Abstract
A haloarchaeal strain G41 showing lipolytic activity was isolated from the saline soil of Yuncheng Salt Lake, China. Biochemical and physiological characterizations along with 16S rRNA gene sequence analysis placed the isolate in the genus Haloarcula. Lipase production was strongly influenced by the salinity of growth medium with maximum in the presence of 20 % NaCl or 15 % Na2SO4. The lipase was purified to homogeneity with a molecular mass of 45 kDa. Substrate specificity test revealed that it preferred long-chain p-nitrophenyl esters. The lipase was highly active and stable over broad ranges of temperature (30–80 °C), pH (6.0–11.0), and NaCl concentration (10–25 %), with an optimum at 70 °C, pH 8.0, and 15 % NaCl, showing thermostable, alkali-stable, and halostable properties. Enzyme inhibition studies indicated that the lipase was a metalloenzyme, with serine and cysteine residues essential for enzyme function. Moreover, it displayed high stability and activation in the presence of hydrophobic organic solvents with log P ow ≥ 2.73. The free and immobilized lipases from strain G41 were applied for biodiesel production, and 80.5 and 89.2 % of yields were achieved, respectively. This study demonstrated the feasibility of using lipases from halophilic archaea for biodiesel production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipases (EC 3.1.1.3) are ubiquitous hydrolytic enzymes that preferentially hydrolyze triglycerides composed of long-chain fatty acids and catalyze the reverse reaction under certain conditions (Teo et al. 2003). They have diverse industrial applications ranging from use in detergent and paper manufacturing to the production of structured lipids, biodiesel, and biosurfactants (Jaeger et al. 1999). However, industrial processes often require aggressive reaction conditions. For example, wastewaters may contain high salt contents, and high temperatures may be required either to favor stereoselectivity or simply to solubilize high-melting-point lipids in biocatalysis processes. Furthermore, catalysis with lipases is often carried out within organic solvents in order to promote synthesis reactions by providing a low-water environment (Lima et al. 2004a, b). Many lipases lose their activity rapidly under these conditions, and therefore, novel lipases with better catalytic efficiency and specific properties suitable for special reaction conditions are highly demanded.
Halophilic archaea are characterized as the extremophiles that grow from around 8 % (1.5 mol/L) sodium chloride to approximately 36 % (5 mol/L) NaCl, which is at saturation for NaCl (Litchfield 2011). Based on the unique stability of archaeal enzymes under high temperature, salt concentration, and extreme pH, they are expected to be a very powerful tool in industrial biotransformation processes that run under harsh conditions (Ozcan et al. 2009). Since salt tends to greatly reduce water activity, enzymes from halophilic archaea may become the choice for biocatalytic processes performed in low-water activity environments like aqueous/organic and nonaqueous media (Sellek and Chaudhuri 1999). Many lipases from bacteria and fungi have been purified and characterized; however, reports about the haloarchaeal lipases were scarce. Recently, screening for lipase activity in halophilic archaea isolated from the Sebkha of El Golea (Algerian Sahara) was carried out (Bhatnagar et al. 2005). This work reported the discovery of the first true lipase from Archaea domain, and a preliminary characterization was later performed using a crude enzymatic preparation obtained from Natronococcus sp. (Boutaiba et al. 2006). Besides, several haloarchaeal strains showing lipolytic activities were reported (Camacho et al. 2009; Ozcan et al. 2009). However, up to date, few lipase from halophilic archaea has been purified and characterized (Müller-Santos et al. 2009), and meanwhile, the potential usefulness of haloarchaeal lipases in biotechnological processes is still an open question.
In this paper, a haloarchaeal strain G41 producing extracellular lipase was isolated and identified. The purification and characteristics of the lipase, especially its activity and stability in the presence of organic solvents, were reported. In addition, the lipase was shown to be potentially useful for biodiesel production based on its excellent organic solvent tolerance.
Materials and methods
Strain identification and culture conditions
The strain G41 was isolated from the saline soil of Yuncheng, China. Production of extracellular lipase was performed in the complex medium (CM) containing the following (g/L): casein peptone 7.5, yeast extract 10.0, sodium citrate 3.0, MgSO4 · 7H2O 20.0, KCl 2.0, FeSO4 · 7H2O 0.01, NaCl 200.0, and pH 7.0–8.0. Morphological, physiological, and biochemical characteristics of strain G41 were studied either on CM agar plate (2 % agar, w/v) or in CM broth plus 16 % NaCl. 16S ribosomal RNA (rRNA) gene of strain G41 was amplified using the primers 22 F (5′-ATTCCGGTTGATCCTGC-3′) and 1540R (5′-AGGAGGTGATCCAGCCGCAG-3′) (Xu et al. 2005), and it has been submitted to GenBank with the accession number JN112010. The strain was deposited at China Center of Industrial Culture Collection with the accession number CICC 10510.
Extracellular lipase production and effect of different salts
The haloarchaeal growth and extracellular lipase production of strain G41 were determined at different time intervals. It was inoculated in CM broth and incubated at 37 °C with shaking. Samples were withdrawn aseptically every 6 h, and strain growth along with lipase activity was measured using spectrometric method (Shimadzu model UV-160A).
Effect of salts on lipase production was determined in CM broth containing different concentrations of KCl, NaCl, Na2SO4, and NaNO3, respectively. After incubation at 37 °C for 120 h, the culture broths were centrifuged at 10,000 g for 15 min, and cell-free supernatant was used for lipase activity assay.
Lipase activity assay
Lipase activity was determined using p-nitrophenyl palmitate (p-NPP) as a substrate according to Winkler and Stuckmann (1979), with some modifications. The substrate p-NPP was dissolved in 2-propanol to give a final concentration of 1 mmol/L and mixed with 9 mL of 0.01-M Tris-HCl buffer (pH 8.0) containing gum arabic (0.1 %), Triton X-100 (0.6 %), and 16.3 % NaCl. The reaction was carried out at 70 °C by adding 20 μL of appropriately diluted enzyme solution to 240 μL of substrate solution after preincubation for 5 min, and incubation was continued for further 10 min. Following the addition of 100 μL of Na2CO3 solution (0.1 M) to stop the reaction, the amount of p-nitrophenol (p-NP) released was measured at 410 nm against a blank. One unit was defined as the amount of enzyme liberating 1 μmol of p-NP per minute under the standard assay conditions. The specific activity was expressed as the units of enzyme activity per milligram of protein.
Enzyme purification
Culture supernatant obtained by centrifugation was treated with solid ammonium sulfate to 75 % saturation and stirred overnight at 4 °C. The precipitate collected by centrifugation was dissolved in buffer A (10 mmol/L Tris-HCl containing 15 % NaCl, pH 8.0). After dialysis against Tris-HCl buffer (10 mmol/L, pH 8.0) overnight, the sample was applied to a Diethylaminoethyl (DEAE) cellulose column (2.5 cm × 30 cm). The bound proteins were eluted with a linear gradient of 0.1–1 mol/L NaCl in Tris-HCl buffer at a flow rate of 0.5 mL/min. Active fractions showing lipase activity were pooled and concentrated by freeze-drying. The resulting concentrate was dissolved in buffer A, and then loaded on a Sephacryl S-100 gel filtration column (1.6 cm × 60 cm). The samples were eluted with buffer A at a flow rate of 0.5 mL/min. Active fractions were pooled and used for further characterization. Molecular mass of the purified lipase was estimated using the same column, which was calibrated previously with bovine serum albumin (BSA, 67 kDa), ovalbumin (43 kDa), bovine carbonic anhydrase (29 kDa), and cytochrome C (12.4 kDa). Blue Dextran was used to determine the void volume of the column. Protein concentration was determined by the method of Bradford (1976), using BSA as standard.
Molecular mass determination
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed to determine the purity and molecular mass of the purified lipase on 12 % (w/v) polyacrylamide gel (Laemmli 1970). After electrophoresis, the gel was stained with Coomassie Brilliant Blue R-250.
Substrate specificity
To determine the substrate specificity of the lipase, p-nitrophenyl esters with different chain lengths (acetate, C2; butyrate, C4; hexanoate, C6; octanoate, C8; decanoate, C10; laurate, C12; myristate, C14; palmitate, C16; stearate C18) were used as the substrates with the final concentration of 1 mmol/L, respectively, and then, the released amounts of p-NP were measured at 410 nm using p-NPP method. Data were expressed as the percentage of the observed maximal activity obtained with p-nitrophenyl myristate (p-NPM) (C14).
Effects of metal ions and chemical reagents
Effects of different metal ions and chemical reagents (ethylenediaminetetraacetic acid (EDTA), phenylmethylsulfonyl fluoride (PMSF), phenylarsine oxide (PAO), diethyl pyrocarbonate (DEPC), β-mercaptoethanol) on the lipase activity were examined by preincubating the enzyme with them at 30 °C for 1 h, respectively, and residual activity was determined under the standard assay conditions. Lipase activity in the absence of any additives was taken as 100 %.
Effects of temperature, pH, and NaCl concentration on lipase activity and stability
The temperature optimum of the purified lipase was determined under temperatures from 20 to 90 °C. To assess its thermostability, the enzyme was preincubated under different temperatures for 24 h, and residual activity was measured using p-NPP method as described above. Effect of pH was measured over a pH range of 5.0–11.0. The buffers (10 mmol/L) used were as follows: sodium acetate (pH 5.0–5.5), sodium phosphate (pH 6.0–7.5), Tris-HCl (pH 8.0–9.0), and glycine-NaOH (pH 9.5–11.0). The pH stability was examined by preincubating the enzyme at different pHs at 50 °C for 24 h, and residual activity was measured as described above. Effect of NaCl was determined by measuring the lipase activity in the reaction mixture containing different concentrations of NaCl (0–25 %). To determine its salt stability, the lipase was preincubated in Tris-HCl buffer (10 mmol/L, pH 8.0) containing various concentrations of NaCl at 50 °C for 24 h. The residual activity was measured under the standard assay conditions.
Lipase activity and stability in organic solvents
Effect of organic solvents with different log P ow values at 30 % (v/v) concentration on the purified lipase was determined by incubating the enzyme solution with different organic solvents at 30 °C with shaking, respectively. At different time intervals, aliquots were withdrawn, and residual activity was measured under the standard assay conditions. If residual activity was more than 50 % after 5 days, half-life was taken as “>5 days.” While activity was less than 50 % after 1 day, half-life was taken as “<1 day.”
Immobilization of the purified lipase
The purified lipase was lyophilized and dissolved in Tris-HCl buffer (10 mmol/L, pH 8.0). The method for immobilization of the lipase was described by Ji et al. (2010). Briefly, 1 g of macroporous anion exchange resin (Amberlite IRA-93, America) was added to 5 mL of solution containing 0.5 mg of purified lipase (about 80.1 units), and the mixture was stirred for 12 h at 20 °C with shaking at 150 rpm. The immobilized lipase was recovered through centrifugation, dried in a vacuum desiccator, and stored at 4 °C. The activity of the immobilized lipase was determined to be about 31.1 units/g. In addition, a blank using the resin without enzyme on p-NPP hydrolysis was performed to evaluate the effect of the anion exchange resin.
Production of biodiesel catalyzed by the lipase
The soybean oil was selected for lipase-catalyzed transesterification as described by Ji et al. (2010). Mixtures of soybean oil (6 mmol, about 5.4 g), methanol (24 mmol, 960 μL), and water (500 μL) were dissolved in tert-butanol (6 mL), and then, free (0.2 mg) or immobilized lipase (1 g) was added, respectively. Methanol was added by four steps according to Yu et al. (2013). Briefly, the first portion of methanol and 5.4 g of plant oil were added at the start of the reaction; the second, the third, and the fourth portions of methanol were added at an interval of 8 h. The transesterification reactions were carried out at 70 °C with shaking at 180 rpm. Aliquots (20 μL) of the reaction mixture were withdrawn at different time intervals, and then diluted with n-heptane for gas chromatograph (GC) analysis. In addition, the resin without lipase was used for transesterification reaction as a blank.
The methyl ester contents of the reaction mixture were measured on a GC (6820, Agilent) equipped with an SE-30 capillary column (0.33 μm × 0.25 mm × 30 m, Agilent). Nitrogen was used as carrier gas at a flow rate of 1.0 mL/min. The column temperature was kept at 200 °C for 1 min, raised to 240 °C at 4 °C/min, and then maintained at 240 °C for 6 min. The injector and detector temperatures were both set at 280 °C. Methyl esters of palmitic, stearic, oleic, and linoleic acids were purchased from Sigma as the standards and were detected by GC at 8.9, 11.8, 12.0, and 12.5 min, respectively.
Results and discussion
Strain identification and production of extracellular lipase
The strain G41 is a gram-negative, strictly aerobic, and motile rod. Colonies are red-pigmented on CM agar plate. It is able to grow in media containing 10–30 % (w/v) NaCl with optimum at 16–20 % NaCl. No growth was observed in the absence of NaCl. Thus, this strain was considered to be an extremely halophilic archaea (Kushner and Kamekura 1988). Optimal growth was observed at 37–40 °C and pH 6–8. Nitrate reduction, methyl red, catalase, Tween 60, starch, and gelatin hydrolysis are positive, while H2S production, indole production, Voges-Proskauer test, oxidase, and casein hydrolysis are negative. Acid is produced from starch, d-galactose, and inositol. As shown in Fig. 1, phylogenetic analysis based on 16S rRNA gene sequence comparisons revealed that the strain G41 belonged to the genus of Haloarcula and was most close to Haloarcula salaria HST01-2RT (97.4 % 16S rRNA gene sequence similarity). Thus, it was tentatively named as Haloarcula sp. G41.
Phylogenetic tree based on 16S rRNA gene sequence of the strain G41 to other members of the genus Haloarcula. Accession numbers of the sequences used in this study are shown in parentheses after the strain designation. Numbers at nodes are percentage bootstrap values based on 1,000 replications; only values greater than 50 % are shown. Bar 0.01 substitutions per nucleotide position
As shown in Fig. 2, the lipase was produced from the early-exponential phase (30 h) and reached a maximum level during the early-stationary phase (96 h). Moreover, extracellular lipase production of strain G41 was observed to be strongly influenced by the salinity of the culture medium (Table 1). Maximal lipase production (about 12.0 units/mL) occurred when 20 % NaCl or 15 % Na2SO4 was added. In addition, it was able to produce lipase in the presence of KCl. Although NaNO3 provided some haloarchaeal growth, no enzyme production was found. These results clearly revealed the halophilic nature of Haloarcula sp. G41, which the salt appeared to be a prerequisite for haloarchaeal growth and enzyme production. Similar behaviors were reported for other halophilic bacteria with the capability of producing extracellular enzymes (Coronado et al. 2000; Amoozegar et al. 2003).
Lipase purification
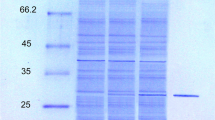
The extracellular lipase from strain G41 was well purified by ammonium sulfate precipitation, DEAE-Sepharose anion exchange chromatography, and Sephacryl S-100 gel filtration chromatography. It was purified 7.7-fold with 18.6 % recovery and a specific activity of 160.4 units/mg protein using p-NPP as the substrate (Table 2). The purified lipase showed a single protein band on SDS-PAGE with an estimated molecular mass of 45 kDa (Fig. 3, lane 2), corresponding with that determined by gel filtration. Together, these results indicated that the lipase was a single polypeptide chain. Similarly, Pérez et al. (2011) reported a novel lipase (LipBL) with a molecular mass of 45.3 kDa from a halophilic bacterium Marinobacter lipolyticus SM19, and it was high identity to class C β-lactamases. Although some halophilic archaea with lipolytic activity were obtained (Boutaiba et al. 2006; Ozcan et al. 2009), to the best of our knowledge, few haloarchaeal lipase has been purified and characterized (Müller-Santos et al. 2009).
Properties of the purified lipase
The substrate specificity of the lipase was determined using p-NP esters with different acyl chain lengths (Fig. 4). Maximal hydrolytic activity was obtained with p-NPM (C14). A trend of preferential specificity toward p-NP esters with acyl chain lengths longer than C10 is clearly evident. However, enzyme activity declined along with shorter chain length, reaching 30 % activity with p-NPH (C6), 20 % with p-NPB (C4), and 12 % with p-NPA (C2), respectively. Meanwhile, lipolytic activity on rhodamine B agar plates indicated that the purified lipase could hydrolyze olive oil (data not shown). These results confirmed the lipase nature of this enzyme, with it being more active on longer-chain fatty acid esters. The crude lipase from haloarchaeal strain Natronococcus sp. showed similar substrate specificity to ours (Boutaiba et al. 2006).
Effects of temperature, pH, and NaCl concentration
The temperature profile of lipase activity is shown in Fig. 5a. The purified lipase displayed optimal activity at 70 °C. Most of the known lipases from halophilic archaea have been reported to show maximal activities between 45 and 65 °C (Boutaiba et al. 2006; Camacho et al. 2009; Ozcan et al. 2009). Thus, the present lipase from Haloarcula sp. G41 can be rated among the higher thermoactive lipases. Moreover, excellent thermostability was observed over temperature ranging from 20 to 80 °C, and it was worth noting that the purified lipase retained more than 60 % activity after preincubating it under 90 °C for 24 h. These characteristics made it obviously different from other fungus lipases reported previously, which were usually neither active nor stable at temperatures above 70 °C (Dheeman et al. 2011; Ji et al. 2010). The excellent thermostability of the enzyme might be due to the presence of NaCl, which has been described in the lipase from Natronococcus sp. (Boutaiba et al. 2006). Thus, high activity and stability of purified lipase made it potentially useful in biocatalytic processes operating under high temperatures.
Effects of temperature (a), pH (b), and NaCl concentration (c) on activity (solid lines) and stability (dotted lines) of the purified lipase. Relative activity was defined as the percentage of activity detected with respect to the maximum enzyme activity. For determining the stability, the lipase activity without any treatment was taken as 100 %. Data are the average of three independent experiments. See “Materials and methods” for further details
Effect of pH on lipase activity and stability is shown in Fig. 5b. Optimal pH for the enzyme activity was 8.0. Meanwhile, it was highly stable in the pH range of 7.0–11.0 after 24-h incubation, indicating its alkali-stable nature. Similarly, Bhatnagar et al. (2005) found that the highest lipase activity of Natronococcus TC6 was at pH 7.0 for p-NPP hydrolysis; while in another study, Ozcan et al. (2009) reported that optimal lipase activities of five halophilic archaeal strains were at pH 8.0. Alkaline enzymes have received considerable interest because of their tremendous potentiality in industrial processes (Chakraborty et al. 2011).
Lipase activity was measured in the presence of different NaCl concentrations with optimum at 15 % NaCl (Fig. 5c). However, no activity was detected in the absence of NaCl. These results were quite interesting since they demonstrated that the lipase was completely adapted to high salt concentrations and probably optimized, at the molecular level, to be fully efficient only when salt was present in high amount. In fact, halophilic enzymes usually required the presence of NaCl or KCl concentrations in the range of 1–4 M for optimum activity and stability (Mevarech et al. 2000). They can be divided into two groups on the basis of whether or not their activity is irreversibly lost upon exposure to low ionic strength. Most of the halophilic enzymes studied so far are inactivated irreversibly. For example, β-galactosidase from Haloferax alikantei irreversibly lost its activity within minutes at NaCl concentrations below 0.5 M (Holmes et al. 1997). On the other hand, some halophilic enzymes recovered their activity upon reestablishment of the high salt concentration. Such was the case for the lipase of Natronococcus sp. (Boutaiba et al. 2006). The purified lipase of strain G41 clearly fell into the latter group since it recovered 82.5 % of the initial activity after treatment in the absence of NaCl for 24 h (Fig. 5c). This property is quite useful, since the enzyme purification can be run without salt using classical chromatographic methods such as ion exchange chromatography and the activity assayed on line in the presence of salt after reactivation. Furthermore, the lipase from strain G41 showed strong tolerance to NaCl. It was highly stable in the presence of NaCl concentrations from 2.5 to 25 %. Similar extreme halotolerance has been observed in other lipases from Natronococcus sp. (Boutaiba et al. 2006) and Haloarcula marismortui (Camacho et al. 2009).
Effects of metal ions and chemical reagents
As shown in Table 3, lipase activity was found to be stimulated in the presence of Ca2+ and Mg2+. Many lipases were reported to display enhanced activity in the presence of Ca2+ (Ji et al. 2010). A possible explanation for this phenomenon is that Ca2+ bound to the active site of the lipase and changes the conformation of the protein (Rahman et al. 2005). Conversely, Hg2+ inhibited the lipase with about 88.6 % activity lost, as reported for other lipases from Penicillium species (Dheeman et al. 2011). Strong inhibition by Hg2+ suggested the presence of cysteine residues in the enzyme (Sugihara et al. 1996).
Effects of some known enzyme inhibitors on the lipase activity were also studied. After incubation with EDTA, only 7.9 % of its original activity was retained, indicating that it was a metalloenzyme. β-Mercaptoethanol and DEPC showed only marginal effect in lipase activity, which indicated that disulfide bonds and histidine residues may be buried or less accessible in this lipase. However, significant inhibition by PMSF (a serine modifier) and PAO (a cysteine modifier) was observed, implying that serine and cysteine residues were essential for enzyme function. The crude lipase from Penicillium chrysogenum 9’ showed similar behavior in the presence of PMSF and EDTA (Bancerz et al. 2005).
Effect of organic solvents
High activity and stability of lipases in organic solvents are an essential prerequisite for applications in organic synthesis (Doukyu and Ogino 2010); hence, activity and stability in organic solvents are considered novel attributes in a lipase. So far, there have been some reports about organic solvent-tolerant lipases; however, published studies on the enzymatic behavior of lipases from halophilic archaea in nonaqueous media are scarce. As described above, the purified lipase from G41 was active and stable under high salinities, making it quite possible to remain stable in organic solvents.
Effect of various organic solvents on the activity and stability of the purified lipase is shown in Table 4. More than 80 % activity was retained in the presence of glycerol, DMSO, tert-butanol, cyclohexane, n-hexane, or isooctane compared to the control. Interestingly, glycerol and n-hexane even increased the lipase activity to 112.1 and 116.9 %, respectively. The activation of lipase could be explained that organic solvent molecules could interact with hydrophobic amino acid residues present in the lid that covers the catalytic site of the enzyme, thereby maintaining the lipase in its open conformation and conducting to catalyze (Rúa et al. 1993). Although there is a tendency for hydrophilic solvents to cause more significant enzyme inactivation than hydrophobic solvents (Doukyu and Ogino 2010), the lipase from strain G41 retained 50 % activity in the presence of hydrophilic solvents (Table 4).
Furthermore, the lipase displayed considerable stability in the presence of hydrophobic organic solvents (log P ow 2.73–4.7), which showed longer half-lives in these solvents than those in the absence of organic solvent or in the presence of hydrophilic solvents (Table 4). Together, these results indicated that the stability of the lipase was probably dependent on the polarity of the solvents, which increased only in hydrophobic organic solvents with higher log P ow values. These results were in agreement with the general behaviors of most lipases, which showed high tolerance toward hydrophobic solvents with significant instability in hydrophilic solvents (Dheeman et al. 2011; Lima et al. 2004a, b). The reasonably high stability of the purified lipase in organic solvents made it potentially useful for practical application in synthetic reactions with nonconventional media.
Application of the lipase for biodiesel production
Considering that the lipase was stable in organic solvents and its preferential specificity toward long-chain substrates, the lipase was deemed likely to be very suitable for biodiesel production. Short-chain alcohols, especially methanol, have low solubility in oils, and therefore, a new liquid phase appears in the system leading to an inactivation of the enzyme and decreased yields of ester. Therefore, tert-butanol was used as the solvent to reduce the influence of methanol and glycerol on the lipase (Royon et al. 2007). Meanwhile, the resin for enzyme immobilization was found to have no effect on p-NPP hydrolysis and transesterification reaction of the lipase.
Biodiesel production with 80.5 and 89.2 % yields was achieved by free and immobilized lipase, respectively (Fig. 6). The immobilized lipase was found to catalyze biodiesel synthesis in tert-butanol with higher yield, and this might be due to larger surface area of the immobilized lipase (Noureddini et al. 2005). However, free lipases were known to have mass transfer problem since these form aggregates in low-water media (Shah and Gupta 2007). In contrast to other lipases used in biodiesel production (Ji et al. 2010; Sivaramakrishnan and Muthukumar 2012; Yu et al. 2013), the lipase from strain G41 showed higher efficiency. Moreover, the values obtained were for an unoptimized process, and there are ample scopes to improve the efficiency of the process to obtain higher biodiesel yields.
Lipases from different sources have significant variation in properties especially with respect to regiospecificity, fatty acid specificity, thermostability, pH optimum, and kinetics in nonaqueous system (Gupta et al. 2004). A broad spectrum of substrate utilization coupled with enantioselectivity and enhanced efficiency in nonaqueous media is always desirable in new lipases. In this study, the purified lipase from Haloarcula sp. G41 displayed excellent thermostable, alkali-stable, halotolerant, and organic solvent-tolerant properties. These features rendered the lipase more potentially valuable for biotechnological applications in nonaqueous catalysis. This study formed the basic trials conducted to test the feasibility of using haloarchaeal lipases for transesterification and subsequent biodiesel production. Attempts will be made to optimize the enzyme reaction conditions so as to obtain a better yield of biodiesel.
References
Amoozegar MA, Malekzadeh F, Malik KA (2003) Production of amylase by newly isolated moderate halophile, Halobacillus sp. strain MA-2. J Microbiol Methods 52:353–359
Bancerz R, Ginalska G, Fiedurek J, Gromada A (2005) Cultivation conditions and properties of extracellular crude lipase from the psychrotrophic fungus Penicillium chrysogenum 9'. J Ind Microbiol Biotechnol 32:253–260
Bhatnagar T, Boutaiba S, Hacene H, Cayol JL, Fardeau ML, Ollivier B, Baratti JC (2005) Lipolytic activity from halobacteria: screening and hydrolase production. FEMS Microbiol Lett 248:133–140
Boutaiba S, Bhatnagar T, Hacene H, Mitchell DA, Baratti JC (2006) Preliminary characterization of a lipolytic activity from an extremely halophilic archaeon, Natronococcus sp. J Mol Catal B-Enzym 41:21–26
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Camacho RM, Mateos JC, González-Reynoso O, Prado LA, Córdova J (2009) Production and characterization of esterase and lipase from Haloarcula marismortui. J Ind Microbiol Biotechnol 36:901–999
Chakraborty S, Khopade A, Biao R, Jian W, Liu XY, Mahadik K, Chopade B, Zhang LX, Kokare C (2011) Characterization and stability studies on surfactant, detergent and oxidant stable α-amylase from marine haloalkaliphilic Saccharopolyspora sp. A9. J Mol Catal B-Enzym 68:52–58
Coronado M, Vargas C, Hofemeister J, Ventosa A, Nieto JJ (2000) Production and biochemical characterization of an alpha-amylase from the moderate halophile Halomonas meridiana. FEMS Microbiol Lett 183:67–71
Dheeman DS, Antony-Babu S, Frias JM, Henehan GTM (2011) Purification and characterization of an extracellular lipase from a novel strain Penicillium sp. DS-39 (DSM 23773). J Mol Catal B-Enzym 72:256–262
Doukyu N, Ogino H (2010) Organic solvent-tolerant enzymes. Biochem Eng J 48:270–282
Gupta R, Gupta N, Rathi P (2004) Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol 64:763–781
Holmes ML, Scopes RK, Moritz RL, Simpson RJ, Englert C, Pfeifer F, Dyall-Smith ML (1997) Purification and analysis of an extremely halophilic beta-galactosidase from Haloferax alicantei. Biochim Biophys Acta 1337:276–286
Jaeger KE, Dijkstra BW, Reetz MT (1999) Bacterial biocatalysts: molecular biology, three-dimensional structures, and biotechnological applications of lipases. Ann Rev Microbiol 53:315–351
Ji Q, Xiao S, He B, Liu X (2010) Purification and characterization of an organic solvent-tolerant lipase from Pseudomonas aeruginosa LX1 and its application for biodiesel production. J Mol Catal B-Enzym 66:264–269
Kushner D, Kamekura M (1988) Physiology of halophilic eubacteria. In: Rodriguez-Valera F (ed) Halophilic bacteria. CRC Press, Boca Raton, pp 109–138
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of becteriophage T4. Nature 227:680–685
Lima VMG, Krieger N, Mitchell DA, Baratti JC, Filippis ID, Fontana JD (2004a) Evaluation of the potential for use in biocatalysis of a lipase from a wild strain of Bacillus megaterium. J Mol Catal B-Enzym 31:53–61
Lima VMG, Krieger N, Mitchell DA, Fontana JD (2004b) Activity and stability of a crude lipase from Penicillium aurantiogriseum in aqueous media and organic solvents. Biochem Eng J 18:65–71
Litchfield CD (2011) Potential for industrial products from the halophilic archaea. J Ind Microbiol Biotechnol 38:1635–1647
Mevarech M, Frolow F, Gloss LM (2000) Halophilic enzymes: proteins with a grain of salt. Biophys Chem 86:155–164
Müller-Santos M, de Souza EM, Pedrosa Fde O, Mitchell DA, Longhi S, Carrière F, Canaan S, Krieger N (2009) First evidence for the salt-dependent folding and activity of an esterase from the halophilic archaea Haloarcula marismortui. Biochim Biophys Acta 1791:719–729
Noureddini H, Gao X, Philkana RS (2005) Immobilized Pseudomonas cepacia lipase for biodiesel fuel production from soybean oil. Bioresour Technol 96:769–777
Ozcan B, Ozyilmaz G, Cokmus C, Caliskan M (2009) Characterization of extracellular esterase and lipase activities from five halophilic archaeal strains. J Ind Microbiol Biotech 36:105–110
Pérez D, Martín S, Fernández-Lorente G, Filice M, Guisán JM, Ventosa A, Mellado E (2011) A novel halophilic lipase, LipBL, showing high efficiency in the production of eicosapentaenoic acid (EPA). PLoS One 6:e23325
Rahman RN, Baharum SN, Basri M, Salleh AB (2005) High-yield purification of an organic solvent-tolerant lipase from Pseudomonas sp. strain S5. Anal Biochem 341:267–274
Royon D, Daz M, Ellenrieder G, Locatelli S (2007) Enzymatic production of biodiesel from cotton seed oil using t-butanol as a solvent. Bioresour Technol 98:648–653
Rúa L, Díaz-Mauriño T, Fernández VM, Otero C, Ballesteros A (1993) Purification and characterization of two distinct lipases from Candida cylindracea. Biochim Biophys Acta 1156:181–189
Sellek GA, Chaudhuri JB (1999) Biocatalysis in organic media using enzymes from extemophiles. Enzyme Microb Technol 25:471–482
Shah S, Gupta MN (2007) Lipase catalyzed preparation of biodiesel from Jatropha oil in a solvent free system. Process Biochem 42:409–414
Sivaramakrishnan R, Muthukumar K (2012) Isolation of thermo-stable and solvent-tolerant Bacillus sp. lipase for the production of biodiesel. Appl Biochem Biotechnol 166:1095–1111
Sugihara A, Shimada Y, Takada N, Nagao T, Tominaga Y (1996) Penicillium abeanum lipase: purification, characterization, and its use for docosahexaenoic acid enrichment of tuna oil. J Ferment Bioeng 82:498–501
Teo JWP, Zhang LH, Poh CL (2003) Cloning and characterization of a novel lipase from Vibrio harveyi strain AP6. Gene 312:181–188
Winkler UK, Stuckmann M (1979) Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J Bacteriol 138:663–670
Xu XW, Liu SJ, Tohty D, Oren A, Wu M, Zhou PJ (2005) Haloterrigena saccharevitans sp. nov., an extremely halophilic archaeon from Xinjiang, China. Int J Syst Evol Microbiol 55:2539–2542
Yu XW, Sha C, Guo YL, Xiao R, Xu Y (2013) High-level expression and characterization of a chimeric lipase from Rhizopus oryzae for biodiesel production. Biotechnol Biofuels 6:29
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (grant no. 31300002), Program for the Top Young Academic Leaders of Higher Learning Institutions of Shanxi, and PhD Start-up Foundation of Yuncheng University (grant no. YQ-2011043).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Yu, HY. Characterization of an organic solvent-tolerant lipase from Haloarcula sp. G41 and its application for biodiesel production. Folia Microbiol 59, 455–463 (2014). https://doi.org/10.1007/s12223-014-0320-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-014-0320-8