Abstract
This study reports on the effects of growth temperature on the secretion and some properties of the xylanase and β-xylosidase activities produced by a thermotolerant Aspergillus phoenicis. Marked differences were observed when the organism was grown on xylan-supplemented medium at 25 °C or 42 °C. Production of xylanolytic enzymes reached maximum levels after 72 h of growth at 42 °C; and levels were three- to five-fold higher than at 25 °C. Secretion of xylanase and β-xylosidase was also strongly stimulated at the higher temperature. The optimal temperature was 85 °C for extracellular and 90 °C for intracellular β-xylosidase activity, independent of the growth temperature. The optimum temperature for extracellular xylanase increased from 50 °C to 55 °C when the fungus was cultivated at 42 °C. At the higher temperature, the xylanolytic enzymes produced by A. phoenicis showed increased thermostability, with changes in the profiles of pH optima. The chromatographic profiles were distinct when samples obtained from cultures grown at different temperatures were eluted from DEAE–cellulose and Biogel P-60 columns.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microorganisms capable of growth at high temperatures produce more thermostable enzymes [7, 17]. Such enzymes are frequently more resistant and more efficient as catalysts than homologous proteins synthesized by mesophiles. Despite this, the majority of enzymes used in industrial processes have been isolated from mesophiles, due to the limited knowledge and difficulties of growing thermophiles on a large scale.
The xylanolytic complex has potential applications in food, feeding and the pulp/paper industry [4, 6, 21]. β-1,4-Endoxylanase (1,4-β-d-xylan xylohydrolase; EC 3.2.1.8) hydrolyzes xylan, a hemicellulose which consists of a backbone of β-1,4-linked xylopyranoside residues. β-d-Xylosidase (β-d-xyloside xylohydrolase; EC 3.2.1.37) hydrolyzes xylooligomers and xylobiose from the nonreducing end, liberating xylose. Fermentation technology to produce ethanol or xylitol from xylose is available [1, 8, 24] but, so far, only a few thermophilic microorganisms are able to grow on xylan and secrete thermoactive xylanolytic enzymes [10]. Some heat-stable xylanases have been described from archaea and bacteria, but these enzymes are mainly intracellular or cell-associated [14]. Although thermophilic/thermotolerant filamentous fungi are not as extreme as thermophilic/thermotolerant eubacteria or archaea, they typically excrete xylanases into the medium and produce several accessory xylanolytic enzymes which are necessary for debranching substituted xylan. Furthermore, the xylanase levels from fungal cultures are generally much higher than those from bacteria or yeast [3].
On an industrial scale, xylanases are produced mainly by Aspergilli, but the large majority of the strains employed for submerged fermentation develop at 28–30 °C [3, 18]. In our laboratory, during a screening program for β-d-xylosidase and xylanase production, a strain of Aspergillus phoenicis was isolated which grew well at 42 °C, apparently a characteristic of a thermotolerant fungus [16]. In this report, the production and secretion of xylanases is evaluated in this fungus, at two distinct temperatures: i.e. at a temperature sub-optimal for growth (25 °C) and at a temperature which is close to the upper limit for growth (42 °C). Some physiological and biochemical properties are compared, such as thermal stability, pH and optimum temperature of the xylanolytic enzymes produced at 25 °C and 42 °C.
Materials and methods
Organism and culture conditions
An Aspergillus strain was isolated from bagasse sugar cane compost and classified as A. phoenicis [15]. Stock cultures were propagated at 35 °C on slants of solid Vogel medium [22] supplemented with 0.75% yeast extract, 0.75% peptone and 1% glucose. SR liquid medium [16] supplemented with 1% xylan birchwood, pH 6.0, was used to grow A. phoenicis. Flasks were inoculated with a spore suspension to give a final concentration of 4×105 spores/ml of medium. Growth and production of enzymes were followed in 250-ml Erlenmeyer flasks, each containing 50 ml of culture medium and shaken at 100 rpm, at 25 °C or 42 °C, for 72 h.
Extraction of xylanolytic enzymes and protein determinations
Mycelia were harvested by filtration, rinsed with distilled water, blotted on filter paper and stored at −15 °C until used. The mycelial mass was homogenized in a mortar with acid-washed sea sand at 4 °C. After the addition of 10 ml of McIlvaine buffer [11], pH 4.0, cell disruption was continued for an additional 15 min. The slurry was then centrifuged (12,100 g, 15 min) and the supernatant was used to determine intracellular activities and protein levels [9]. The culture filtrate was dialyzed overnight against the same buffer, and used to determine extracellular activities.
Growth (1–24 h) was estimated in terms of total protein, as follows: culture aliquots were precipitated with 10% trichloroacetic acid at 4 °C. The precipitate was collected by centrifugation (12,000 g, 10 min) and resuspended with 1 N NaOH. After boiling the precipitate for 10 min, proteins in the supernatant were estimated by the method of Lowry [9], using bovine serum albumin as standard.
Enzymatic assays
β-d-Xylosidase was determined with p-nitrophenyl-β-d-xyloside [16]. One unit was defined as the amount of enzyme that liberated 1 μmol p-nitrophenol/min.
Xylanase was assayed by measuring the reducing groups released from birchwood xylan [13]. The reaction mixture consisted of 250 μl of 1% xylan, 150 μl of McIlvaine buffer, pH 4.0, and 100 μl of enzymatic extract appropriately diluted. The reaction mixture was incubated at 50 °C for 15 min. One unit was defined as the amount of enzyme that released 1 μmol xylose/min under the assay conditions.
Temperature, pH and thermostability of xylanase and β-xylosidase
The effect of temperature on extracellular and intracellular β-xylosidase and xylanase activities was analyzed using cultures of A. phoenicis grown in SR medium at 25 °C or 42 °C. The assays were performed in McIlvaine buffer, pH 4.0, at 5 °C intervals over 30–70 °C (xylanase), or 30–95 °C (β-xylosidase). The effect of pH was assayed using the same buffer in the pH range 3.0–8.0 at 50 °C for xylanase and 75 °C for β-xylosidase. Thermal inactivation was analyzed with enzymes incubated at 70 °C or 80 °C (β-xylosidase) and 50 °C or 60 °C (xylanase). At different time intervals, aliquots were withdrawn and residual activities were measured as described above.
Purification of xylanases
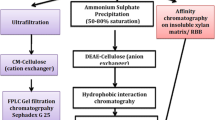
After cultivation of the microorganism at 25 °C or 42 °C, the culture filtrate was dialyzed overnight against 10 mM sodium phosphate buffer, pH 8.0, and applied to a DEAE–cellulose column (6.4×2.0 cm) previously equilibrated with the same buffer. A 200-ml gradient of NaCl (0.0–0.3 M) was applied and 10 ml fractions were collected at a flow rate of 20 ml/h. Absorbance at 280 nm (A 280) and xylanase activity were determined for all fractions. Fractions exhibiting xylanase activity were pooled, dialyzed against distilled water and lyophilized. The concentrated samples were resuspended in 100 mM sodium acetate buffer, pH 4.0, and then applied to a Biogel P-60 filtration column (89.0×1.8 cm) previously equilibrated with the same buffer. Fractions (1 ml) were collected and A 280 and xylanase activity were determined. Fractions containing enzymatic activity were pooled and dialyzed for subsequent analyses.
Reproducibility of results
All results are the mean of at least three independent experiments.
Results
Influence of temperature on the growth and secretion of proteins by A. phoenicis
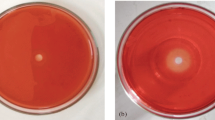
Figure 1 shows germinating spores of A. phoenicis cultures supplemented with xylan as carbon source, incubated for 8 h in SR medium, either at 25 °C (Fig. 1A) or 42 °C (Fig. 1B). Spores germinated faster at 42 °C and the growth yield was higher than at 25 °C (Fig. 1C). The upper limit of temperature for growth of the fungus might be close to 42 °C. At 45 °C, the rate of growth decreased (data not shown), although it still offered conditions for high levels of β-xylosidase production [16]. In view of these results, it was considered worthwhile to investigate the effect of the growth temperature on the production of xylanolytic enzymes.
Growth of Aspergillus phoenicis. A, B Spore germination of A. phoenicis cultivated at 25 °C (A) and 42 °C (B) for 8 h in SR medium [16], using xylan as carbon source. C Growth at 25 °C (○) and 42 °C (●). Growth was expressed as total protein (see Materials and methods)
Influence of temperature on the production of xylanolytic activities
The temperature of incubation exerted a marked influence on the production and secretion of xylanolytic enzymes (Fig. 2). The highest levels of intracellular and extracellular β-d-xylosidase (Fig. 2A) and xylanase (Fig. 2B) were observed at 42 °C and resulted in three- to five-fold higher levels than at 25 °C. At the higher temperature, the release of both enzymes into the culture medium was achieved in about 10 h.
Influence of temperature on the intrinsic properties of β-d-xylosidase and xylanase
Maximum temperatures for β-xylosidase activities, both extracellular and intracellular, were 85–90 °C, regardless of growth temperature (Fig. 3A, B). This result differed from data obtained with extracellular β-xylosidase (75 °C) previously purified from the same fungus [16], suggesting that some components of the medium might stabilize the enzyme.
The temperature profile for xylanase activities, in contrast, shifted toward a higher temperature range. For the extracellular enzyme, the optimum temperature increased from 50 °C to 55 °C for cultures grown at 42 °C, and the enzyme activity was greater within the range 55–65 °C than that of cultures grown at 25 °C (Fig. 3C). The temperature profile of the intracellular xylanase activity (Fig. 3D) also showed a shift toward a higher temperature range, but this was not as striking as that of the extracellular enzyme.
While the pH profile for β-xylosidase activities (optimum at pH 3.5–5.5; Fig. 4A, B) and that for the intracellular xylanase (Fig. 4D) were not significantly affected by the growth temperature, that of the extracellular xylanase was altered when the fungus was cultivated at 42 °C, compared with the culture grown at 25 °C (Fig. 4C). Maximum levels of extracellular xylanase activity were pH 3.5–5.5, for cultures incubated at 25 °C. However, for cultures grown at 42 °C, the optimum pH of the extracellular xylanase was shifted to a more acidic range, with a maximum at pH 3.5.
β-Xylosidase and xylanase were completely stable up to 60 min at 70 °C and 50 °C, respectively (data not shown). Higher temperatures (80 °C for β-xylosidase, 60 °C for xylanase) significantly decreased the enzymatic activity, but the enzymes from cultures incubated at 42 °C were somewhat more resistant to thermal inactivation than those from cultures grown at 25 °C (Fig. 5). Comparing these results with those previously reported for β-xylosidase [16], it was verified that the crude enzyme obtained at 42 °C was more thermostable than the purified enzyme.
Thermal inactivation of β-xylosidase (A) and xylanase (B) from A. phoenicis. The organism was cultivated in SR medium at 25 °C (□, ○) and 42 °C (■, ●). β-Xylosidase was incubated at 80 °C and xylanase at 60 °C. The extracellular and intracellular specific activities are represented by squares and circles, respectively
Chromatographic profile of extracellular xylanase activities
In order to know whether the xylanase activities produced at 25 °C and 42 °C were composed of the same or different isoforms, the fungus was cultivated at these temperatures and the enzymes produced were partially purified by chromatographic separation (data not shown). When the microorganism was cultivated at 42 °C, three distinct peaks with xylanase activity were resolved by DEAE–cellulose chromatography. Pool I (approx. 52% of total recovered activity) did not bind to the resin, while pools II (27%) and III (21%) were eluted with 0.1 M and 0.2 M NaCl, respectively. Pools I, II and III were separately submitted to gel-sieving in a Biogel P-60 column. Pool I was resolved into three fractions (approx. 39.8 kDa, 22.2 kDa, 8.8 kDa). Pool II gave a single sharp peak of xylanase activity (approx. 13.7 kDa). Pool III was resolved as two xylanase activity peaks (approx. 14.4 kDa, 5.7 kDa).
A different chromatographic profile of xylanase activity was obtained when the organism was cultivated at 25 °C. Only two xylanase activity peaks were resolved by DEAE–cellulose chromatography. Pool I (approx. 50% of recovered activity) did not interact with the resin. Pool II eluted as a wide peak, spreading along the gradient. Pools I and II were submitted to gel-sieving in Biogel P-60. Pool I was resolved into three xylanase peaks (approx. 16.9 kDa, 13.6 kDa, 6.1 kDa), but pool II was resolved into two peaks (approx. 17.8, 7.4 kDa). Therefore, the chromatographic profiles of the xylanases produced at lower and higher temperatures indicate that the observed differences in biochemical properties must be a consequence of different isoforms.
Discussion
The adaptation of a microbial metabolism to perform efficiently at different temperatures is fascinating. It seems evident that thermal adaptation cannot be attributed to any specific characteristic, but results from various changes which contribute to the stability of the organism in an additive manner. This might be the result of many intracellular adjustments, such as membrane lipids composition, structures of proteins and protein synthesis machinery, or cytoplasmic thermostabilizing substances, such as polyamines [5, 7, 17]. Nevertheless, the molecular basis of protein thermostability is still not totally understood [2].
In the present study, it was shown that the growth temperature exerted a marked influence on the production and secretion of the xylanolytic enzymes of A. phoenicis. A similar effect of temperature has been reported in other fungal species (for a review, see [3]). Hydrolases, such as glucoamylase and invertase, increased two- to three-fold when Arxula adeninivorans grew at 45 °C, compared with cultures grown at 30 °C [23]. Also, a gradual increase in xylanase and endoglucanase activities was reported for two Trichoderma reesei strains, QM 6a and the hypersecretory mutant RL-P37, cultivated at 25 °C, 30 °C and 37 °C in lactose- and xylan-supplemented media. Both strains grew equally well at the three temperatures, with no significant change in protein secretion for Trichoderma QM 6a, whereas overall protein secretion increased three-fold for strain RL-P37 at 37 °C. Both strains secreted more xylanase and endoglucanase at higher temperatures [20].
This study also showed that some intrinsic properties of the xylanases produced by Aspergillus phoenicis, grown at 42 °C, were significantly different from those produced at 25 °C. Not only were the xylanase-specific activities three- to five-fold higher than those produced at 25 °C, but also the optima of pH and temperature; and to some extent the thermostability changed significantly. The chromatographic profiles were distinct, suggesting that A. phoenicis, when cultivated at 42 °C or 25 °C, produced different xylanase forms.
A change in the intrinsic properties of the xylanase from Aspergillus FP-470 was also reported by Mendicuti-Castro [12]. In that case, a change from 37 °C to 45 °C resulted in a switch of optimum pH from 6.5 to 4.3 and a switch of temperature optimum from 80 °C to 50 °C. While the pH change reported by those authors was in the same direction as that observed in the present study, the change in temperature optimum was the opposite; i.e. the enzyme produced at 45 °C displayed a lower temperature optimum than that produced at 37 °C. For A. phoenicis, these changes in the properties of xylanase may have an adaptative value, by improving the ability of the organism to degrade xylan-containing materials at more elevated temperatures. This seems to confirm the opinion that the thermophilic attributes, including improved enzyme thermostability, result from a series of modifications of pre-existing mesophilic functions, in order to optimize survival at higher temperatures [19].
All properties cited may be important for improving biotechnological applications of this important xylanolytic complex in its several purposes.
References
Aristidou A, Penttilä M (2000) Metabolic engineering applications to renewable resource utilization. Curr Opin Biotechnol 11:187–198
Arnold FH, Wintrode PL, Miyazaki K, Gershenson A (2001) How enzymes adapt: lessons from directed evolution. Trends Biochem Sci 26:100–106
Haltrich D, Nidetzky B, Kulbe KD, Steiner W, Zupancic S (1996) Production of fungal xylanases. Bioresour Technol 58:137–161
Harris GW, Pickersgill RW, Connerton I, Debeire P, Touzel JP, Breton C, Pérez S (1997) Structural basis of the properties of an industrially relevant thermophilic xylanase. Proteins Struct Funct Gen 29:77–86
Jaenicke R, Böhm G (1998) The stability of proteins in extreme environments. Curr Opin Struct Biol 8:738–748
Kulkarni N, Shendye A, Rao M (1999) Molecular aspects of xylanases. FEMS Microbiol Rev 23:411–456
Lasa I, Berenguer J (1993) Thermophilic enzymes and their biotechnological potential. Microbiologia 9:77–89
Lee J (1997) Biological conversion of lignocellulosic biomass to ethanol. J Biotechnol 56:1–24
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:267–275
Maheshwari R, Bharadwaj G, Bhat MK (2000) Thermophilic fungus: their physiology and enzymes. Microbiol Mol Biol Rev 4:461–488
McIlvaine TC (1921) A buffer solution for colorimetric comparison. J Biol Chem 49:183–186
Mendicuti-Castro LPM, Trejo-Aguilar BA, Osorio GA (1997) Thermostable xylanases produced at 37 °C and 45 °C by a thermotolerant Aspergillus strain. FEMS Microbiol Lett 146:97–102
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–429
Niehaus F, Bertoldo C, Kähler M, Antranikian G (1999) Extremophiles as a source of novel enzymes for industrial application. Appl Microbiol Biotechnol 51:711–729
Raper KB, Fennell DI (1965) The genus Aspergillus. Williams & Wilkins, Baltimore
Rizzatti ACS, Jorge JA, Terenzi HF, Rechia CGV, Polizeli MLTM (2001) Purification and properties of a thermostable extracellular β-d-xylosidase produced by a thermotolerant Aspergillus phoenicis. J Ind Microbiol Biotechnol 26:156–160
Slapack GE, Russell I, Stewart GG (1987) General aspects of thermophily. In: Slapack GE, Russell I, Stewart GG (eds) Thermophilic microbes in ethanol production. CRC Press, Boca Raton, Fla., pp 3–25
Smith DC, Blat KM, Wood TM (1991) Xylan-hydrolysing enzymes from thermophilic and mesophilic fungi. World J Microbiol Biotechnol 7:475–484
Somero GN (1995) Proteins and temperature. Annu Rev Physiol 57:43–68
Suh DH, Thomas CB, Sands JA, Montenecourt BS (1988) Effects of temperature on xylanases secretion by Trichoderma reesei. Biotechnol Bioeng 32:821–825
Sunna A, Antranikian G (1997) Xylanolytic enzymes from fungi and bacteria. Crit Rev Biotechnol 17:39–67
Vogel HF (1964) Distribution of lysine pathways among fungi: evolutionary implications. Am Nat 98:435–446
Wartmann T, Krüger A, Adler K, Duc BM, Kunze I, Kunze G (1995) Temperature-dependent dimorphism of the yeast Arxula adeninivorans Ls3. Antonie Van Leeuwenhoek 68:215–223
Wheals AE, Basso LC, Alves DMG, Amorim HV (1999) Fuel ethanol after 25 years. Trends Biotechnol 17:482–487
Acknowledgements
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). J.A.J., H.F.T. and M.L.T.M.P. are Research Fellows of CNPq. A.C.S.R. was the recipient of a CNPq fellowship. This study is part of a doctoral thesis submitted by A.C.S.R. The authors thank Ricardo Alarcon and Mauricio de Oliveira for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rizzatti, A.C.S., Sandrim, V.C., Jorge, J.A. et al. Influence of temperature on the properties of the xylanolytic enzymes of the thermotolerant fungus Aspergillus phoenicis . J IND MICROBIOL BIOTECHNOL 31, 88–93 (2004). https://doi.org/10.1007/s10295-004-0120-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-004-0120-2