Abstract
Ten Mile Creek (TMC) is a major tributary of the Indian River Lagoon (IRL), one of the largest and most ecologically diverse estuaries of the east coast of Florida. Recent algal blooms within the IRL have focused attention on the role of different watersheds playing in the supply of growth-limiting nutrients. The goal of this study was to determine the nutrient-limiting status of the TMC outflow, which is influenced by both agricultural input and urban development. Four laboratory experiments were conducted with water samples from TMC, adding different concentrations of phosphorus (P) and nitrogen (N) under controlled conditions. The results showed that turbidity and phytoplankton biomass (in terms of chlorophyll a concentration) in TMC water samples were responsive to N additions. Turbidity and phytoplankton biomass increased with addition of available N, but were not affected by addition of reactive P. The results indicate that available N is the limiting nutrient for the growth of phytoplankton in the TMC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutrients entering rivers, lakes, and oceans from surface runoff water of agricultural lands and urban areas have become a major environmental concern around the world (Nixon, 1995). Eutrophication of aquatic environments stimulates primary production and leads to deleterious impacts on the structure and function of ecosystems, including the proliferation of harmful algal blooms (Paerl, 1988; Rosenberg et al., 1990; Harding, 1994; Vargo et al., 1996; Katz et al., 1999; Mortazav et al., 2000; Phlips, 2002; Bledsoe et al., 2004; Webster et al., 2005). Some algal bloom species produce toxins that can enter the food chain, harming animal and even human health (Chorus & Bartram, 1999; Landsberg, 2002).
The occurrence and growth of phytoplankton in fresh waters are affected by nutrient level, and hydrological and meteorological conditions (An et al., 2003; Li et al., 2004; Jeppesen et al., 2007). The specific effects of increases in nutrient loads are strongly dependent on the structure of the ecosystem in question and the nature of nutrient availability prior to changes in load. In many ecosystems, phytoplankton biomass is correlated with the availability of nitrogen (N) or phosphorus (P) (Hecky & Kilham, 1988; Tomasky & Valiela, 1995; Phlips et al., 1997; Sarnelle et al., 1998; Bledsoe & Phlips, 2000; Cloern, 2001; Bledsoe et al., 2004). The composition of phytoplankton species are also affected by the concentrations of N and P (Smayda & Reynolds, 2001; Reynolds, 2006). The eutrohpication thresholds of P for fresh waters (rivers and lakes) are from 20 to 100 μg P l−1 and of N from 500 to 1,000 μg N l−1. In Florida, these critical values are 10 μg P l−1 and 520 μg N l−1 for lakes and reservoirs and 40 μg P l−1 and 900 μg N l−1 for rivers and streams (US EPA, 2000). Water bodies with naturally low P or N concentration will, therefore, be highly sensitive to external inputs of P or N (Ruley & Rusch, 2002; An, 2003; Vos & Roos, 2005). In addition, the ratio of N:P in the water body (referred to as the “Redfield ratio”) is an important indicator of which nutrient is limiting eutrophication. If the Redfield ratio is 16:1, P is most likely the limiting factor for algal growth; lower ratios indicate that N is of great importance (Redfield et al., 1963; Hodgkiss & Lu, 2004).
Phosphorus has been shown to be the principal limiting nutrient for primary production of phytoplankton in many freshwater environments (Schindler, 1978; Vollenweider & Kerekes, 1980; Hecky & Kilham, 1988; Tilzer, 1990; Tomasky & Valiela, 1995; Phlips, 2002), while N is commonly limiting in marine ecosystems (Cloern, 2001), however, there are many exceptions to this general pattern. In some freshwater environments, particularly in the tropics and subtropics, N has been found to be the primary limiting nutrient for phytoplankton production, due in large part to excessive P load and long growing seasons (Toerien et al., 1975; Henry et al., 1985; Thornton, 1987; Rast et al., 1989; Phlips et al., 1997; Phlips, 2002). Likewise, some marine environments may become predominantly P-limited due to an excess of N availability (Phlips et al., 1999).
The Indian River Lagoon (IRL), a nutrient-rich environment that stretches 350 km along the east coast of Florida, is one of the most important water ways in Florida, and its health has been a concern for years due to growing pressures from anthropogenic sources of nutrients and pollutants (Chamberlain & Hayward, 1996; Phlips et al., 2002). One of the most important watersheds flooding into the southern IRL is Ten Mile Creek (TMC) (Fig. 1), which annually contributes approximately 150,000 acre-feet (or 185.2 million m3) of water to the IRL. TMC has been identified as having poor water quality due to pesticide and nutrient contamination from soil and stormwater runoff (Hand et al., 1994; Chamberlain & Hayward, 1996). The sources of N and P for TMC include runoff not only from agricultural activities, but also from rapidly growing commercial and residential communities in the region. Mean total N (TN) and total P (TP) concentrations in TMC range from 385 to 1,058 μg l−1 and 158 to 336 μg l−1, respectively (Powell & He, unpublished; Phlips et al., 2002). The relative TN and TP levels in TMC suggest the existence of N-limiting conditions for phytoplankton growth.
The environmental impacts of eutrophication on water quality, and subsequently on human and animal health, have resulted in major efforts to develop management strategies to reduce nutrient inputs to surface waters and to remediate eutrophic water bodies. Controlling eutrophication requires both N and P enrichment of surface waters be minimized. The objective of this study was to test this hypothesis using nutrient limitation bioassay methods, as a means of determining the relative bioavailability of N and P entering TMC via the watershed. This information is essential to the development best management practices to reduce N or P transport from land to surface waters and models for the prediction of future trends in eutrophication of TMC and the downstream environments.
Materials and methods
Physical and chemical characteristics
The water used in the nutrient limitation bioassay experiments was collected from Site 2, one of seven sites in an on-going monitoring study of water quality of the St. Lucie estuary and TMC. This site is strongly influenced by surface water runoff from a watershed dominated by agricultural land uses and urban development. Water was collected with a grabbing sampler at 0.5 m from the surface. The electrical conductivity (EC), dissolved oxygen (DO), and temperature of the water samples were determined using an YSI environmental multi-probe in field. Water pH and turbidity were measured immediately after water samples were transported into the lab using a pH/ion/conductivity meter (pH/Conductivity Meter, Model 220, Denver Instrument, Denver, CO) and a Turbidity meter (DRT 100B, HF Scientific, Inc., USA), respectively. Total dissolved solid (TDS) concentrations of unfiltered water samples were measured using a gravimetric method. The concentrations of NO −3 -N, NH +4 -N, total Kjeldahl N (TKN), total N (TN), PO −34 -P, dissolved total P (DTP), reactive P, and TP of water were determined following EPA standard methods (USEPA 300.0 for NO −3 -N; USEPA 350.1 for NH +4 -N and TN; USEPA 351.2 for TKN; USEPA 300.0 for PO −34 -P; USEPA 200.7 for DTP; USEPA 365.1 for reactive P; and USEPA 365.2 for TP) using an inductively couple plasma atomic emission spectrometer (ICP-AES) (Ultima, YJ Horiba, Inc., Edison, N.J.), a N/P Discrete Autoanalyzer (EasyChem, Systea Scientific LLC., Oak Brook, IL), and an Ion Chromatograph (IC) (DX 500, Dionex Corporation, Synnyvale, CA), respectively.
For chlorophyll a determination, water samples were filtered on to glass fiber filters (47 mm, 0.7 μm, Whatman, GF/F). The filters were frozen at −20°C prior to extraction. The samples were removed from the freezer (−70°C), thawed at room temperature for 15 min, then, placed into the appropriate test tubes. Chlorophyll a was extracted using the hot ethanol method (Sartory & Grobbelaar, 1984; APHA, 1998; Bledsoe & Phlips, 2000; Phlips et al., 2000). Absorption (Å) values of the extracts were determined using a spectrophotometer (Digital Ab. Hitachi, model U-2810) in accordance with standard methods (APHA, 1998).
Nutrient limitation bioassay experiments
The nutrient limitation experiments were conducted in a climate controlled culture room at the Indian River Research and Education Center, University of Florida, Ft. Pierce, FL. Twenty-one 37.85-l (10-gallon) glass aquaria were used as the experimental mesocosms. Water samples collected from TMC were pooled together in a large container, mixed well, and divided among the tanks (20 l per tank). Tanks were aerated with a SL56 Sweetwater air pump (Aquatic Eco-System, Inc., Apopka, FL, USA). The temperature was maintained at 23 ± 1°C, and the light intensity above the surface of the tanks was 52 ± 6 μmol m−2 s−1, with a photoperiod of 12:12 (dark: light, hours).
Four experimental treatment groups were established for the study: (1) various N concentrations added to the TMC water, (2) various P concentrations added to the TMC water, (3) various N concentrations with an enforced higher available P level added to the TMC water, and (4) various P concentrations with an enforced higher available N level added to the TMC water. The sources of available N (NO −3 and NH +4 -N) and P (PO −34 -P) used in the experiment were ammonium nitrate (NH4 NO3) (for N) and sodium phosphate monobasic dehydrate (NaH2PO4 H2O) (for P) (Sigma, St. Louis, MO, USA).
The first experiment was initiated on March 02, 2006 and included six N levels, i.e., 187.5, 375, 750, 1,500, 3,000, and 6,000 μg N l−1. The second experiment was initiated on April 04, 2006 with six P levels, i.e., 62.5, 125, 250, 500, 1,000, and 2,000 μg P l−1. The third experiment was initiated on June 01, 2006, with six N levels (187.5, 375, 750, 1,500, 3,000, and 6,000 μg N l−1) and an enforced higher-background reactive-P level (1,000 μg P l−1) by adding sodium phosphate. The fourth experiment started on July 19, 2006 with six reactive P levels (150, 300, 600, 1,200, 2,400, 4,800 μg P l−1) and an enforced higher-background available N concentration (750 μg N l−1) by adding ammonium nitrate. The final concentrations of available N and/or reactive P in the treatments in 20 l of water were obtained by analyzing the back ground available N and reactive P levels in the water and adding supplement N and P amount to it. A control (TMC water without any nutrient additions) was included in each of the four experiments. There were three replicates for each treatment (including the control), and all the tanks were arranged randomly with a factorial design.
Water samples from the tanks were collected at day 1, 7, 14, 21, 28, and 35 of the experiment and analyzed for physical, chemical, and biological properties. The amount of water lost from each tank due to evaporation and sampling was measured weekly and replenished with ultra-pure deionized water, which was obtained from a Barnstead Ultrapure Water System (Nanopure Infinity Inc.). Prior to each sampling, the water in the tanks was thoroughly mixed by stirring, and 1 l of water was sampled from each tank. Three hundred milliliters of each sample were used for chlorophyll a determination and 500 ml for determining the concentrations of P and N, and the changes in phytoplankton biomass were estimated in two ways, as chlorophyll a concentration and turbidity.
Results
Physical, chemical and biological characteristics
The range of values for selected water column characteristics observed on the four collection dates are shown in Table 1. Some parameters showed relatively small variation between sampling dates, including pH, electrical conductivity, salinity, dissolved oxygen, turbidity, TKN, TN, DTP, and TP. Other parameters showed an over 3-fold range of values, including nitrate, ammonium, orthophosphate, and chlorophyll a concentration.
Response of turbidity to N addition
The turbidity of the TMC water was most affected by the addition of N (Fig. 2A-1). Greater N addition resulted in higher turbidity of the water after 21 days of culture (Fig. 2A-1). T-test analysis showed that the turbidity of the TMC water treated with 750–6,000 μg l−1 of N was significantly higher than that of the control (P < 0.01). In the treatments with added P, N additions of 375–6,000 μg l−1 yielded higher turbidities than the control (Fig. 2A-1).
Comparison of water turbidity in the Ten Mile Creek (TMC) water with additions of nitrogen (N), phosphorus (P) or the combinations of N and P at 21 day after treatment. (A-1) Treatments of TMC water with addition of N, but no addition of P. The background reactive P (PO 3+4 -P) concentration was 0.13 mg l−1. (A-2) Treatments of TMC water with addition of N and P. The final reactive P concentration in all the treatments was 1.0 mg l−1. (A-1) and (A-2) 1, Control; 2, 0.1875 mg N l−1; 3, 0.375 mg N l−1; 4, 0.75 mg N l−1; 5, 1.5 mg N l−1; 6, 3.0 mg N l−1; 7, 6.0 mg N l−1). (B-1) Treatments of TMC water with addition of P, but no addition of N. The background available N concentration was 0.19 mg l−1. 1, control; 2, 0.0625 mg P l−1; 3, 0.125 mg P l−1; = 4, 0.25 mg P l−1; 5, 0.5 mg P l−1; 6, 1.0 mg P l−1; 7, 2.0 mg P l−1. (B-2) Treatments of TMC water with addition of P and N. The final available N in the treatments was 0.75 mg l−1. 1, control; 2, 0.3 mg P l−1; 3, 0.45 mg P l−1; 4, 0.6 mg P l−1; 5, 1.2 mg P l−1; 6, 2.4 mg P l−1; 7, 4.8 mg P l−1. Means ± SD (n = 3)
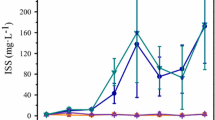
Over the 35-day culture period, water turbidities leveled off or declined after 21 days in the treatment group without P addition, with a maximum turbidity of 20 NTU (Fig. 4A-1). By contrast, for the treatment with P addition (1,000 μg l−1) turbidities in the 1,500–6,000 μg l−1 N addition groups increased throughout the entire culture period, with a maximum observed value of 35 NTU (Fig. 4A-2). This result indicates that the water samples in the group without P addition reached a condition of P-limitation in approximately 14–21 days of culture.
Response of turbidity to P addition
The turbidity of the TMC water was not affected by the addition of P (reactive P) (Fig. 2B-1). The turbidity of the TMC water treated with 62.5–2,000 μg l−1 of reactive P was not significantly different from that of the control at day 21 of culture (t-test, P < 0.05). In the treatment group with added available N (750 μg l−1), the turbidity of TMC water treated with additions of 450–4,800 μg l−1 reactive P were higher than the control (P < 0.05), but there were no significant differences in turbidity among the different levels of reactive P (Fig. 2B-2). Over the course of the 35-day culture period, turbidity increased moderately for all the treatment groups, including the control (Fig. 4B-1, B-2), perhaps reflecting release of N from the decomposition of existing biomass.
Response of chlorophyll a to N addition
Chlorophyll a concentrations in TMC water treated with available N additions of 373–6,000 μg l−1 were significantly higher than the control after 21 days of culture (Fig. 3A-1). Chlorophyll a concentrations were proportional to available N additions, up to 3,000 μg l−1. The same trend was observed for the relationship between available N addition and chlorophyll a concentration in the treatment group with reactive P addition (Fig. 3A-2).
Comparison of phytoplankton biomass (chlorophyll a, μg l−1) in the Ten Mile Creek (TMC) water with additions of nitrogen (N), phosphorus (P) or the combinations of N and P at 21 day after treatment. (A-1) Treatments of TMC water with addition of N, but without addition of P. The background reactive P (PO 3+4 -P) concentration was 0.13 mg l−1. (A-2) Treatments of TMC water with addition of N and P. The final reactive P concentration in all the treatments was 1,000 μg l−1. (A-1) and (A-2) 1, Control; 2, 0.1875 mg N l−1; 3, 0.375 mg N l−1; 4, 0.75 mg N l−1; 5, 1.5 mg N l−1; 6, 3.0 mg N l−1; 7, 6.0 mg N l−1. (B-1) Treatments of TMC water with addition of P, but no addition of N. The background available N concentration was 0.19 mg l−1. 1, control; 2, 0.0625 mg P l−1; 3, 0.125 mg P l−1; 4, 0.25 mg P l−1; 5, 0.5 mg P l−1; 6, 1.0 mg P l−1; 7, 2.0 mg P l−1. (B-2) Treatments of TMC water with addition of P and N. The available N concentration in all the treatments was 0.75 mg l−1. 1, Control; 2, 0.3 mg P l−1; 3, 0.45 mg P l−1; 4, 0.6 mg P l−1; 5, 1.2 mg P l−1; 6, 2.4 mg P l−1; 7, 4.8 mg P l−1. Means ± SD (n = 3)
Over the course of culture, chlorophyll a concentrations increased over time for up to 21–28 days at the higher N addition levels (Fig. 5A-1, A-2). In the treatment groups without additional reactive P, chlorophyll a levels peaked at 210 μg l−1 for the 6,000 μg l−1 available N addition group, after 21 days of culture. In the treatment groups with P added, chlorophyll a levels peaked at 280 μg l−1 for the 6,000 μg l−1 N addition group, after 28 days of culture.
Response of chlorophyll a to phosphorus additions
Chlorophyll a concentration of the TMC water was not affected by the addition of reactive P (Fig. 4B-1). Chlorophyll a concentrations in the TMC water treated with 62.5–2,000 μg l−1 of reactive P were not significantly different from that of the control at day 21 of culture (t-test, P < 0.05). In the treatment groups with added available N (750 μg l−1), chlorophyll a concentrations in the TMC water treated with additions of 300–4,800 μg l−1 reactive P were higher than the control (P < 0.05), but there were no significant differences in the turbidity among the reactive P addition groups (Fig. 2B-2). Over the course of the 35-day culture period, chlorophyll a concentrations increased moderately for all treatment groups, including the control (Fig. 5B-1, B-2).
Changes in N and P concentration over the culture period
Available N concentrations in water of the N treatment groups and the control declined over the culture period. In the treatment groups without added P, available N (NO3-N and NH4-N) concentrations declined to below detection limit (10 and 15 μg l−1 for NO3-N and NH4-N, respectively) in all addition groups except 6,000 μg l−1 over the 35-day culture period (Fig. 6A-1). The same result was observed for the treatment groups with reactive P added (Fig. 6A-2).
Changes in available nitrogen (NH4-N plus NO3-N) concentration in the TMC water with additions of nitrogen, phosphorus, or in combinations at different concentrations under laboratory conditions. (A-1) N addition only; (A-2) N addition plus 1.000 mg P l−1; (B-1) P addition only; (B-2) P addition plus 0.750 mg N l−1
Available N concentrations in water of the reactive P treatment groups and the control declined to detection limits within 14 days of the culture period (Fig. 6B-1). In the treatment group without added N, available N concentrations declined to near detection limits for most addition groups (Fig. 6B-2).
Reactive P concentrations in the N addition treatment groups and control declined over time in the higher N addition samples (Fig. 7A-1). In the treatment group with added P, P concentrations declined moderately over the culture period, except in the control group (Fig. 7A-2).
Changes in available phosphorus (reactive phosphorus) concentrations in the TMC water with additions of nitrogen, phosphorus, or in combinations at different concentrations under laboratory conditions. (A-1) N addition only; (A-2) N addition plus 1.000 mg P l−1; (B-1) P addition only; (B-2) P addition plus 0.750 mg N l−1
Reactive P concentrations in the P addition treatment groups remained relatively constant (Fig. 7B-1). In the treatment group wit added N, P concentrations declined only in the 4,800 μg l−1 addition group (Fig. 7B-2).
Discussion
The results of this study show that the phytoplankton biomass, in terms of turbidity and chlorophyll a concentrations, in TMC water are responsive to the addition of available N, but not reactive P, indicating that available N is the primary limiting nutrient for the growth of phytoplankton in this body of water. This result agrees with previous finding that in the southern Indian River Lagoon (IRL), N is the primary limiting nutrient affecting the abundance of phytoplankton (Phlips et al., 2002). The basis for N limitation in TMC and IRL is the high P emanating from the watershed where high P concentrations were detected from surface runoff water from agricultural fields (He et al., 2003). Reactive P levels in the TMC are well above the critical P concentration identified by the US EPA as excessive (i.e., 40 μg l−1), while available N levels are below the critical level (900 μg l−1) (Grobbelaar & House, 1995; USEPA, 2000; Phlips et al., 2002). Over the study period, the TMC water contained 120–326 μg l−1 of reactive P, but only 0–150 μg l−1 of NO −3 -N and 0.007–193 μg l−1 of NH +4 -N.
If phytoplankton production follows the general stoichiometry defined by the Redfield Ratio, i.e., 7 g N:1 g P (Redfield et al., 1963), reactive P levels observed in TMC are roughly an order of magnitude higher than that the available N levels can support, in terms of phytoplankton production. In the bioassay experiments, available N enrichment (up to 1,500–6,000 μg l−1) should be sufficient to cause P limitation. The consumption of added available N by phytoplankton was rapid. For example, in the 1,500 μg l−1 available N addition treatment (the N:P ratio was about 12.5, much higher than 7:1), the available N in the water was completely depleted in less than 3 weeks of phytoplankton growth. These uptake relationships would be expected from the Redfield Ratio, which predicts that 120–300 μg l−1 of reactive P should be used up with the addition of 840–2,100 μg l−1 of bio-available N. Given typical literature values of chlorophyll/dry weight and P/dry weight ratios in freshwater phytoplankton, which range from 0.5 to 2 and 0.8 to 1.45, respectively (Reynolds, 2006), the reactive P observed in TMC should roughly yield 100–300 μg l−1 of chlorophyll. The increases in chlorophyll observed in the bioassays for the highly available N enrichment treatments fell within this expected range. This observation indicates that reactive P load to the SLE and IRL from TMC represents a substantial nutrient potential for algal growth and bloom formation. The highest levels of chlorophyll observed in TMC during the study period fell within the range of 25–35 μg l−1, far below the potential represented by the reactive P present in the water, highlighting the effects of N limitation on phytoplankton growth. Outside of the peak periods of phytoplankton biomass, chlorophyll concentrations in TMC were typically below 10 μg l−1, most probably a reflection of the additional effects of short water residence times within TMC limiting the time available for the accumulation of phytoplankton biomass, as observed in other lotic ecosystems (Phlips et al., 2007). The results also highlight the fact that further increases in available N load to the ecosystem would be expected to greatly enhance algal bloom potential in the St. Lucie estuary and IRL, given the continued presence of surplus P emanating from the agriculturally enriched watersheds of TMC. Currently, great emphasis was placed on the reduction of P loads into fresh water bodies (St. Lucie Estuary and Indian River Lagoon). Results from this study strongly indicate that P load reduction is important, but N load reduction is probably more critical for controlling algal boom in these water bodies. Therefore, the development of best management practices in the St. Lucie watershed should take consideration of reducing loads for both N and P.
References
An, K. G., 2003. Determination of a limiting nutrient regulating algal biomass using in situ experiments of nutrient enrichment bioassay (NEB) and empirical relations of nutrients and chlorophyll-a. Journal of Environmental Biology 24: 229–239.
An, K. G., S. S. Park, K. H. Ahn & C. G. Urchin, 2003. Dynamics of nitrogen, phosphorus, algal biomass, and suspended solids in an artificial lentic ecosystem and significant implications of regional hydrology on trophic status. Journal of Environmental Biology 24: 29–38.
APHA, 1998. Standard Methods for the Analysis of Water and Wastewater, 17th ed. American Public Healthy Association, Washington, DC, USA: 1067–1074.
Bledsoe, E. L. & E. J. Phlips, 2000. Relationship between phytoplankton standing crop and physical, chemical and biological gradient in the Suwannee River and plume region, USA. Estuaries 23: 458–473.
Bledsoe, E. L., E. J. Phlips, C. E. Jett & K. A. Donnelly, 2004. The relationships among phytoplankton biomass, nutrient loading and hydrodynamics in an inner-shelf estuary. Ophelia 58: 29–47.
Chamberlain, R. & D. Hayward, 1996. Evaluation of water quality and monitoring in the St. Lucie Estuary. Journal of American Water Research Association 32: 681–696.
Chorus, I. & J. Bartram (eds), 1999. Toxic Cyanobacteria in Water: A Guide to Public Health Significance, Monitoring and Management. Chapman & Hall, London.
Cloern, J. E., 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series 210, 223–253.
Grobbelaar, J. U. & W. A. House, 1995. Phosphorus as a limiting resource in inland waters; interactions with nitrogen. In H. Tiessen (ed.), Phosphorus in the Global Environment: Transfers, Cycles and Management. Wiley, New York: 255–276.
Hand, J., J. Col & E. Grimison, 1994. Southeast and South Florida District Water Quality Assessment 1994 305(b) Technical Appendix. FDEP, November 1994.
Harding, L. W. Jr., 1994. Long-term trends in the distribution of phytoplankton in Chesapeake Bay: roles of light, nutrients and streamflow. Marine Ecology Progress Series 104: 267–291.
He, Z. L., M. K. Zhang, D. V. Calvert, P. J. Stoffella & Y. C. Li, 2003. Loading of phosphorus in surface runoff in relation to management practices and soil properties. Soil Crop Science Society of Florida Proceedings 62: 12–19.
Hecky, R. E. & P. Kilham, 1988. Nutrient limitation of phytoplankton in freshwater and marine environments: A review of recent evidence on the effect of enrichment. Limnology and Oceanography 33: 796–822.
Henry, R., K. Hino, J. G. Tundisi & J. S. B. Riberio, 1985. Responses of phytoplankton in Lake Jacaretinga to enrichment with nitrogen and phosphorus in concentrations similar to those of the River Solimoes (Amazon, Brazil). Archiv Für Hydrobiologie 103: 453–477.
Hodgkiss, I. J. & S. H. Lu, 2004. The effects of nutrients and their ratios on phytoplankton abundance in Junk Bay, Hong Kong. Hydrobiologia 512: 215–229.
Jeppesen, E., M. Sondergraard, M. Meerhoff, T. L. Lauridsen & J. P. Jensen, 2007. Shallow lake restoration by nutrient loading reduction-some recent findings and challenges ahead. Hydrobiologia 584: 239–252.
Katz, B. G., H. D. Hornsby, J. E. Bohlke & M. F. Mokray, 1999. Sources and chronology of nitrate contamination in spring water, Suwannee River basin, Florida. U.S. Geological Survey, Water Resources Investigations Reports 99-4252. Tallahassee, FL.
Landsberg, J. H., 2002. The effects of harmful algal blooms on aquatic organisms. Reviews in Fisheries Science 10: 113–390.
Li, Y. S., X. Chen, O. W. H. Wai & B. King, 2004. Study on the dynamics of algal bloom and its influence factors in Tolo Harbour, HongKong. Water Environmental Research 76: 2643–2654.
Mortazav, B., R. L. Iverson, W. M. Landing, G. G. Lewis & W. Huang, 2000. Control of phytoplankton production and biomass in a river-dominated estuary: Apalachicola Bay, Florida, USA. Marine Ecology Progress Series 198: 19–31.
Nixon, S. W., 1995. Coastal marine eutrophication: a definition, social causes, and future concerns. Ophelia 41: 199–219.
Paerl, H. W., 1988. Nuisance phytoplankton blooms in coastal estuaries and inland waters. Limnology and Oceanography 33: 823–847.
Phlips, E. J., 2002. Algae and eutrophication. In Bitton, G. (ed.), Encyclopedia of Environmental Microbiology. Wiley, New York.
Phlips, E. J., S. Badylak & T. Grosskopf, 2002. Factors affecting the abundance of phytoplankton in a restricted subtropical lagoon, the Indian River Lagoon, Florida, USA. Estuarine, Coastal and Shelf Science 55: 385–402.
Phlips, E. J., S. Badylak & T. C. Lynch, 1999. Blooms of the picoplanktonic cyanobacterium Synechococcus in Florida Bay, a subtropical inner-shelf lagoon. Limnology and Oceanography 44: 1166–1175.
Phlips, E. J., M. Cichra, E. J. Aldridge & J. Jembeck, 2000. Light availability and variations in phytoplankton standing crops in a nutrient-rich black-water river. Limnology and Oceanography 45: 916–929.
Phlips, E. J., M. Cichra, K. Havens, C. Hanlon, S. Badylak, B. Rueter, M. Randall & P. Hansen, 1997. Relationships between phytoplankton dynamics and the availability of light and nutrients in a shallow subtropical lake. Journal of Plankton Research 19: 319–342.
Phlips, E. J., J. Hendrickson, E. L. Quinlan & M. Cichra, 2007. Meteorological influences on algal bloom potential in a nutrient-rich blackwater river. Freshwater Biology 52: 2141–2155.
Rast, W., V. H. Smith & J. A. Thornton, 1989. Characteristics of eutrophication. In Ryding, S., & W. Rast (eds), The Control of Eutrophication of Lakes and Reservoirs. Unesco, Paris, and the Parthenon Publishing Group, Park Ridge, NJ: 37–64.
Redfield, A. C., B. H. Ketchum & F. A. Richards, 1963. The influence of organisms on the composition of seawater. In Hill, M. N. (ed.), The Sea, Vol. 2. Wiley-Interscience, New York: 26–77.
Reynolds, C., 2006. Ecology of Phytoplankton. Cambridge University Press, Cambridge: 535 pp.
Rosenberg, R., E. Elmgren, S. Fleischer, P. Jonsson, G. Persson & H. Dahlin, 1990. Marine eutrophication studies in Sweden. Ambio 19: 102–108.
Ruley, J. E. & K. A. Rusch, 2002. An assessment of long term post-restoration water quality trends in a shallow, subtropical, urban hypertrophic lake. Ecological Engineering 19: 265–280.
Sarnelle, O., S. D. Cooper, S. Wiseman & K. M. Mavuti, 1998. The relationship between nutrients and trophic-level biomass in turbid tropical ponds. Freshwater Biology 40: 65–75.
Sartory, D. P. & J. U. Grobbelaar, 1984. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114: 177–187.
Schindler, D. W., 1978. Factors regulating phytoplankton production and standing crops in the worlds freshwater. Limnology and Oceanography 23: 478–486.
Smayda T. J. & C. S. Reynolds, 2001. Community assembly in marine phytoplankton: application of recent models to harmful dinoflagellate blooms. Journal of Plankton Research 23: 447–461.
Thornton, J. A., 1987. Aspects of eutrophication management in tropical/subtropical regions: a review. Journal of Limnology of the Society of South Africa 13: 25–43.
Tilzer, M. M., 1990. Environmental and physiological control of phytoplankton productivity in large lakes. In Tilzer M. M. & C. Serruya (eds), Large Lake, Ecological Structure and Function. Springer-Verlag, Berlin: 339–367.
Toerien, D. F., K. L. Hyman & M. J. Brewer, 1975. A preliminary trophic status classification of some South African impoundments. Water South Africa 1: 15–23.
Tomasky, G. & I. Valiela, 1995. Nutrient limitation of phytoplankton growth in Waquoit Bay, Massachusetts. Biological Bulletin 189: 257–258.
United States Environmental Protection Agency (USEPA), 2000. Ambient Water Quality Criteria Recommendations, Information Supporting the Development of State and Tribal Nutrient Criteria: Rivers and Streams in Nutrient Ecoregion XII. EPA 822-B-00-021. Office of Water, Washington, DC.
Vargo, G. A., R. Erdman, G. Kleppel & C. Burkhart, 1996. Controls on phytoplankton populations in Florida Bay: clams and eggs. Florida Bay Science Conference. University of Florida and Sea Grant of Florida: 97–99.
Vollenweider, R. A. & J. Kerekes, 1980. The loading concept as basis for controlling eutrophication, philosophy and preliminary results of the OECD program on eutrophication. Progress in Water Technology 12: 5–38.
Vos, A. T. & J. C. Roos, 2005. Causes and consequences of algal blooms in Loch Logan, an urban impoundment. Water South Africa 31: 385–392.
Webster, I. T., N. Rea, A. V. Padovn, P. Dostine, S. A. Townsend & S. Cook, 2005. An analysis of primary production in the Daly River, a relatively unimpacted tropical river in northern Australia. Marine and Freshwater Research 56: 303–316.
Acknowledgment
This study was, in part, supported by a grant from the St. Lucie River Issues Team Program (contract # OT050690).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: L. Naselli-Flores
Rights and permissions
About this article
Cite this article
Lin, Y., He, Z., Yang, Y. et al. Nitrogen versus phosphorus limitation of phytoplankton growth in Ten Mile Creek, Florida, USA. Hydrobiologia 605, 247–258 (2008). https://doi.org/10.1007/s10750-008-9360-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-008-9360-x