animals, hypoxia can also affect the exchange of nutrients between the sediments and overlying water column. The distribution of hypoxia may also influence the composition and abundance of phytoplankton due to the ability of dinoflagellate cysts to survive low oxygen conditions in surface sediments (Millie et al. 2004).
Autochthonous Phytoplankton Blooms
As a consequence of regional differences in water residence time in the SLE, North Fork is the most likely breeding ground for autochthonous phytoplankton blooms due to the potential for extended water residence times (i.e., weeks). Periods of high freshwater discharge are often accompanied by elevated nutrient levels in the SLE, but the accompanying reductions in water residence times and variable salinities can restrict the buildup of phytoplankton biomass derived from autochthonous production. Therefore, bloom potential depends on the balance between water residence time and phytoplankton population growth rates, which in turn are dependent on environmental conditions (e.g., light and nutrient availability). During 2006 and spring–early summer of 2007 below average rainfall resulted in long water residence times, which coincided with peaks in marine dinoflagellate abundance in the inner estuary. Several sources of nutrients may have supported the blooms in the 2006 and spring–early summer of 2007, such as freshwater discharges following rainfall events, as indicated by coincidental increases in nitrogen concentration. In addition, the nutrients added to the SLE during the preceding high discharge period of 2005 could have resulted in time-delayed enhancement of biomass through re-cycling of nutrients entrained in the benthos, as observed in some other estuaries (Malone et al. 1988; Eyre 2000; Caffrey et al. 2007; Murrell et al. 2007; Paerl et al. 2010). The high organic content of the muddy flocculent sediments found in the inner SLE (Borja and Tunberg 2010) also represent a latent internal nutrient pool for primary production, given favorable hydrologic conditions.
By contrast to the former periods of high phytoplankton biomass, phytoplankton biomass was low during the 2006/2007 winter dry period, likely due to the combined effects of low nutrient inputs from the watershed, increased rates of tidal water exchange with the Atlantic Ocean, and lower winter temperatures and light fluxes which reduce growth rates. After July 2007, there was a 6-month period of above average rainfall, elevated nutrient levels and low total phytoplankton biomass (i.e., <106 μm3 ml−1). The low phytoplankton biomass also coincided with short water residence times, which likely played a significant role in lowering biomass potential. The results of the nutrient limitation bioassays carried out in this study support this conclusion, within the context of existing ambient nutrient levels and zooplankton populations. Surplus bioavailable phosphorus and nitrogen levels present in the summer and fall samples of 2007 were sufficient to support substantial increases in phytoplankton biomass in experimental mesocosms, despite the presence of the ambient zooplankton. Estimates of biomass potential using DIN concentrations observed during the summer of 2007, combined with the Redfield Ratio (i.e., N/P of 16) (Reynolds 2006), indicate that biovolumes in excess of 20 × 106 μm3 ml−1 should have been attainable but were not observed. Such biovolume levels were observed during the same time period in the nearby northern Indian River Lagoon ecosystem where water residence times are much longer than in the SLE (i.e., weeks–months) (Phlips et al. 2004, 2010, 2011). Highly variable salinities during the latter time period, which ranged from <1 to 20 psu, may also have contributed to reduced biomass potential in relation to the negative responses of the phytoplankton community to osmotic stress (Rijstenbil 1988; Kirst 1989; Thessen et al. 2005). Due to variable salinities encountered in the SLE, autochthonous phytoplankton blooms were dominated by euryhaline marine species, including the dinoflagellates A. sanguinea, P. minimum, Protoperidnium sp., and G. instriatum, and the diatoms S. costatum, O. regia, R. setigera, and L. minimus, all of which are common features in estuaries along the coast of the Florida peninsula (Badylak et al. 2007; Badylak and Phlips 2004; Quinlan and Phlips 2007) and many estuaries around the world (Kennish 1990; Horner et al. 1997; Heil et al. 2005; Nagasoe et al. 2006; Reynolds 2006; Matsubara et al. 2007; Heisler et al. 2008).
Climate, Ecosystem Structure, and Phytoplankton Biomass Potential
Relatively high winter temperatures and solar insolation in subtropical and tropical estuaries provide the potential for elevated phytoplankton productivity and biomass throughout the year; however, the realization of that potential within individual systems is dependent on other factors, such as local climatic conditions (e.g., rainfall regimes), hydrologic features (e.g., water residence times), watershed characteristics (e.g., size and trophic state), and biological communities present (e.g., grazer communities, competing benthic primary producers) (Boynton et al. 1982; Paerl 1988; Monbet 1992; Valiela et al. 1997; Smayda 2008; Cloern and Jassby 2010; Zingone et al. 2010). It is useful to compare and contrast the SLE with other subtropical and tropical estuaries in terms of how these factors operate to define biomass potential and distribution.
Regional differences in the character of wet and dry seasons associated with different tropical and subtropical estuaries influence phytoplankton dynamics. For example, along the eastern coast of Australia, generally dry conditions are punctuated by wet season flood events, which result in a cycle of depressed phytoplankton biomass during periods of high freshwater discharge, due to high flushing rates and reduced water residence times, followed by re-stabilization of the estuaries and an increase in biomass (Eyre and Twigg 1997; Eyre 2000). By comparison, more consistent rainfall in the Southeastern United States provides a steadier stream of freshwater and nutrients into estuaries from the watershed. Tropical storms and hurricanes during the wet season can result in large pulses of nutrient-rich water that support major bloom events, particularly in estuaries with persistently long water residence times, such as the Neuse River estuary in North Carolina (Paerl et al. 2010) and the Indian River Lagoon in Florida (Phlips et al. 2010). By contrast, large storm events in the SLE and other systems subject to large shifts in water residence time, have a negative effect on autochthonous phytoplankton biomass because large freshwater discharges result in short water residence times (i.e., <2 days).
From a physical perspective, the small size of the SLE (i.e., 29 km2) relative to the size of the watershed (i.e., 1,800 km2) makes the system sensitive to even small shifts in rainfall. In this sense, the SLE is similar to a number of tropical and subtropical estuaries in Australia (Eyre 2000), Brazil (Abreu et al. 2010), and the Gulf of Mexico (Lehrter 2008), in which nutrient loads associated with moderate increases in rainfall and freshwater inflows from nutrient-enriched watersheds enhance phytoplankton productivity and increase biomass potential, although strong flood periods have negative impacts on biomass due to reductions in water residence times. Similar relationships have been observed in small temperate estuaries in California (Caffrey et al. 2007) and North Carolina (Hubertz and Cahoon 1999). Variability in freshwater discharge is further accentuated in the SLE by the fact that all of its major tributaries include water control structures, which result in periodic regulated releases of water.
In contrast to the SLE and the other aforementioned estuaries linked to freshwater inputs from nutrient-enriched watersheds, some tropical and subtropical estuaries are associated with discharges from rivers of comparatively low nutrient content, such as Escambia Bay (Murrell et al. 2007), Apalachicola Bay (Mortazawi et al. 2000), and Perdido Bay (Livingston 2007), located along the northern Gulf of Mexico. In these systems, reductions in river inflows during drought periods have been linked to reductions in overall ecosystem productivity. For example, in Apalachicola Bay, which supports a large oyster harvesting industry, such drops in phytoplankton production are considered a threat to the sustainability of harvests.
The SLE and other small estuaries with highly variable flushing rates also function differently than open and unrestricted estuaries associated with discharges from large eutrophic rivers, such as the Amazon River in Brazil (Smith and DeMaster 1996; Santos et al. 2008), the Pearl (Lin et al. 2008; Dai et al. 2006) and Huange (Turner et al. 1990) Rivers in China, the Mississippi (Liu et al. 2004; Rabalais et al. 2004) and Suwannee (Bledsoe et al. 2004; Quinlan and Phlips 2007) Rivers in the Gulf of Mexico, and the Nile River in Africa (Oczlowski et al. 2009). Phytoplankton maxima in these systems often occur in the nearshore transition zone between light-limited river water and the nutrient-limited offshore water. The location and magnitude of the phytoplankton maxima are dictated by the rates of discharge from the river and its light attenuation properties, which in turn can influence the productivity of higher trophic levels, as well as the potential for and distribution of hypoxia. Similar relationships have been observed in the temperate estuaries associated with large rivers in Europe (Lemaire et al. 2002).
Other potential contributors to the control of phytoplankton biomass potential involve biological processes, including grazing losses and competition for nutrients by benthic primary producers. The importance of zooplankton grazing in phytoplankton biomass, dynamics, and succession is well established (Calbet 2001; Calbet and Landrey 2004; Smayda 2008). Broad comparisons between ecosystems from around the world suggest that the percentage of primary production grazed per day can be high in tropical and subtropical ecosystems (i.e., average of 75%) (Calbet and Landrey 2004). In the southeastern USA, significant impacts of zooplankton grazing on phytoplankton biomass have been reported for several estuaries, such as Apalachicola Bay (Mortazawi et al. 2000) and Pensacola Bay (Murrell and Lores 2004). By contrast, lesser impacts have been indicated for the Suwannee River estuary (Quinlan et al. 2009). Although zooplankton grazing studies have not been carried out in the SLE, the results of the bioassay experiments performed in this study do not suggest a major impact on bloom dynamics. Benthic grazers have also been shown to play a major role in the control of phytoplankton biomass in some shallow estuaries (Officer et al. 1982), such as San Francisco Bay (Cloern 1982). However, the role of benthic grazers may be less important in the SLE due to limited densities of bivalves and other benthic filter-feeding macroinvertebrates (Sime 2005; Arnold et al. 2008; Parker and Geiger 2009; Borja and Tunberg 2010).
Another issue in defining biomass potential in shallow estuaries is competition for nutrients and light between phytoplankton and macroalgae. Increases in nutrient loads have been linked to both phytoplankton (Glibert and Burkholder 2006; Anderson et al. 2008; Heisler et al. 2008) and macroalgal blooms in estuaries throughout the world (Valiela et al. 1997; Theichberg et al. 2010). Macroalgae can have an advantage in estuaries with high flushing rates and short water residence times because they are not as easily washed out (Valiela et al. 1997). Conversely, estuaries with long water residence times allow for the buildup of phytoplankton biomass, which adversely affects benthic macroalgae by limiting light reaching the benthos and nutrients in the water column. Based on these principles, there should be a potential for macroalgae blooms in the SLE; however, rapid shifts from freshwater to saline conditions relatively high light attenuation in the water column, and the presence of large areas of flocculent muddy sediments appears to limit the development of such blooms.
Summary
The spatial and temporal patterns in phytoplankton composition and biomass observed in the SLE highlight the influence of climate on phytoplankton dynamics, particularly as it relates to changes in rainfall and freshwater discharge from the watershed and their effects on water residence time, salinity, nutrient levels, and allochthonous inputs of phytoplankton biomass. From a temporal perspective, both drought and flood conditions can negatively impact phytoplankton biomass potential. In the SLE, prolonged drought periods are associated with reduced nutrient loads and phytoplankton inputs from the watershed and increased water exchange with the Atlantic Ocean, all of which restrict biomass potential. Conversely, under flood conditions, nutrient loads are elevated, but high freshwater flushing rates in the estuary diminish water residence times and increase salinity variation, thereby restricting the buildup of phytoplankton biomass. An exception to the latter condition exists when large inputs of allochthonous algal biomass enter the estuary from the watershed, such as the incursion of a cyanobacterial bloom from Lake Okeechobee via the St. Lucie Canal in the summer of 2005. Maximum autochthonous biomass potential lies in between flood and drought conditions, when water residence times are long enough to permit bioaccumulation of biomass, and periodic rainfall events or internal nutrient recycling inject sufficient nutrients into the system to support high phytoplankton growth rates.
From a spatial perspective, regional differences in water residence times, sources of watershed inputs, and the proximity to the Atlantic Ocean influence the composition and biomass of the phytoplankton community. Long water residence times in the North Fork region of the SLE provide an environment conducive to the development of blooms of autochthonous origin. Conversely, the short residence times in South Fork and mid-estuary limit autochthonous increases in biomass, but allochthonous sources of biomass can result in bloom concentrations of phytoplankton. By contrast, phytoplankton dynamics and composition in the outer estuary are primarily controlled by tidal interchange with Atlantic Ocean coastal waters.
References
Abreu, P. C., M. Bergesch, L. A. Proença, C. A. E. Garcia and C. Odebrecht. 2010. Short- and long-term chlorophyll a variability in the shallow microtidal Patos Lagoon estuary, Southern Brazil. Estuaries and Coasts 33:554–569.
Aldridge, F.J., E.J. Phlips, and C.L. Schelske. 1995. The use of nutrient enrichment bioassays to test for spatial and temporal distribution of limiting factors affecting phytoplankton dynamics in Lake Okeechobee, Florida, USA. Ergebnisse der Limnologie 45: 177–190.
Anderson, D.M., J.M. Burkholder, W.P. Cochlan, P.M. Glibert, C.J. Gobler, C.A. Heil, R.M. Kudela, M.L. Parsons, J.E.J. Rensel, D.W. Townsend, V.L. Trainer, and G.A. Vargo. 2008. Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States. Harmful Algae 8: 39–53.
APHA. 1998. Standard Methods for the Analysis of Water and Wastewater, 20th ed. Washington, D.C.: American Public Health Association.
Arnold, W., M.L. Parker, and S.P. Stephenson. 2008. Oyster monitoring in the northern estuaries, 156. St. Petersburg: Florida Fish and Wildlife Research Institute.
Atkins, R., T. Rose, R.S. Brown, and M. Robb. 2001. The Microcystis cyanobacterial bloom in the Swan River – February 2000. Water Science and Technology 43: 107–114.
Badylak, S., and E.J. Phlips. 2004. Phytoplankton communities of a restricted subtropical lagoon, the Indian River Lagoon, Florida, USA. Journal of Plankton Research 26: 1–22.
Badylak, S., E. J. Phlips, P. Baker and J. Fajans and R. Boler. 2007. Distributions of phytoplankton in Tampa Bay, USA. Bulletin of Marine Science 80:295–317.
Black, K., M. Yilmaz, and E.J. Phlips. 2011. Growth and toxin production by Microcystis aeruginosa PCC7806 (Kutzing) Lemmerman at elevated salt concentrations. Journal of Environmental Protection 2: 669–674.
Bledsoe, E., E.J. Phlips, C.E. Jett, and K.A. Donnelly. 2004. The relationships among phytoplankton biomass, nutrient loading and hydrodynamics in an inner-shelf estuary, the Suwannee River estuary, Florida, USA. Ophelia 58: 29–47.
Borja, A., and B.G. Tunberg. 2010. Assessing benthic health in stressed subtropical estuaries, eastern Florida, USA using AMBI and M-AMBI. Ecological Indicators 11: 295–303.
Boynton, W.R., W.M. Kemp, and C.W. Keefe. 1982. A comparative analysis of nutrients and other factors influencing estuarine phytoplankton production. In Estuarine Comparisons, ed. V.S. Kennedy, 69–90. New York: Academic.
Brinceño, H.O., and J.N. Boyer. 2010. Climatic controls on phytoplankton biomass in a sub-tropical estuary, Florida Bay, USA. Estuaries and Coasts 33: 541–553.
Caffrey, J.M., T.P. Chapin, H.W. Jannasch, and J.C. Haskins. 2007. High nutrient pulses, nutrient mixing and biological response in a small California estuary: Variability in nutrient concentrations from decadal to hourly time scales. Estuarine Coastal and Shelf Science 71: 368–380.
Calbet, A. 2001. Mesozooplankton grazing impact on primary production: A global comparative analysis in marine ecosystems. Limnology and Oceanography 46: 1824–1830.
Calbet, A., and M. Landrey. 2004. Phytoplankton growth, microzooplankton grazing and carbon cycling in marine systems. Limnology and Oceanography 49: 51–57.
Carmichael, W.A. 2008. A world overview one hundred twenty seven years of research on toxic cyanobacteria: Where do we go from here. Advances in Experimental Medicine and Biology 619: 105–127.
Chamberlain, R., and D. Hayward. 1996. Evaluation of water quality and monitoring in the St. Lucie Estuary, Florida. Water Resources Bulletin 32: 681–696.
Chorus, I., and J. Bartram. 1999. Toxic cyanobacteria in water: A guide to their public health consequences, monitoring, management, 416. London: E & FN Spon.
Cloern, J.E. 1982. Does the benthos control phytoplankton biomass in South San Francisco Bay? Marine Ecology Progress Series 9: 191–202.
Cloern, J.E. 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series 210: 223–253.
Cloern, J.E., and A.D. Jassby. 2010. Patterns and scales of phytoplankton variability in eastuarine-coastal ecosystems. Estuaries and Coasts 33: 230–241.
Dai, M., X. Guo, W. Zhai, L. Yuan, B. Wang, L. Wang, P. Cai, T. Tang, and W. Cai. 2006. Oxygen depletion in the upper reach of the Pearl River estuary during a winter drought. Marine Chemistry 102: 159–169.
Doering, P.H. 1996. Temporal variability of water quality in the St. Lucie Estuary, South Florida. Water Resources Bulletin 32: 1293–1306.
Eyre, B.D. 2000. Regional evaluation of nutrient transformation and phytoplankton growth in nine river-dominated subtropical east Australian estuaries. Marine Ecology Progress Series 205: 61–83.
Eyre, B., and C. Twigg. 1997. Nutrient behaviour during post-flood recovery of the Richmond River estuary Northern NSW, Australia. Estuarine, Coastal and Shelf Science 44: 311–326.
Glibert, P.M., and J.M. Burkholder. 2006. The complex relationships between increases in fertilization of the earth, coastal eutrophication and proliferation of harmful algal blooms. In Ecology of Harmful Algae, ed. E. Granéli and J.T. Turner, 341–354. Berlin: Springer.
Hallegraeff, G.M. 2010. Ocean climate change, phytoplankton community responses and harmful algal blooms: A formidable predictive challenge. Journal of Phycology 46: 220–235.
Haunert, D.E., and R.J. Startzman. 1985. Some short-term effects of a freshwater discharge on biota of the St. Lucie Estuary, Florida. West Palm Beach: South Florida Water Management.
Havens, K.E., N.G. Aumen, R.T. James, and V.H. Smith. 1996. Rapid ecological changes in a large subtropical lake undergoing cultural eutrophication. Ambio 25: 150–155.
Havens, K.E., J.R. Beaver, D.A. Casamatta, T.L. East, R.T. James, P. McCormick, E.J. Phlips, and A.J. Roduskey. 2011. Hurricane effects on the planktonic food web of a large subtropical lake. Journal of Plankton Research 33: 1091–1094.
Heil, C.A., P.M. Glibert, and C. Fan. 2005. Prorocentrum minimum (Pavillard) Schiller: A review of a harmful algal bloom species of growing worldwide importance. Harmful Algae 4: 449–470.
Heisler, J., P.M. Glibert, J.M. Burkholder, D.M. Anderson, W. Cochlan, W.C. Dennison, Q. Dortch, C.J. Gobler, C.A. Heil, E. Humphries, A. Lewitus, R. Magnien, H.G. Marshall, K. Sellner, D.A. Stockwell, D.K. Stoecker, and M. Suddleson. 2008. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 8: 3–14.
Horner, R.A., D.L. Garrison, and F.G. Plumley. 1997. Harmful algal blooms and red tide problems on the U.S. west coast. Limnology and Ocenaography 42: 1076–1088.
Hubertz, E. D. and L. B. Cahoon. 1999. Short-term variability of water quality parameters in two shallow estuaries of North Carolina. Estuaries 22:814–823.
Ibelings, B.W., and I. Chorus. 2007. Accumulation of cyanobacterial toxins in freshwater “seafood” and its consequences for public health: A review. Environmental Pollution 150: 177–192.
Ibelings, B.W., and K.E. Havens. 2008. Cyanobacterial toxins, a qualitative meta-analysis of concentrations, dosage and effects in freshwater, estuarine and marine biota. Advances in Experimental Medicine and Biology 619: 675–732.
Ji, Z., G. Hu, J. Shen, and Y. Wan. 2007. Three-dimensional modeling of hydrodynamic processes in the St. Lucie Estuary. Estuarine Coastal and Shelf Science 73: 188–200.
Kardinaal, W.E.A., and P.M. Visser. 2005. Dynamics of cyanobacterial toxins: Sources of variability in microcystin concentrations. In Harmful cyanobacteria, ed. J. Huisman, 41–57. Dordrecht: Springer.
Kennish, M.J. 1990. Ecology of estuaries, Volume II, Biological Aspects. Boca Raton: CRC.
Kirst, G.O. 1989. Salinity tolerance of eukaryotic marine algae. Annual Reviews of Plant Physiology and Plant Molecular Biology 40: 21–53.
Knoppers, B., B. Kjerfve, and J.P. Carmouze. 1991. Trophic state and water turn-over time in six choked coastal lagoons in Brazil. Biogeochemistry 14: 147–166.
Landsberg, J.H. 2002. The effects of harmful algal blooms on aquatic organisms. Reviews in Fisheries Science 10: 113–390.
Lehman, P.W., G. Boyer, C. Hall, S. Waller, and K. Gehrts. 2005. Distribution and toxicity of a new colonial Microcystis aeruginosa bloom in the San Francisco Bay esuary, California. Hydrobiologia 541: 87–99.
Lehrter, J.C. 2008. Regulation of eutrophication susceptibility in oligohaline regions of a northern Gulf of Mexico estuary, Mobile Bay, Alabama. Marine Pollution Bulletin 56: 1446–1460.
Lemaire, E., G. Abril, R. De Wit, and H. Etcheber. 2002. Distribution of phytoplankton pigments in nine European estuaries and implications for an estuarine typology. Biogeochemistry 59: 5–23.
Lewitus, A.J., L.M. Brock, M.K. Burke, K.A. DeMattio, and S.B. Wilde. 2008. Lagoonal stormwater detention ponds as promoters of harmful algal blooms and eutrophication along the South Carolina coast. Harmful Algae 8: 60–65.
Lin, Y., Z. He, Y. Yang, P.J. Stoffella, E.J. Phlips, and C.A. Powell. 2008. Nitrogen versus phosphorus limitation of phytoplankton growth in Ten Mile Creek, Florida, USA. Hydrobiologia 605: 247–258.
Liu, H., M. Dagg, L. Campbell, and J. Urban-Rich. 2004. Picoplankton and bacterioplankton in the Mississippi River plume and its adjacent waters. Estuaries 27: 147–158.
Livingston, R.J. 2007. Phytoplankton bloom effects on a Gulf estuary: Water quality changes and biological response. Ecological Applications 17: S110–S128.
Magalhães, V.F., R.M. Soares, and S.M.F.O. Azevedo. 2001. Microcystin contamination in fish from the Jacarepaguá Lagoon (Rio de Janeiro, Brazil): ecological implication and human health risk. Toxicon 39: 1077–1085.
Magalhães, V.F., M.M. Marinho, P. Domingos, A.C. Oliveira, S.M. Costa, L.O. Azevedo, and S.M.F.O. Azevedo. 2003. Microcystins (cyanobacteria hepatotoxins) bioaccumulation in fish and crustaceans from Sepetiba Bay (Brazil, RJ). Toxicon 42: 289–295.
Malone, T.C., L.H. Crocker, S.E. Pike, and B.W. Wendler. 1988. Influences of river flow on the dynamics of phytoplankton production in a partially stratified estuary. Marine Ecology Progress Series 48: 235–249.
Matsubara, T., S. Nagasoe, Y. Yamasaki, T. Shikata, Y. Shimasaki, Y. Oshima, and T. Honjo. 2007. Effects of temperature, salinity, and irradiance on the growth of the dinoflagellate Akashiwo sanguinea. Journal of Experimental Marine Biology and Ecology 342: 226–230.
Metcalf, J.S., and G.A. Codd. 2000. Microwave oven and boiling waterbath extraction of hepatotoxins from cyanobacterial cells. FEMS Microbiology Letters 184: 241–246.
Millie, D.F., H.J. Carrick, P.H. Doering, and K.A. Steidinger. 2004. Intra-annual variability of water quality and phytoplankton in the North Fork of the St. Lucie Estuary, Florida (USA): a quantitative assessment. Estuarine Coastal and Shelf Science 61: 137–149.
Monbet, Y. 1992. Control of phytoplankton biomass in estuaries: A comparative analysis of microtidal and macrotidal estuaries. Estuaries 15: 563–571.
Mortazawi, B., R.L. Iverson, W.M. Landing, F.G. Lewis, and W. Huang. 2000. Control of phytoplankton production and biomass in ariver-dominated estuary: Apalachicola Bay, Florida, USA. Marine Ecology Progress Series 198: 19–31.
Murrell, M.C., and E.M. Lores. 2004. Phytoplankton and zooplankton seasonal dynamics in a subtropical estuary: Importance of cyanobacteria. Journal of Plankton Research 26: 372–382.
Murrell, M.C., J.D. Hagey III, E.M. Lores, and R.M. Greene. 2007. Phytoplankton production and nutrient distributions in a subtropical estuary: Importance of freshwater flow. Estuaries and Coasts 30: 390–402.
Nagasoe, S., D. Kim, Y. Shimasaki, Y. Oshima, M. Yamaguchi, and T. Honjo. 2006. Effects of temperature, salinity and irradiance on the growth of the red tide dinoflagellate Gyrodinium instriatum Freudenthal et Lee. Harmful Algae 5: 20–25.
Nikulina, V.N. 2003. Seasonal dynamics of phytoplankton in the inner Neva Estuary in the 1980’s and 1990’s. Oceanologia 45: 25–39.
Nixon, S.W. 1995. Coastal marine eutrophication: A definition, social consequences and future concerns. Ophelia 41: 199–220.
Oczlowski, A.J., S.W. Nixon, S.L. Granger, A.M. El-Sayed, and R.A. McKinney. 2009. Anthropogenic enhancement of Egypt’s Mediterranean fishery. Proceedings of the National Academy of Science 106: 1364–1367.
Officer, C.B., T.J. Smayda, and R. Mann. 1982. Benthic filter feeding: A natural eutrophication control. Marine Ecology Progress Series 9: 203–210.
Oliviera, A.M., and B. Kjerfve. 1993. Environmental responses of a tropical coastal lagoon system to hydrological variability: Mundau-Manguaba, Brazil. Estuarine, Coastal and Shelf Science 37: 575–591.
Orr, P.T., G.J. Jones, and G.B. Douglas. 2004. Response of cultured Microcystis aeruginosa from The Swan River, Australia, to elevated salt concentration and consequences for bloom and toxin management in estuaries. Marine and Freshwater Research 55: 277–283.
Paerl, H.W. 1988. Nuisance phytoplankton blooms in coastal, estuarine and inland waters. Limnology and Oceanography 33: 823–847.
Paerl, H.W., K.L. Rossignol, S.N. Hall, B.L. Peierls, and M.S. Wetz. 2010. Phytoplankton community indicators of short- and long-term ecological change in the anthropogenically and climatically impacted Neuse River Estuary, North Carolina, USA. Estuaries and Coasts 33: 485–497.
Parker, M.L., and S.P. Geiger. 2009. Oyster monitoring in the northern estuaries on the Southeast coast of Florida, 68. St. Petersburg: Florida Fish and Wildlife Research Institute.
Parmer, K., K. Laskis, R. McTear, and R. Peets. 2008. TMDL Report: Nutrient and dissolved oxygen TMDL for the St. Lucie Basin. Tallahassee: Florida Department of Environmental Protection.
Parsons, T.R., Y. Maita, and C.M. Lalli. 1984. A manual of chemical and biological methods for seawater analysis. New York: Pergamon.
Phlips, E.J., M. Cichra, K. Havens, C. Hanlon, S. Badylak, B. Rueter, M. Randall, and P. Hansen. 1997. The control of phytoplankton abundance and structure by nutrient and light availability in a shallow subtropical lake. Journal of Plankton Research 19: 319–342.
Phlips, E.J., S. Badylak, and T.L. Lynch. 1999. Blooms of the picoplanktoni cyanobacterium Synechococcus in Florida Bay. Limnology and Oceanography 44: 1166–1175.
Phlips, E.J., N. Love, S. Badylak, P. Hansen, C.V. John, and R. Gleeson. 2004. A comparison of water quality and hydrodynamic characteristics of the Guana Tolomato Matanzas National Estuarine Research Reserve and the Indian River Lagoon in Florida. Journal of Coastal Research, Special Issue 45: 93–109.
Phlips, E.J., J. Hendrickson, E.L. Bledsoe, and M. Cichra. 2007. Meteorological influences on algal bloom potential in a nutrient-rich blackwater river. Journal of Freshwater Biology 52: 2141–2155.
Phlips, E.J., S. Badylak, M. Christman, and M. Lasi. 2010. Climatic trends and temporal patterns of phytoplankton composition, abundance and succession in the Indian River Lagoon, Florida, USA. Estuaries and Coasts 33: 498–513.
Phlips, E.J., S. Badylak, M. Christman, J. Wolny, J. Garland, L. Hall, J. Hart, J. Landsberg, M. Lasi, J. Lockwood, R. Paperno, D. Scheidt, A. Staples, and K. Steidinger. 2011. Scales of variability of harmful algae blooms in the Indian River, Florida, USA. Harmful Algae 10: 277–290.
Pinckney, J.L., D.F. Millie, B.T. Vinyard, and H.W. Paerl. 1997. Environmental controls of phytoplankton bloom dynamics in the Neuse River estuary, North Carolina, USA. Canadian Journal of Fisheries and Aquatic Sciences 54: 2491–2501.
Quinlan, E.L., and E.J. Phlips. 2007. Phytoplankton assemblages across the marine to low-salinity zone in a blackwater dominated estuary. Journal of Plankton Research 29: 410–416.
Quinlan, E.L., C.H. Jett, and E.J. Phlips. 2009. Microzooplankton grazing and the control of phytoplankton biomass in the Suwannee River and estuary, USA. Hydrobiologia 632: 127–137.
Rabalais, N.N., N. Atilla, C. Normandeau, and R.E. Turner. 2004. Ecosystem history of Mississippi River-influenced continental shelf revealed through preserved phytoplankton pigments. Marine Pollution Bulletin 49: 537–547.
Redfield, A.C., B.H. Ketchum, and F.A. Richards. 1963. The influence of organisms on the composition of seawater. In The Sea, ed. M.N. Hill, 26–77. New York: Wiley.
Reynolds, C.S. 2006. Ecology of phytoplankton. Cambridge: Cambridge University Press.
Rijstenbil, J.W. 1988. Selection of phytoplankton species in culture by gradual salinity changes. Netherlands Journal of Sea Research 22: 291–300.
Robson, B.J., and D.P. Hamilton. 2003. Summer flow event induces a cyanobacterial bloom in a seasonal Western Australian estuary. Marine and Freshwater Research 54: 139–151.
Rocha, C., H. Galvao, and A. Barbosa. 2002. Role of transient silicon limitation in the development of cyanobacteria blooms in the Guadiana estuary, south-western Iberia. Marine Ecology Progress Series 228: 35–45.
Santos, M.L.S., K. Muniz, B. Barros-Neto, and M. Aruajo. 2008. Nutrient and phytoplankton biomass in the Amazon River shelf waters. Annals of the Brazilian Academy of Sciences 80: 703–717.
Sartory, D.P., and J.U. Grobbelaar. 1984. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114: 177–187.
Sellner, K.G., R.V. Lacouture, and C.R. Parrish. 1988. Effects of increasing salinity on a Cyanobacterial bloom in the Potomac River estuary. Journal of Plankton Research 10: 49–61.
Sime, P. 2005. St. Lucie Estuary and Indian River Lagoon conceptual ecological model. Wetlands 25: 898–907.
Smayda, T.J. 1978. From phytoplankters to biomass. In Phytoplankton Manual, ed. A. Sournia, 273–279. Paris: UNESCO.
Smayda, T.J. 2008. Complexity in the eutrophication-harmful algal bloom relationship, with comment on the importance of grazing. Harmful Algae 8: 140–151.
Smetacek, V., and J. Cloern. 2008. On phytoplankton trends. Science 319: 1346–1348.
Smith Jr., W.O., and D.J. DeMaster. 1996. Phytoplankton biomass and productivity in the Amazon River plume: correlation with seasonal river discharge. Continental Shelf Research 16: 291–319.
Sun, D. 2009. Development of CH3D hydrodynamic/salinity model for the St. Lucie Estuary: Appendix D in Technical Document to Support a Water Reservation Rule for the North Fork of the St. Lucie River. West Palm Beach: South Florida Water Management.
Sun, D. 2011. Estimate of residence time using a box model approach. West Palm Beach: South Florida Water Management. Technical Memorandum.
Theichberg, M., S.E. Fox, Y.S. Olsen, I. Valiela, P. Martinettos, O. Iribarnes, E.Y. Muto, M.A.V. Petti, T.N. Corbisier, M. Soto-Jiménez, F. Páez-Osuna, P. Castro, H. Freitas, A. Zitelli, M. Cardinaletti, and D. Tagliapietrass. 2010. Eutrophication and macroalgal blooms in temperate and tropical coastal waters: nutrient enrichment experiments with Ulva spp. Global Change Biology 16: 2624–2637.
Thessen, A.E., Q. Dortch, M.L. Parsons, and W. Morrison. 2005. Effect of salinity on Pseudo-nitzschia species (Bacillariophyceae) growth and distribution. Journal of Phycology 41: 21–29.
Tonk, L., K. Bosch, P.M. Visser, and J. Huisman. 2007. Salt tolerance of the harmfu cyanobacterium Microcystis aeruginosa. Aquatic Microbial Ecology 46: 117–123.
Turner, R.E., N.N. Rabalais, and Z.Z. Nan. 1990. Phytoplankton biomass, production and growth limitations on the Huanghe (Yellow River) continental shelf. Continental Shelf Research 10: 545–571.
Utermohl, H. 1958. Zur vervollkommnung der quantitativen phytoplankton-methodik. Mitteilingen-Internationale Vereinigung fur Theoretische und Angewandte Limnologie 9: 1–38.
Valiela, I., J. McClelland, J. Hauxwell, P.J. Behr, D. Hersh, and K. Foreman. 1997. Macroalgal blooms in shallow estuaries: Controls and ecophysiological and ecosystem consequences. Limnology and Oceanography 42: 1105–1118.
Verspagen, J.M.H., J. Passarge, K.D. Johnk, P.M. Visser, L. Peperzak, P. Boers, H.J. Laanbroek, and J. Huisman. 2006. Water management strategies against toxic Microcystis blooms in the Dutch Delta. Ecological Applications 16: 313–327.
Williams, D.E., S.C. Dawe, M.L. Kent, R.J. Andersen, M. Craig, and C.F.B. Holmes. 1997. Bioaccumulation and clearance of microcystin from saltwater mussels, Mytilus edulis, and in vivo evidence for covalently bound microcystins and mussel tissues. Toxicon 35: 1617–1625.
Winder, M., and J.E. Cloern. 2010. The annual cycles of phytoplankton biomass. Philosphical Transactions of the Royal Society B 365: 3215–3226.
Yang, Y., Z. He, Y. Lin, C.A. Powell, E.J. Phlips, J. Yang, G. Chen, and P.J. Stofella. 2008. Temporal and spatial variations of nutrients in Ten Mile Creek of South Florida, USA and effects on phytoplankton biomass. Journal of Environmental Monitoring 10: 508–516.
Yunes, J.S., P.S. Salomon, A. Matthiensen, K.A. Beattie, S.L. Raggett, and G.A. Codd. 1996. Toxic blooms of cyanobacteria in the Patos Lagoon Estuary, Southern Brazil. Journal of Aquatic Ecosystem Health 5: 223–229.
Zingone, A., E.J. Phlips, and P. Harrison. 2010. Multiscale variability of twenty-two coastal phytoplankton time series: A global comparison. Estuaries and Coasts 33: 224–229.
Ackowledgements
We thank Joel Steward and Margaret Lasi of the St. Johns River Water Management District for their review of the manuscript. Special thanks to the O’Donnell family of Port St. Lucie for their logistical assistance. The research was funded by the South Florida Water Management District.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phlips, E.J., Badylak, S., Hart, J. et al. Climatic Influences on Autochthonous and Allochthonous Phytoplankton Blooms in a Subtropical Estuary, St. Lucie Estuary, Florida, USA. Estuaries and Coasts 35, 335–352 (2012). https://doi.org/10.1007/s12237-011-9442-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-011-9442-2