Abstract
This study presents the production of biodiesel from algae oil by transesterification using thermophilic microorganism. The microorganism used in this study was isolated from the soil sample obtained near the furnace. The organism was identified as Bacillus sp., and the lipase obtained was purified by ammonium sulfate precipitation and ion exchange chromatography leading to 8.6-fold purification and 13% recovery. Molecular weight of the enzyme was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and it was found to be 45 kDa. The effect of pH, temperature, and solvent addition on lipase activity was investigated. The enzyme showed maximum activity at 55 °C and at pH 7 and was also found to be highly active in the presence of organic solvents such as hexane and t-butanol. The isolated lipase was successfully used for the production of biodiesel. The transesterification activity of the isolated lipase showed 76% of fatty acid methyl esters yield in 40 h, which indicated that this enzyme can be used as a potential biocatalyst for the biodiesel production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipases are the second largest group of industrial enzymes after bacterial amylolytic enzymes. It is the most commonly used enzyme to catalyze hydrolysis and ester synthesis reactions. It is a familiar valuable biocatalyst in food, pharmaceutical, detergent, and chemical industries [1]. Lipase successfully catalyzes interesterification, acidolysis, esterification, alcoholysis, and aminolysis in addition to its hydrolytic activity on triglycerides [2]. The performance of lipase in the presence of various solvents is related to the efficiency of synthetic and hydrolytic reactions. Esterification and transesterification are carried out in low-water-content media and using non-polar solvents. Furthermore, lipase activity is slightly enhanced when solvents like diethyl ether, n-hexane, benzene, toluene, and t-butanol were added. Particularly, for the biodiesel applications, solvent tolerant lipases are preferred. Most of the lipases are not stable in organic solvents and solvent like methanol strips water molecules from the enzyme surface, which in turn deactivates the enzyme [3]. To overcome this limitation, several methods were tried, and such methods include chemical modification, protein engineering, etc. [4]. Instead of modifying enzymes for solvent stability, naturally evolved solvent-tolerant enzymes would be more appropriate for industrial applications. Temperature is also an important operational parameter that influences the lipase activity significantly, and higher reaction temperatures accelerate the reaction rate. However, higher temperatures cause significant conformational unfolding of the enzyme and denaturation [5]. On the other hand, thermophiles contain proteins which are thermo-stable and resistant to denaturation and proteolysis. Lipases from thermophiles are also stable towards organic solvents [6]. It should be noted that lipase from Bacillus sp. is different from that of Rhizopus oryzae or Candida antartica in terms of the biochemical properties and three-dimensional structures. For example, Nthangeni et al. [7] reported the absence of lid domain in the Bacillus sp. lipase. Unlike other lipases, Bacillus sp. lipase catalytic machinery is adjusted upon transition of the enzyme from closed to open conformation, and this suggests that these proteins have a flexible tertiary structure [7]. Pouderoyen et al. [8] also found no lid structure in Bacillus sp. lipase and reported an uncomplicated interaction between enzyme and substrate. Lipases are usually less stable compared with chemical catalysts. The process stability is usually higher for the enzymes with high thermo-stability and in the resistance to denaturation in organic solvents [9]. Utilization of lipase for biodiesel production has been attractive since the by-product formed, glycerol, can easily be recovered; the purification of fatty acid methyl esters is also easy [10]. The transesterification can be carried out with immobilized lipase, and many extracellular lipases were successfully used for the transesterification [11, 12]. Nevertheless, the use of whole-cell biocatalysts makes the process economic and also efficiently catalyzes the transesterification reaction [13–15]. However, the development of solvent-tolerant microorganism is essential for the effective production of biodiesel using microorganisms. Recently, various solvent-tolerant bacteria, which have the ability to live in the presence of organic solvents, have been reported in the literature [16, 17]. An extracellular Bacillus lipase had an activity optimum at 50 °C, and it was stable in the presence of surfactants and in organic solvents [18]. There are many studies with respect to purification, and characterization of solvent-tolerant lipases was reported in the literature [1]. However, it remains a challenge to meet the requirements for efficient catalysis with solvent and at higher temperatures. Hence, it is necessary to investigate new solvent and thermo-stable lipases. In the present study, we have isolated a thermo-tolerant as well as solvent-tolerant lipase-producing bacteria identified as Bacillus sp. The purification and characterization of isolated lipase, particularly its activity in the various organic solvents and its thermal stability, was reported. The isolated lipase also showed good catalytic efficiency towards biodiesel synthesis.
Materials and Methods
Microorganism
Microorganism was isolated from the soil sample collected from the metallurgical industry. The soil sample was collected near to the furnace in the industry. One milliliter of sample was suspended in 10 ml sterile normal saline, and after shaking for few minutes, 5 ml of the suspension was transferred into a 250-ml Erlenmeyer flask containing 50 ml of enriching medium containing—0.5% olive oil, 0.1% yeast extract, 0.2% NaCl2, 0.04% MgSO4, 0.07% MgCl2, 0.05%CaCl2, 0.03% KH2PO4, 0.03%K2HPO4, and 0.05% (NH4)2SO4, pH 7.0. The culture was incubated at 55 °C under static conditions for 2 days.
Screening of Lipase-Producing Bacteria
Screening of lipase-producing bacteria was carried out using rhodamine B-olive oil agar plate method [19, 20]. The growth medium was prepared by suspending nutrient agar (28 g) and sodium chloride (4 g) in distilled water (1 l) and was autoclaved at 121 °C for 15 min after the adjustment of its pH to 7.0 using 0.1 N sodium hydroxide. The medium was added with 10 ml of rhodamine B solution (1 mg/ml) and olive oil (31.25 ml) and stirred vigorously using a magnetic stirrer. Then, the contents were allowed to stand for few minutes. Aliquots of 20 ml were poured into Petri dishes and were allowed to solidify. The enriched culture was streaked and incubated at 55 °C for 1 day. After serial dilutions, the colonies were re-streaked on the rhodamine B-olive oil agar plate and incubated at 55 °C for 2 days. The lipase-producing bacterium was identified by the orange fluorescent halos around the colonies when the plates were irradiated with UV illuminator. These organisms were isolated and sub-cultured in nutrient broth containing oil (200 mg/l). Isolated and purified high-lipase-producing bacterial cultures were identified according to cell morphology, gram staining, and spore production properties.
Lipase Production
One loopful of culture from a nutrient agar slant was inoculated into 50 ml tryptone soy broth medium and was incubated at 55 °C overnight. Then, 5 ml of sample was inoculated into a 250-ml Erlenmeyer flask containing 100 ml of basal medium containing—1% olive oil, 0.2% CaCl2·2H2O, 0.01% MgSO4·7H20, and 0.04% FeCl3·6H2O (1% stock solution). The contents were incubated for 72 h at 55 °C under shaking condition (150 rpm) after adjusting the initial pH of the medium to 7.0 [21]. After incubation, the cells were harvested by centrifugation at 10,000 rpm at 4 °C for 10 min, and the supernatant was used for further purification.
Protein Determination
Protein concentration was determined by Lowry method [22] using bovine serum albumin as the standard.
Purification of Lipase and Gel Electrophoresis
The extracellular lipase from Bacillus sp. was collected by centrifugation at 10,000×g for 10 min at 4 °C. Cellular debris and undisrupted cells were removed. Ammonium sulfate was added to the supernatant to give a concentration of 80% (w/v) saturation at 4 °C. The precipitate formed was allowed to settle overnight, which was followed by centrifugation at 12,000×g for 30 min. The pellet obtained was dissolved in 250 mM phosphate buffer (pH 7.0) and dialyzed overnight in the same buffer. The dissolved pellet was loaded on a phenyl sepharose CL-4B column pre-equilibrated with 250 mM buffer (pH 7.0). Column was washed with a gradient of 250–1 mM phosphate buffer (pH 7.0). The lipase was eluted first with 50% ethylene glycol followed by 80% ethylene glycol in 1 mM phosphate buffer (pH 7.0). The fractions showing lipase activity were collected and lyophilized for further use. Molecular weight analysis was done by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) [23]. The molecular weight of the enzyme was determined by comparing with the protein standards.
Lipase Assay and Activity
The enzyme activity was measured by titrimetric analysis [24]. The mixture containing 2.5 ml water, 1 ml Tris–HCL buffer, and 3 ml olive oil was mixed well, and then 1 ml of lipase (260 U/ml) was added. The contents were incubated in a shaker at 125 rpm at 55 °C for 30 min. The blank was prepared and stored at 4 °C to prevent the reaction [24]. After 30 min, 3 ml of ethanol was added to terminate the lipase reaction. Then, 4 ml of 0.9% thymolpthalein indicator was added and titrated against 50 mM NaOH until the end point was observed. One unit of lipase activity was equivalent to 1 μmol of fatty acid released per milliliter per minute at 55 °C.
Effect of pH on lipase activity was investigated at 55 °C by varying the pH from 3.0 to 9.0 using different buffers. For optimum pH determination, reaction mixture was incubated at different pH for 30 min, and residual activity was measured by lipase assay as described. The optimum temperature was arrived by incubating the enzyme in the temperature range from 40–65 °C, and the activity was measured. The purified lipase dissolved in phosphate buffer (pH 7.0) was filtered using cellulose acetate membrane filter (pore size 0.22 μm). One milliliter of organic solvent was added to 3.0 ml of the filtrate and was pre-incubated in a shaker at 125 rpm at 55 °C for 30 min. The activity towards the solvent was determined by lipase assay, and for control experiments, distilled water was added instead of solvent.
Effect of Metal Ions
The effect of different metal ions such as Ca 2+, Mg 2+, Mn2+, Zn2+, Fe2+, K+, and metal chelating agent (ethylene diamine tetraacetic acid (EDTA)) on the lipase activity was investigated. The phosphate buffered (pH 7.0) enzyme solution was pre-incubated with the 1 mM of respective metal ions for 30 min at 55 °C, and the activity was measured by lipase assay.
Substrate Specificity
Palm oil, sunflower oil, castor oil, groundnut oil, coconut oil, waste cooking oil, and triolein were used as a substrate instead of olive oil to find out the substrate specificity of lipase at static conditions, and the activity was measured.
Partial Amino Acid Sequencing
Partial amino acid sequencing was analyzed using matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) [50]. After digestion of enzyme into mixture of peptides, it was subjected to MALDI-TOF analysis for enzyme sequencing using Ultraflex MALDI-TOF/TOF mass spectrometer (Bruker Daltonics, Germany). MALDI–TOF is a well-established technique for enzyme identification and sequencing.
The enzyme was subjected to tryptic digestion, and the mixture of peptides was transferred to a sample position on a ground steel MALDI target plate and allowed to dry at room temperature. Subsequently, the sample was overlaid with 2 μl of MALDI matrix (a saturated solution of α cyano-4-hydroxy-cinnamic acid in 50% acetonitrile–2.5% trifluoroacetic acid) and dried again.
For database construction and validation, measurements were performed with a TOF mass spectrometer (Bruker Daltonics) equipped with a 20 Hz nitrogen laser (parameter settings, ion source 1 (IS1), 25 kV; IS2, 18.5 kV; lens, 8.5 kV; detector gain, 2,650 V; and gating, none). Spectra were recorded in the positive linear mode at 100 Hz for the mass range of 2,000 to 50,000 Da at the maximum laser frequency. The database references (main spectra) for the newly investigated enzyme sequence were constructed using the automated functionality of the MALDI BioTyper (version 1.1) software package (Bruker Daltonik GmbH, Germany). After smoothing of the spectra, baseline correction, and peak picking, the resulting peak lists were used by the program to calculate and to store a main spectrum containing the average peak mass, average peak intensity, and frequency information. A single sample could be sequenced in approximately 10 min.
Lipase-Catalyzed Transesterification
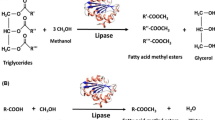
Lipase-catalyzed transesterification was carried out in 8-ml glass-vials. One gram of Oedogonium sp. oil (oil content of Oedogonium sp. was reported in Table 1) was added with methanol in stepwise (1:1 mol ratio for each step), and the reaction was initiated by adding enzyme (10% w/w of oil) and 0.75 ml of t-butanol. Then the contents were incubated in an orbital shaker at 150 rpm at 55 °C. At specified time intervals, 10-μl aliquots were withdrawn and subjected to centrifugation. Since the density of methyl ester is low, glycerin tends to collect at the bottom. Some amount of monoglycerides and di-glycerides also present in the product, and due to their polarity, partially reacted glycerides were preferentially attracted towards the glycerin phase. The glycerin phase was separated, and the phase containing biodiesel was washed to remove the entrained glycerol and excess methanol [25, 26]. To reduce the number of washing cycles, the initial washing was done with dilute acetic acid, which was followed by distilled water. Finally, biodiesel obtained was dried in a hot air oven at 105 °C until the water content was below 0.05% to avoid the formation of excess smoke and poor combustion [27, 28].
Analytical Methods
The fatty acid composition of the Oedogonium sp. oil and methyl ester content in the reaction mixtures were analyzed by gas chromatography (Sigma) equipped with an AC30 Carbowax column (3 met and 1/8”) and a flame ionization detector. Nitrogen was used as a carrier gas; hydrogen and oxygen were employed for the purpose of ignition. The column temperature was kept at 150 °C and raised to 240 °C and maintained for 10 min. The injector and detector port temperatures were both set at 250 °C. Methyl esters of palmitic, stearic, oleic, and linoleic acids standards were purchased from Sigma. Finally, fuel properties of biodiesel like flash point, fire point, cetane number, aniline point, smoke point, and diesel index were analyzed by ASTM standard methods.
Results and Discussion
Identification of Microorganism
In the present study, 12 strains were isolated by following the screening method described, and among those isolated organisms, one showed a maximum lipase activity and a clear zone formation on the agar plates indicating the production of extracellular lipase. The organism showed maximum activity was selected for the production of lipase. The isolated microorganism was analyzed by morphological and biochemical tests (Table 2). From the results, the isolated organism was identified as Bacillus sp. [29] and was used for further studies.
Purification of Lipase
The extracellular lipase from Bacillus sp. was purified by ammonium sulfate precipitation (Table 3). The SDS-PAGE results of the purified lipase obtained from Bacillus sp. is shown in Fig. 1. A single major band with molecular mass of around 45 kDa was observed on SDS-PAGE analysis with about 8.6-fold purification and 13% recovery. Lipolytic enzymes from Bacillus subtilis, Bacillus pumilus, and Bacillus lichenformis were grouped in subfamily 1.4 of true lipases. These lipases have the conservative peptide Ala-His-Ser-Met-Gly and molecular weight in the range of 20 kDa [1, 30, 31]. On the other hand, the molecular weight of Bacillus mageterium, B. subtilis, and Bacillus sp. J33 lipase was reported as 40, 45, and 45 kDa, respectively [32–34]. The molecular weight of solvent-tolerant lipases reported to falls into two groups: viz., 30–45 and 50–60 kDa [35]. The lipase obtained in the present study was found to fall under the first group.
Effect of pH
The effect of pH on the relative activity of purified lipase is shown in Fig. 2. The results showed that the purified lipase was found to be stable in the pH range from 3 to 9. However, the maximum lipase activity was observed at pH 7.0. Although bacteria prefer pH around 7 for best lipase production, maximum activity at higher pH values were also reported in the literature [36]. For the extracellular lipase-producing Pseudomonas sp., the optimum pH range reported was pH 7.0–9.0, and this enzyme was stable between pH 6 and 12 [31]. The lipase from the isolated Bacillus sp. was found to tolerate a wide range of pH, and hence, this can be successfully used for the production of biodiesel. The stability of lipase under variations in pH is mainly due to the stable secondary structure of the enzyme, and a similar kind of result was observed for lipase derived from Antrodia cinnamomea [37].
Effect of Temperature
The effect of temperature on relative lipase activity is shown in Fig. 3. The lipase activity increased sharply when temperature was increased from 45 °C to 55 °C, and then the enzyme activity was found to decrease gradually. At 55 °C, the isolated enzyme exhibited the maximum activity. Thermo-stable lipases reported in the literature are summarized in Table 4, and thermo-stable lipases were isolated from many sources. Wang et al. isolated the thermo-stable enzyme from a Bacillus strain, and it showed a maximum activity at 60 °C [38]. An extracellular Bacillus lipase isolated by Sidhu et al. showed an optimum activity at 50 °C [39]. Thermal stability of lipase is related to its structure and is influenced by environmental factors such as pH and the presence of metal ions. In some cases, thermal denaturation appears to occur through intermediate states of unfolding of the polypeptide. Mutations in the lid region of the enzyme also significantly affect the thermal stability [40]. In addition, temperature was also reported to affect the rate of reaction and mass transfer resistances considerably [41].
Effect of Organic Solvents
Organic solvents have significant effect on the lipase activity, and recently, studies relevant solvent-tolerant lipases were reported in the literature [39]. In the present study, isolated enzyme was incubated with various organic solvents, and the results obtained are shown in Table 5. The relative activity of lipase observed with polar solvents such as methanol, ethanol, and acetone was found to be low. Polar solvents are not well preferred compared with non-polar solvents as they disrupt the thin layer of water molecules behaving as protective sheath surrounding the active site of the enzyme. Therefore, polar solvent-tolerant lipases are efficient for catalysis at lower water medium [42]. Moreover, the lipase activity also depends on the quality of an oil–water interface, and water-miscible solvents cause protein denaturation. Furthermore, solvents such as dimethyl sulfoxide/dimethyl formamide dissolve enzymes and invariably inactivate them [30]. A good measure of polarity of an organic solvent is its polarity through the log P value, which is defined as the logarithm of its partition coefficient in standard n-octane/water two-phase systems [43]. The log P values obtained for the organic solvents used in the present study are presented in Table 5. In general, organic solvents with log P values <2.0 are not considered for biocatalysis [44]. However, Pseudomonas aeruginosa secreted organic solvent protease PST-01, which is very stable in the presence of methanol or ethanol has log P <2.0. On the other hand, the stability and activity of enzyme in polar solvents like methanol, ethanol, and acetone were rarely observed [45]. In the present study, organic solvents such as n-hexane and t-butanol were not affected the lipase activity much. The lipase activity was stable on benzene, toluene, methyl acetate, and ethyl acetate with relative activity of 74%, 53%, 42%, and 46%, respectively. The Bacillus sp. lipase activity on solvents like n-hexane and t-butanol is an important aspect for the use of this lipase as a potential catalyst for biodiesel production.
Effect of Metal Ions
A number of enzymes require metal ions for the maintenance of their stable and active structures. These ions bound strongly with specific binding sites present on the surface, and such binding sites usually consist of negatively charged carboxylate side-chain groups of aspartyl and glutamyl residues brought together by folding of the polypeptide chain [46]. The effect of different metal ions on the relative activity of lipase was studied, and the results are presented in Table 6. The lipase activity was significantly enhanced by Ca2+, Mg2+, and K+ ions. The other metal ions used in this study were found to significantly inhibit the lipase activity. Many lipases were found to display enhanced activity in the presence of Ca2+. This is due to the fact that the polypeptide chain in the active site is cross-linked by the metal ion bridge, and enzyme–calcium ion complex should, therefore, be more rigid and stable, and also may be due to the changes in reaction equilibrium by interacting with the organic acids [47, 48]. The chelating agent EDTA also showed an inhibitory effect, and this is mainly due to the effect of EDTA on the enzyme structure. The other metal ions such as Fe2+ and Zn2+ were also found to be inhibitors and to decrease the enzyme activity.
Substrate Specificity
The hydrolytic reaction catalyzed by lipases generally takes place at the oil–water interface, and the hydrolytic activity is the basic characteristic of lipases. In the present study, catalytic property of the enzyme toward different oils was tested. Since, single lipase was reported to have different hydrolytic activity towards different oils from different sources [49]. The effect of different substrates on relative lipase activity is shown in Fig. 4, and the results indicate that the purified lipase had highest hydrolytic activity towards olive oil, palm oil, and triolein. The enzyme could hydrolyze all the oils used in this study, and hence, it can be confirmed that the purified lipase has the potential to hydrolyze various triglycerides. Therefore, lipase with high hydrolytic activity towards large chain fatty acids is the potential source for catalyzing transesterification of oils from plants and algae for the production of biodiesel (Fig. 5).
Substrate specificity activity (Reaction conditions, pH 7, temperature, 55 °C at 125 rpm for 30 min). The activities are shown as values relative to the maximum activity measured towards olive oil (taken as 100%). Data points were means of triplicate values with the error bars showing standard deviations
Transesterification of Oedogonium sp. oil. Time course of biodiesel production: Oedogonium sp. oil (1 g), 0.1 g of lipase, 1:3 molar ratio of methanol to oil, 0.75 ml of t-butanol, and 55 °C at 150 rpm for free lipase. Methyl esters were analyzed by gas chromatography. Data points were means of triplicate values with the error bars showing standard deviations
Partial Amino Acid Sequencing
MALDI-TOF MS, used to analyze the protein sequences of a bacterial cell, has emerged as a new technology for species identification. By measuring the exact sizes of peptides and small proteins, which are assumed to be characteristic for enzyme, it is possible to determine the enzyme within a few minutes [51–53].
The partial amino acid sequence for the lipase produced from Bacillus sp. is shown in Fig. 6. The activity of a variety of lipases majorly depends on the catalytic triad usually formed by Ser, His, and Asp residues. The serine residue usually appears in the conserved pentapeptide, Gly-Xaa-Ser-Xaa-Gly. This kind of peculiar catalytic property of lipases makes them very attractive for industrial applications [54].The catalytic triad consisting of amino acids Ser, Asp, and His was found to be present in the isolated Bacillus sp. lipase sequence. The presence of this catalytic triad amino acid sequence confirms that the sequence obtained in present belongs to lipase enzyme [55, 56]. Cysteine residues which are responsible for the formation of disulfide bridge were also present in the lipase sequence. Aspartic acid residues involved in the Ca2+ binding site were identified at 240 and 320 amino acid positions [57]. The lipase enzyme produced from Bacillus sp. was found to contain 375 amino acids. The results of the partial amino acid sequencing confirm that the enzyme isolated in the present has the characteristics of lipase enzyme family.
Transesterification
Experiments were carried out to asses the ability of lipase derived from Bacillus sp. for the biodiesel production from Oedogonium sp. Oil, and the results are shown in Fig. 5. The yield of fatty acid methyl esters (FAMEs) reached 76% after 40 h considering that the isolated lipase was stable in t-butanol, and better yield was obtained. Short-chain alcohols, especially methanol, have low solubility in oils, and therefore, a new liquid phase appears in the system leading to an inactivation of the enzyme and decreased yields of ester. t-Butanol was used as the solvent to reduce the influence of methanol and glycerol on the lipase [58]. Usage of t-butanol as a solvent could hence be recognized as one possible solution for reducing the inhibitory effects of methanol and for the industrial implementation of the process [59]. This study suggests that the thermo- and organic-tolerant lipase isolated can be effectively used for the biodiesel production from algae oil. The diesel properties of the biodiesel like its flash point, fire point, smoke point, etc., were analyzed adopting the standard procedures from ASTM (American standard testing method), and the results are tabulated in Table 7. The higher values of flash and fire points obtained ensure better storage and handling properties of the fuel.
Conclusions
In the present study, lipolytic bacteria producing organic solvent and thermo-stable lipase, identified as Bacillus sp., was isolated. Lipase was purified by simple purification procedure, and the molecular mass was determined as 45 kDa. The optimum temperature and pH for the better enzyme activity was found to be 55 °C and 7, respectively. The enzyme showed high activity toward the organic solvents such as hexane and t-butanol. The lipase activity was significantly enhanced by Ca2+, Mg2+, and K+ ions. The purified lipase was used for the production of biodiesel from Oedogonium sp., and 76% FAME yield was obtained at the end of the 40 h. These results conclude that the isolated solvent-tolerant and thermo-stable lipase can potentially be used for the biodiesel production.
References
Guncheva, M., & Zhiryakova, D. (2011). Catalytic properties and potential applications of Bacillus lipases. Journal of Molecular Catalysis B: Enzymatic, 68, 1–21.
Joseph, B., Ramteke, P. W., & Thomas, G. (2008). Cold active microbial lipases: some hot issues and recent developments. Biotechnology Advances, 26, 457–470.
Yang, L., Dordick, J. S., & Garde, S. (2004). Hydration of enzyme in non-aqueous media is consistent with solvent dependence of its activity. Biophysical Journal, 87, 812–821.
Polizzi, K., Bommarius, A., Broering, J., & Chaparro-Riggers, J. (2007). Stability of enzymes. Current Opinion in Chemical Biology, 11, 220–225.
Zhen-qian, Z., & Chun-yun, G. (2009). Screening for lipase-producing Enterobacter agglomerans for biodiesel catalyzation. African Journal of Biotechnology, 8(7), 1273–1279.
Haki, G. D., & Rakshit, S. K. (2003). Developments in industrially important thermostable enzymes: a review. Bioresource Technology, 89, 17–34.
Nthangeni, M. B., van Patterton, H. G., Tonder, A., Vergeer, W. P., & Litthauer, D. (2001). Over-expression and properties of a purified recombinant and Bacillus licheniformis lipase: a comparative report on Bacillus lipases. Enzyme and Microbial Technology, 28, 705–712.
Ying, M., & Chen, G. (2007). Study on the Production of Biodiesel by Magnetic Cell Biocatalyst Based on Lipase-Producing Bacillus subtilis. Applied Biochemistry and Biotechnology, 136(140), 793–804.
Owusu, R. K., & Cowan, D. A. (1989). Correlation between microbial protein thermostability and resistance to denaturation in aqueus: organic solvent two-phase systems. Enzyme and Microbial Technology, 11, 568–574.
Ranganathan, S. V., Narasimhan, S. L., & Muthukumar, K. (2008). An overview of enzymatic production of biodiesel. Bioresource Technology, 99, 3975–3981.
Xu, Y., Du, W., Zeng, J., & Liu, D. (2004). Conversion of soyabean oil to biodiesel fuel using lipozyme TL IM in a solvent free medium. Biocatal. Biotransform., 22, 45–48.
Samukawa, T., Kaieda, M., Matsumoto, T., Ban, K., Kondo, A., Shimada, Y., Noda, H., & Fukuda, H. (2000). Pretreatment of Immobilized Candida antarctica lipase for Biodiesel fuel production from plant oil. J. Bioresour. Bioeng., 90, 180–183.
Ban, K., Kaieda, M., Matsumoto, T., Kondo, A., & Fukuda, H. (2001). Whole-cell biocatalyst for Biodiesel fuel production utilizing Rhizopus oryzae cells immobilized within biomass support particles. Biochem. Eng., 8, 39–43.
Narita, J., Okano, K., Tateno, T., Tanino, T., Sewaki, T., Sung, M. H., Fukuda, H., & Kondo, A. (2006). Display of active enzymes on the cell surface of Escherichia coli using PgsA anchor protein and their application to bioconversion. Applied Microbiology and Biotechnology, 70, 564–572.
Ban, K., Hama, S., Nishizuka, K., Kaieda, M., Matsumoto, T., Kondo, A., Noda, H., & Fukuda, H. (2002). Repeated use of whole cell biocatalysts immobilized within biomass support particles for biodiesel fuel production. Journal of Molecular Catalysis B: Enzymatic, 17, 157–165.
Sellek, G. A., & Chaudhari, B. (1999). Biocatalysis in organic media using enzymes from extremophiles. Microb. Technol., 25, 471–482.
Gupta, A., & Khare, S. K. (2009). Enzymes from solvent-tolerant microbes: useful biocatalysts for non-aqueous enzymology. Critical Reviews in Biotechnology, 29, 44–54.
Sidhu, P., Sharma, R., Soni, S. K., & Gupta, J. K. (1998). Production of extracellular alkaline lipase by a new thermophilic Bacillus sp. Folia Microbiologica, 43, 51–54.
Kouker, G., & Jaeger, K. E. (1986). Specific and sensitive plate assay for bacterial lipases. Applied and Environmental Microbiology, 89, 211–213.
Yeap, C.K. (1998)BSTM Thesis, Screening and Purification of Lipase Enzyme from Bacteria, Universiti Putra Malaysia; Serdang, Selangor, Malaysia.
Eltaweel, M. A., Rahman, R. N. Z. A., Salleh, A. B., & Basri, M. (2005). An organic solvent-stable lipase from Bacillus sp. strain 42. Annals of Microbiology, 55(3), 187–192.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randal, J. (1951). Protein measurement with the folin-phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature, 227, 680–685.
Washington, D.C. (1993) ACS Specifications, 8th ed., 95 Reagent chemicals.
Ma, F., Clements, L. D., & Hanna, M. A. (1998). The effects of catalyst free fatty acids and water on transesterification of beef tallow. Transactions of ASAE, 41, 1261–1264.
Sinha, S., Agarwal, A. V., & Garg, S. (2008). Biodiesel development from rice bran oil-Transesterification process optimization and fuel characterization. Energy. Conv. Manag, 49, 1248–1257.
Fernando, S., Karra, P., Hernandez, R., & Jha, S. K. (2007). Effect of incompletely converted soybean oil on biodiesel quality. Energy, 32, 844–851.
Demirbas, A. (2009). Progress and recent trends in biodiesel fuels. Energy. Conv. Manag., 50, 14–34.
Doetsch, R. N. (1981). Determinative methods of light microscopy. In P. Gerhardt (Ed.), Manual of Methods for General Bacteriology (pp. 21–23). D.C. Washington: American Society for Microbiology.
Sugihara, A., Tani, T., & Tominaga, Y. (1991). Purification and characterization of novel thermostable lipase from Bacillus sp. Journal of Biochemistry, 109, 211–216.
Pouderoyen, G. V., Eggert, T., Jaeger, K. E., Bauke, W., & Dijkstra, B. W. (2001). Structural investigations of calcium binding and its role in activity and activation of outer membrane. Journal of Molecular Biology, 309, 215–226.
Lima, V. M. G., Nadia Kriegera, D., Mitchellb, D. A., Barattic, J. C., Filippisd, I. D., & Fontanae, J. D. (2004). Evaluation of the potential for use in biocatalysis of a lipase from a wild strain of Bacillus megaterium. Journal of Molecular Catalysis B: Enzymatic, 31, 53–61.
Olusesan, A.T., Kamaruzaman, L. Forghani, Abu bakar, B.F. Sabo Mohamed, A.K. Radu, S. Yazid, M. Manap, A and Saari, N. (2011). Purification, characterization and thermal inactivation kinetics of a non-regioselective thermostable lipase from a genotypically identified extremophilic Bacillus subtilise NS8. New Biotechnol. 28, 6, October 2011.
Nawani, N., & Kaur, J. (2000). Purification, characterization and thermostability of lipase from a thermophilic Bacillus sp. J33. Molecular and Cellular Biochemistry, 206, 91–96.
Ji, Q., Xiao, S., He, B., & Liu, X. (2010). Purification and characterization of an organic solvent-tolerant lipase from Pseudomonas aeruginosa LX1 and its application for biodiesel production. Journal of Molecular Catalysis B: Enzymatic, 66, 264–269.
Shu, C. H., Xu, C. J., & Lin, G. C. (2006). Purification and partial characterization of a lipase from Antrodia cinnamomea. Process Biochemistry, 41, 734–738.
Sharma, R., Chisti, Y., & Banerjee, U. C. (2001). Production, purification, characterization and applications of lipases. Biotechnology Advances, 19, 627–662.
Wang, Y., Srivastava, K. C., Shen, G. J., & Wang, H. Y. (1995). Production, purification, characterization and applications of lipases. Journal of Fermentation and Bioengineering, 79, 433–438.
Nawani, N., Dosanjh, N. S., & Kaur, J. (1998). A novel thermostable lipase from a thermophilic Bacillus sp.: characterization and esterification studies. Biotechnology Letters, 20(10), 997–1000.
Zhu, K., Jutila, A., Tuominen, E. K. J., Patkar, S. A., Svendsen, A., & Kinnunen, P. K. (2001). Impact of the tryptophan residues of Humicola lanuginosa lipase on its thermal stability. J. Biochim. Biophys. Acta, 1547, 329–338.
Al-Zuhair, S., Ling, F. W., & Jun, L. S. (2007). Proposed kinetic mechanism of the production of biodiesel from palm oil using lipase. Process Biochemistry, 42, 951–960.
Zhao, L., Xu, J., Zhao, J., Pan, J., & Wang, Z. (2008). Biochemical properties and potential applications of an organic solvent tolerant lipase isolated from Serratia marcescens ECU1010. Process Biochemistry, 43, 626–633.
Laane, C., Boeren, S., & Vos, K. V. (1987). Rules for optimization of biocatalysis in organic solvents. Biotech. Bioeng., 30, 81–87.
Inoue, A., Yamamoto, M., & Horikoshi, K. K. (1991). Pseudomonas putida which can grow in the presence of toluene. Applied and Environmental Microbiology, 57(5), 1560–1562.
Ogino, H., & Ishikawa, H. (2001). Enzymes which are stable in the presence of organic solvents. Journal of Bioscience and Bioengineering, 91, 109–116.
Sharma, R., Soni, S. K., Vohra, R. M., Gupta, L. K., & Gupta, J. K. (2002). Purification and characterisation of a thermostable alkaline lipase from a new thermophilic Bacillus sp. RSJ-1. Process Biochemistry, 37, 1075–1084.
Rahman, R., Baharum, S. N., Basri, M., & Salleh, A. B. (2005). High-yield purification of an organic solvent-tolerant lipase from Pseudomonas sp. strain S5. Analytical Biochemistry, 341, 267–274.
Chakraborty, K. P., & Raj, R. (2008). An extra-cellular alkaline metallolipase from Bacillus licheniformis MTCC 6824: Purification and biochemical characterization. Food Chemistry, 109, 727–736.
Wu, X. Y., Jaaskelainen, S., & Linko, Y. (1996). An investigation of crude lipase for hydrolysis, esterification and transesterification. Enzyme and Microbial Technology, 19, 226–231.
Karas, H. F. (1990). Mass spectrometry of peptides and proteins by matrix-assisted ultraviolet laser desorption/ionization. M. Methods Enzymol, 193, 280–295.
Fenselau, C., & Demirev, P. A. (2001). Characterization of intact microorganisms by MALDI mass spectrometry. Mass Spectrometry Reviews, 20, 157–171.
Holland, R. D., Wilkes, J. G., Rafii, F., Sutherland, J. B., Person, C. C., Voorhees, K. J., & Lay, J. O. J. (1996). Rapid identification of intact whole bacteria based on spectral patterns using MALDI-TOF-MS. Rapid Communications in Mass Spectrometry, 10, 1227–1232.
Krishnamurthy, T., Ross, P. L., & Rajamani, U. (1996). Detection of pathogenic and non-pathogenic bacteria by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry, 10, 883–888.
Arpigny, J. L., & Jaeger, K. E. (1999). Bacterial lipolytic enzymes: classification and properties. Biochemical Journal, 343, 177–183.
Brumlik, M. J., & Buckley, J. T. (1996). Identification of the catalytic triad of the lipase/acyltransferase from Aeromonas hydrophila. J. Bact., 178(7), 2060–2064.
Oh, S., Park, A. R., Bae, M. S., Kwon, S. J., Kim, Y. S., Lee, J. E., Kang, N. Y., Lee, S., Cheong, H., & Park, O. K. (2005). Secretome analysis reveals an Arabidopsis lipase involved in defense against alternaria brassicicola. The Plant Cell, 17, 2832–2847.
Nardini, M., Lang, D. A., Liebeton, K., Jaeger, K. E., & Dijkstra, B. W. (2000). Crystal structure of Pseudomonas aeruginosa lipase in the open confirmation. The prototype for family I.1 of bacterial lipases. Journal of Biological Chemistry, 275(40), 31219–31225.
Royon, D., Daz, M., Ellenrieder, G., & Locatelli, S. (2007). Enzymatic production of biodiesel from cotton seed oil using t-butanol as a solvent. Bioresource Technology, 98, 648–653.
Manco, G., Adinolf, E., Pisani, F., Ottolina, G., Carea, G., & Rossi, M. (1998). Overexpression and properties of a new thermophilc and thermostable esterase from Bacillus acidocaldarius with sequence similarity to hormone-sensitive lipase subfamily. Biochemical Journal, 332, 203–212.
Gupta, R., Gupta, K., Sexena, R., & Khan, S. (1999). Bleach-stable, alkaline protease from Bacillus sp. Biotechnology Letters, 21, 135–138.
Klibanov, A. (1983). Immobilized enzymes and cells as practical catalysts. Science, 219, 722–727.
Dong-Woo, L., You-Seok, K., Ki Jun, K., Byung-Chan, K., Hak-Jong, C., Doo-sik, K., Maggy, T., & Yu-ryang, P. (1999). Isolation and characterisation of thermophilic lipase from Bacillus thermoleovorans ID-1. FEMS Microbiology Letters, 179, 393–400.
Abdel-Fattah, Y. (2002). Optimization of thermostable lipase production from a thermophilic Geobacillus sp. using Box–Behnken experimental design. Biotechnology Letters, 24, 1217–1222.
Kulkurani, N., & Gadre, R. (1999). A novel alkaline, thermostable, protease free lipase from Pseudomonas sp. Biotechnology Letters, 21, 897–899.
Rathi, P., Sapna, B., Sexena, R., & Gupta, R. (2000). A hyperthermostable, alkaline lipase from Pseudomonas sp. with the property of thermal activation. Biotechnology Letters, 22, 495–498.
Hotta, Y., Ezaiki, S., Atomi, H., & Imanaka, T. (2002). Extremely stable and versatile carboxyl esterase from a hyperthermophilic archaeon. Applied and Environmental Microbiology, 68, 3925–3931.
Ikeda, M., & Clark, D. (1998). Molecular cloning of extremely thermostable esterase gene from hyperthermophilicarc haeon Pyrococcus furiosus in Escherhia coli. Biotechnology and Bioengineering, 57, 624–629.
Ando, S., Ishida, H., Kosugi, Y., & Ishikawa, K. (2002). Hyperthermostable endoglucanase from Pyrococcus horikoshi. Applied and Environmental Microbiology, 68, 430–433.
Yan, F., Yong-Goe, J., Kazuhiko, I., Hiroyasu, I., Susumu, A., Tohru, Y., Hiroshi, N., Shugui, C., Ikuo, M., & Yoshitsugu, K. (2000). Thermophilicphospholi pase A2 in the cytosolic fraction from the archaeon Pyrococcus horikoshii. Journal of the American Oil Chemists' Society, 77, 1075–1084.
Salleh, A. B., Musani, R., Basri, M., Ampon, K., Yunus, W. M. Z., & Razak, C. N. A. (1993). Extra- and intra-cellular lipases from a thermophilic Rhizopus oryzae and factors affecting their production. Can. J. Microbiol. Canadian Journal of Microbiology., 39(10), 978–981.
Nawani, N., Singh, R., & Kaur, J. (2006). Immobilization and stability studies of a lipase from thermophilic Bacillus sp: The effect of process parameters on immobilization of enzyme. Electron. J. Biotechn., 9(5), 559–565.
Salameh, M. A., & Wiegel, J. (2007). Purification and Characterization of Two Highly Thermophilic Alkaline Lipases from Thermosyntropha lipolytica. Applied and Environmental Microbiology, 73(23), 7725–7731.
Sifour, M., Saeed, H. M., Zaghloul, T. I., Berekaa, M. M., & Abdel-Fattah, Y. R. (2010). Isolation of Lipase Gene of the Thermophilic Geobacillus stearothermophilus Strain-5. Biotechnology, 9, 55–60.
Sathish Kumar, M., Karrunakaran, C. M., & Vikram, M. (2010). Process Facilitated Enhancement of Lipase Production from Germinated Maize Oil in Bacillus Spp. Using Various Feeding Strategies. Australian Journal of Basic and Applied Sciences, 4(10), 4958–4961.
Bora, L. Kalita, M. Production and Optimization of Thermostable lipase from a Thermophilic Bacillus sp LBN 4. Int. J. Microbiol. 4, 1.
Razak, C. N. A., Salleh, A. B., Musani, R., Samad, M., & Basri, Y. (1997). Some characteristics of lipases from thermophilic fungi isolated from palm oil mill effluent. M. Journal of J. Mol. Catal. B: Enzym., 3, 153–159.
Ogundero, V. W. (1982). Hydrolysis of vegetable oils and triglycerides by thermotolerant and zoopathogenic species of AspergUlus from Nigerian palm produce. Mycopathologia, 77, 43–46.
Uttatree, S., Winayanuwattikun, P., & Charoenpanich, J. (2010). Isolation and Characterization of a Novel Thermophilic-Organic Solvent Stable Lipase From Acinetobacter baylyi. Applied Biochemistry and Biotechnology, 162, 1362–1376.
Kumar, S., Kikon, K., Upadhyay, A., Shamsher, S. R., & Gupta, K. (2005). Production, purification, and characterization of lipase from thermophilic and alkaliphilic Bacillus coagulans BTS-3. Protein Expression Purif., 41, 38–44.
Pogaku, P., Suresh, A., Srinivas, P., & Ram Reddy, S. (2010). Optimization of lipase production by Staphylococcus sp. Lp12. African Journal of Biotechnology, 9(6), 882–886.
Acknowledgments
The authors gratefully acknowledge Department of Science and Technology (DST), New Delhi, for providing financial support to carry out this research work under PURSE scheme. One of the authors, Mr. R. Sivaramakrishnan, is grateful to DST, New Delhi, for the award of DST-PURSE fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivaramakrishnan, R., Muthukumar, K. Isolation of Thermo-stable and Solvent-Tolerant Bacillus sp. Lipase for the Production of Biodiesel. Appl Biochem Biotechnol 166, 1095–1111 (2012). https://doi.org/10.1007/s12010-011-9497-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9497-3