Abstract
Endoplasmic reticulum (ER) is the key organelle involved in protein folding and maturation. Emerging studies implicate the role of ER stress in the development of chronic kidney disease. Thus, there is an urgent need for compounds that could ameliorate ER stress and prevent CKD. Piperine and its analogs have been reported to exhibit multiple pharmacological activities; however, their efficacy against ER stress in kidney cells has not been studied yet. Hence, the goal of this study was to synthesize amide-substituted piperine analogs and screen them for pharmacological activity to relieve ER stress using an in vitro model of tunicamycin-induced ER stress using normal rat kidney (NRK-52E) cells. Five amide-substituted piperine analogs were synthesized and their chemical structures were elucidated by pertinent spectroscopic techniques. An in vitro model of ER stress was developed using tunicamycin, and the compounds of interest were screened for their effect on cell viability, and the expression of ER chaperone GRP78, the pro-apoptotic ER stress marker CHOP, and apoptotic caspases 3 and 12 (via western blotting). Our findings indicate that exposure to tunicamycin (0.5 μg/mL) for 2 h induces the expression of GRP78 and CHOP, and apoptotic markers (caspase-3 and caspase-12) and causes a significant reduction in renal cell viability. Pre-treatment of cells with piperine and its cyclohexylamino analog decreased the tunicamycin-induced upregulation of GRP78 and CHOP and cell death. Taken together, our findings demonstrate that piperine and its analogs differentially regulate ER stress, and thus represent potential therapeutic agents to treat ER stress-related renal disorders.

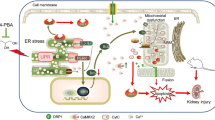
Piperine (PIP) reduces the expression of ER stress markers (GRP78 and CHOP) induced by pathologic stimuli and consequently decreases the activation of apoptotic caspase-12 and caspase-3; all of which contributes to its chemical chaperone and cytoprotective properties to protect renal cells against ER stress and ER stress-induced cell death, and would ultimately prevent the development of chronic kidney disease
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endoplasmic reticulum (ER) is one of the most versatile and adaptable organelles in eukaryotic cells (Brandizzi et al. 2014; Staehelin 1997). It plays a principal role in controlling the synthesis, folding and maturation of luminal, secreted and transmembrane proteins (Brandizzi et al. 2014; Staehelin 1997; Zhuang and Forbes 2014). During protein synthesis, only those proteins folded in proper configuration and underwent specific post-translational modifications will be processed through the ER secretory pathway and translocated to Golgi bodies (Zhuang and Forbes 2014). If cells cannot mitigate the changes caused by unfolded or misfolded proteins and restore homeostasis, it results in ER stress and activation of unfolded protein response (UPR) (Walter and Ron 2011).
The UPR is a collection of phylogenetically conserved signaling pathways that attempts to re-establish homeostasis through inducing several chaperones. It is composed of three principal pathways: PERK (PKR-like endoplasmic reticulum kinase), IRE1 (inositol-requiring enzyme 1), and ATF6 (activating transcription factor 6) (Nagelkerke et al. 2014; Walter and Ron 2011). Under normal conditions, a chaperone named glucose-regulated protein 78 (GRP78) is connected to the three transducers of UPR (i.e., PERK and IRE1 in the amino-terminal and ATF6 in the carboxy-terminal in the intraluminal domain), and retain them inactive. Dissociation of GRP78 from PERK, IRE1, or ATF6 results in the activation of UPR signaling (Nagelkerke et al. 2014). If the ER stress is severe and prolonged, and homeostasis is not restored by the adaptive mechanism of the UPR, then the cell initiates a pro-apoptotic response rather than a pro-survival response (Sovolyova et al. 2014).
Numerous studies have provided evidence for the involvement of ER stress in chronic kidney disease (CKD) (Henriquez et al. 2005; Inagi 2010; Markan et al. 2009; Peyrou and Cribb 2007). Currently, CKD is considered as one of the most significant global health issues, which not only endangers the health of our society but also places a huge burden on our health-care cost (Drawz et al. 2015; Henriquez et al. 2005). Studies have found that once the diagnosis of CKD is established, the rate of decline in kidney function was found to be 2.3 to 4.5 mL/min/year (Drawz et al. 2015). Thus, a prophylaxis to hinder the deterioration of kidney function is a better strategy than treatment, especially in high-risk populations such as patients with diabetes and/or hypertension (Drawz et al. 2015; Markan et al. 2009; Rogers et al. 2013).
Findings from a study conducted on kidney biopsies taken from patients with primary glomerulonephritis, one of the primary glomerular diseases (PGD), revealed increased expression of GRP78 and GADD153/CHOP, and decrease in the expression of anti-apoptotic Bcl-2 proteins in PGD (Markan et al. 2009). Several other studies also confirmed the involvement of ER stress in a wide variety of kidney diseases (Cybulsky et al. 2005; Cybulsky et al. 2009; Fujii et al. 2006; Inagi et al. 2005; Schonthal 2012). Thus, prevention or treatment of ER stress could serve as a therapeutic strategy to combat kidney disease and prevent the onset of CKD in patients.
Natural products have served as a valuable source of drugs and are considered as potential leads for drug development (Doucette et al. 2015). Black pepper (Piper nigrum) is commonly used in conventional medicine especially in Chinese and Indian medicine to treat several ailments (Doucette et al. 2015; Yoon et al. 2015). The medicinal properties of black pepper are mainly attributed to its major phytoconstituent piperine. Piperine [1-[5-[1,3-benzodioxol-5-yl]-1-oxo-2,4-pentadienyl] piperidine] (PIP) is one of the four diastereomeric geometric isomers isolated from black pepper (Greenshields et al. 2015). It is also found in Piper longum plants, which belongs to the family Piperaceae (Meghwal and Goswami 2013). The chemical structure of piperine is composed of three essential components: piperidine moiety linked through carbonylamide linkage to the side chain, methylenedioxyphenyl ring and conjugated double bond chain.
Many recent studies have confirmed the medical properties of piperine and further demonstrated its efficacy as anticarcinogenic, hepatoprotective, anti-inflammatory, anti-arthritic, antidepressant, and antimicrobial (Kumar et al. 2015; Meghwal and Goswami 2013). More recently, a study by Yaffe et al. attempted to investigate the mechanisms by which piperine mediates cell cycle arrest and apoptosis in colon cancer revealed that its pro-apoptotic effects are mediated through increased expression of CHOP and GRP78 in colon cancer cells (Yaffe et al. 2015). Paradoxical to these findings, a study using a high fat diet (HFD)-induced model of hepatic steatosis indicated that piperine decreases the messenger RNA (mRNA) expression of GRP78 in the liver tissues of mice fed an HFD (Jwa et al. 2012). To the best of our knowledge, the effect of piperine on ER stress in kidney has not yet been studied. Furthermore, although piperine and its amide piperine analogs have been reported to exhibit diverse pharmacological activities in various disease models (Faas et al. 2008; Ferreira et al. 2011; Greenshields et al. 2015; Kumar et al. 2015; Meghwal and Goswami 2013; Wattanathorn et al. 2008), none of those studies were targeted to elucidate the impact of piperine and its amide piperine analogs on ER stress markers in kidney cells.
We hypothesize that piperine (and potentially its analogs) would attenuate ER stress and protect renal cells against ER stress-induced cell death. Hence, the objectives of this study were as follows: (1) to synthesize an array of amide-substituted piperine analogs and characterize the prepared analogs using pertinent spectroscopic techniques (2) to establish an in vitro model of ER stress-induced cell injury using tunicamycin in normal rat kidney (NRK-52E) cells, and (3) to evaluate the pharmacological activity of piperine and the prepared piperine analogs to relieve ER stress and associated cell death in the established in vitro model.
Materials and methods
Materials used
All chemicals and reagents used for synthesis of piperine analogs were of analytical grade and obtained from Sigma-Aldrich, Germany. All reactions were monitored by thin layer chromatography (TLC) and the spots were visualized using ultraviolet (UV) transilluminator. TLC was conducted on pre-coated silica gel aluminum plates (Merck, USA). Melting points of the synthesized compounds were measured as range using Stuart SMP40 automatic melting point apparatus. The infrared (IR) spectra were recorded on Perkin Elmer Spotlight 400 Fourier transform-infrared (FT-IR) spectrophotometer. The spectra were acquired using a universal attenuated total reflectance (UATR) sensor to allow the application of the solid samples.
The prepared compounds were analyzed for carbon (C), hydrogen (H), and nitrogen (N) (i.e., elemental analysis) using Thermo Scientific Flash 2000 in the Central Laboratory Unit at Qatar University. Mass spectra (MS) were recorded on an Agilent 6460 Triple Quadrupole LC/MS system using electrospray ionization (ESI) by direct injection technique and reported as [M+1]+. Proton (1H) and carbon (13C) NMR spectra were recorded using Bruker Avance III 400 MHz apparatus, and the chemical shifts were expressed in δ (ppm) with reference to DMSO-d6 peak.
Synthesis of piperic acid (PA1) from piperine (PIP)
Piperine (2 g, 7 mmol) was refluxed with 100 mL of 2 M ethanolic potassium hydroxide for 25 h, and the ethanol was evaporated under reduced pressure. The separated solid (potassium piperate) was filtered and washed with cold ethanol, and then dissolved in warm water and gradually acidified with diluted HCl. The obtained yellow precipitate (piperic acid) was filtered and washed with cold water. The crude product (PA1) was recrystallized from ethanol to yield 1.4 g (6.4 mmol) of yellow crystalline piperic acid (90% yield).
Synthesis of piperine analogs (PA2 to PA4)
The selected amido-piperine analogs (PA2 to PA4) were prepared as described previously (Koul et al. 2000). Briefly, to a solution of piperic acid (10 mmol) in 25 mL dichloromethane (DCM), 2.0 mL (27.6 mmol) of thionyl chloride was added. The mixture was kept under reflux for 1 h. Excess thionyl chloride was removed under reduced pressure using rotary evaporator. The obtained residue (piperoyl chloride) was dissolved in 20 mL DCM and the amine (10 mmol) in 20 mL DCM was added dropwise. The mixture was stirred for 1 h at room temperature. The solvent was evaporated and the residue was crystallized from ethyl acetate. The yield of the final products (PA2 to PA4) was in the range of 40–65%.
Cell culture
NRK-52E (rat renal proximal tubular cell line) was purchased from Health Protection Agency, UK. Dimethyl sulfoxide, piperine, thiazolyl blue tetrazolium bromide (MTT), 4-pheynylbutryric acid (4PBA), and tunicamycin were purchased from Sigma-Aldrich, Germany. Bicinchoninic acid (BCA) protein assay reagent, Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum (FBS), l-glutamine, penicillin-streptomycin (pen-strep), and phosphate-buffered saline (PBS) were purchased from Thermo, UK. All primary and secondary antibodies were purchased from Abcam, UK, except for CHOP (GADD153) and cleaved caspase-3 primary antibodies, which were obtained from Santa Cruz Biotechnology, Germany, and Cell Signaling Technology (CST), Netherlands, respectively.
NRK-52E cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin and 200 mM l-glutamine (complete cell culture media). Cells were grown in 100-mm tissue culture dish and kept in a 5% CO2 humidified incubator at 37 °C. The stock solution of tunicamycin was diluted in media to a final concentration of 0.5 μg/mL. 4PBA was diluted in media and piperine analogs were dissolved in DMSO.
Drug treatments
Twenty-four hours after seeding of cells on to 48-well plates, piperine or its analogs were added to cells at either 250 or 500 nM concentrations for 24 h. After 24 h of pre-treatment, the old media was removed and the cells were washed with PBS and another fresh media containing 0.5 μg/mL tunicamycin was added and cells were incubated for 2 h. After the completion of tunicamycin treatment, the media was removed and cells were washed twice with PBS and new fresh complete media was added for another 22 h. To compare the potency and efficacy of piperine and its analogs to inhibit ER stress and ER stress-induced cell death, we used 4-phenylbutyrate (4PBA), a well-known chemical chaperone, at 1 and 2 mM concentrations in the model.
Cell viability assay
The effect of piperine and its analogs (PA1 to PA4) on cell viability in the developed in vitro model of ER stress was assessed by MTT assay, which is based on the enzymatic conversion of yellow tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide by mitochondrial dehydrogenases into purple formazan. After the completion of the treatment, the media was removed and replaced with fresh media containing 25 μL MTT (0.5 mg/mL) for 3–4 h. Next, the media was removed carefully and 100 μL of DMSO was added to dissolve the formazan crystals. The absorbance was read at 570 nm using SpectraMax M2 multimode plate reader (Molecular Devices, USA).
Quantification of expression of ER stress markers and apoptotic caspases
Total protein lysates from cell culture were normalized by bicinchoninic acid (BCA) protein assay. Proteins were separated using SDS-PAGE with an acrylamide concentration 15%. Equal concentrations (25 μg) of sample protein were mixed with 4× sample loading buffer and electrophoresed for 20 min at 70 V followed by 90 min at 140 V (Bio-Rad, USA). Blots were incubated with rabbit anti-GRP-78 (ab21685, 1:2000), mouse anti-CHOP (ab11419, 1:500), rabbit anti-caspase-12 (ab62484; 1: 1000), and rabbit anti-cleaved caspase-3 (CST 9664; 1:1000) primary antibodies overnight at 4 °C and subsequently with a horseradish peroxidase (HRP)-labeled-goat anti-mouse IgG (1:10,000; Abcam, UK) or HRP-conjugated goat anti-rabbit IgG (1:20,000; Abcam, UK) secondary antibodies respectively for 1 h at room temperature. Bands were visualized using enhanced chemiluminesence (ECL) detection kit (Abcam, UK) and the band intensities were quantified using a FluorChem-M imaging system (Protein Simple, USA). The densitometry values were normalized to beta-actin (used as loading control) and expressed as percentage of DMSO-treated control.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 7. Data were expressed as mean ± SEM relative to vehicle-treated control. One-way analysis of variance (ANOVA) test followed by Tukey’s post hoc test was used to determine statistical differences between the mean values of the different experimental groups. A P value less than 0.05 was considered statistically significant.
Results
Synthesis and characterization of amide-substituted piperine analogs
The spectral and analytical data of the synthesized piperine analogs were consistent with the previously reported data in the literature (Koul et al. 2000; Sangwan et al. 2008; Venkatasamy et al. 2004). Piperic acid (PA1) was obtained by alkaline hydrolysis of piperine (PIP). Amide-substituted analogs (PA2 to PA4) were prepared by conversion of PA1 to the corresponding acyl chloride using thionyl chloride followed by the reaction with the appropriate amine as described by Koul et al. (2000). The physical properties and molecular formulae of the synthesized analogs and the parent compound piperine are shown in Table 1.
All the IR spectra conducted on the solid sample using UATR confirmed the presence of C=O stretching band, in the range of 1650 cm−1. From the mass spectra, it was possible to obtain the mass of the molecular ion. The mass spectrum of the products was recorded on an Agilent 6460 Triple Quadrupole LC/MS system using electrospray ionization (ESI) by direct injection technique and reported as [M+1]+. To further confirm the structures of the synthesized compound elemental analysis, 13C NMR and 1H NMR spectra were analyzed.
Effect of tunicamycin on cell viability and the expression of ER stress markers in NRK-52E cells
Incubation of subconfluent cultures of NRK-52E cells with 0.5 μg/mL tunicamycin for 2 h resulted in a significant loss of cell viability (evidenced by ~64% reduction in cell viability) as demonstrated by decreased reduction of MTT (Fig. 1a). Cells were also subjected to western blot analysis, at which there was a significant increase in the expression of GRP78 and CHOP upon exposure to tunicamycin. These results evidenced that using tunicamycin at a concentration of 0.5 μg/mL for 2 h caused induction of ER stress and consequent activation of UPR in NRK-52E cells (Fig. 1b–d).
a Dose-response studies with tunicamycin (TM) in NRK-52E cells. Cell viability was determined by MTT assay. Values were expressed as percentage of DMSO-treated control (mean ± SEM; n = 3). b Effect of tunicamycin (TM) on GRP78 and CHOP expression in NRK-52E cells as determined by western blotting and quantified using densitometry (c, d). Values were normalized using β-actin and expressed as percentage of DMSO-treated control (mean ± SEM; n = 10). #P < 0.05 compared to DMSO-treated control group
Dose-response studies (tolerability) for piperine and its analogs in NRK-52E cells
We compared the potency and efficacy of piperine and its analogs against 4-phenylbutyrate (4PBA), a well-known chemical chaperone, used at 1 and 2 mM concentrations (Carlisle et al. 2014) (Fig. 2a–d). NRK-52E cells were treated with piperine or its analogs at two concentrations—250 and 500 nM—for 24 h and the vehicle-control group received the highest vehicle concentration (0.1% DMSO) used in the study. Our results demonstrate that single doses of piperine or any of its analogs at up to 500 nM were well tolerated by NRK-52E cells (Figs. 3a, 4a, 5a, 6a, and 7a). The tolerability of NRK-52E cells was measured by MTT assay. Based on the tolerability studies, we chose 250 and 500 nM as optimal concentrations for the prepared analogs.
a Effect of 4PBA on tunicamycin (TM)-induced loss of cell viability (measured by MTT assay) in NRK-52E cells. Values were normalized to TM-treated control and expressed as (mean ± SEM; n = 3). b Effect of 4PBA on tunicamycin (TM)-induced expression of GRP78 and CHOP in NRK-52E cells as determined by western blotting and quantified using densitometry (c, d). Values were normalized to β-actin and expressed as percentage of DMSO-treated control for GRP78 and percentage of tunicamycin (TM)-treated group for CHOP (mean ± SEM; n = 3). #P < 0.05 compared to DMSO-treated control group; *P < 0.05 compared to TM-treated group
a Effect of piperine (PIP) on tunicamycin (TM)-induced loss of cell viability (measured by MTT assay) in NRK-52E cells. Values were normalized to DMSO-treated control and expressed as mean ± SEM (n = 3). b Effect of piperine (PIP) on tunicamycin (TM)-induced expression of GRP78 and CHOP expression in NRK-52E cells as determined by western blotting and quantified using densitometry (c, d). Values were normalized to β-actin and expressed as percentage of DMSO-treated control for GRP78 and percentage of tunicamycin (TM)-treated group for CHOP (mean ± SEM; n = 3). #P < 0.05 compared to DMSO-treated control group; *P < 0.05 compared to TM-treated group
a Effect of piperic acid (PA1) on tunicamycin (TM)-induced loss of cell viability (measured by MTT assay) in NRK-52E cells. Values were normalized to TM-treated control and expressed as mean ± SEM (n = 3). #P < 0.05 compared to DMSO-treated control group; *P < 0.05 compared to TM-treated group. b Effect of piperic acid (PA1) on tunicamycin (TM)-induced expression of GRP78 and CHOP in NRK-52E cells as determined by western blotting and quantified using densitometry (c, d). Values were expressed as percentage of DMSO-treated control for GRP78 and percentage of TM-treated group for CHOP (mean ± SEM; n = 3)
a Effect of cyclohexylamino analog (PA2) on tunicamycin (TM)-induced loss of cell viability (measured by MTT assay) in NRK-52E cells. Values were normalized to DMSO-treated control and expressed as mean ± SEM (n = 3). b Effect of cyclohexylamino analog (PA2) on tunicamycin (TM)-induced expression of GRP78 and CHOP as determined by western blotting and quantified using densitometry (c, d). Values were normalized using β-actin and expressed as percentage of DMSO-treated control for GRP78 or percentage of TM-treated group for CHOP (mean ± SEM; n = 3). #P < 0.05 compared to DMSO-treated control group; *P < 0.05 compared to TM-treated group
a Effect of diethylamino analog (PA3) on tunicamycin (TM)-induced loss of cell viability (measured by MTT assay) in NRK-52E cells. Values were normalized to DMSO-treated control and expressed as mean ± SEM (n = 3). b Effect of diethylamino analog (PA3) on tunicamycin (TM)-induced expression of GRP78 and CHOP in NRK-52E cells as determined by western blotting and quantified using densitometry (c, d). Values were normalized using β-actin and expressed as percentage of DMSO-treated control for GRP78 or percentage of TM-treated group for CHOP (mean ± SEM; n = 3). #P < 0.05 compared to DMSO-treated control group; *P < 0.05 compared to TM-treated group
a Effect of pyrrolidinyl analog (PA4) on tunicamycin (TM)-induced loss of cell viability (measured by MTT assay) in NRK-52E cells. Values were normalized to DMSO-treated control and expressed as mean ± SEM (n = 3). #P < 0.05 compared to DMSO-treated control group; *P < 0.05 compared to TM-treated group. b Effect of pyrrolidinyl analog (PA4) on tunicamycin (TM)-induced expression of GRP78 and CHOP in NRK-52E cells as determined by western blotting and quantified using densitometry (c, d). Values were normalized using β-actin and expressed as percentage of DMSO-treated control for GRP78 or percentage of TM-treated group for CHOP (mean ± SEM; n = 3)
Effect of piperine and its analogs on tunicamycin-induced loss of renal cell viability
NRK-52E cells were pre-treated with the reference standard 4PBA, piperine (PIP), or synthesized analog (PA1 to PA4) at the aforementioned concentrations for 24 h and then exposed to tunicamycin (0.5 μg/mL for 2 h) to induce cell injury. Upon measuring the cell viability (using MTT assay) at 24 h post-tunicamycin exposure, the results have demonstrated the ability of PIP, PA2, and PA4 to protect against TM-induced loss of viability in NRK-52E cells as seen in Table 2. While the rest of the screened compounds including the reference standard revealed no significant changes in cell viability as measured by the MTT assay (Fig. 3a).
Effect of piperine and its analogs on tunicamycin-induced expression of ER stress markers
To investigate the protective effect of pharmacologically evaluated compounds against TM-induced ER stress, NRK-52E cells were seeded on 6-well plates and grown to sub-confluency were treated with piperine for 24 h. After 24-h treatment with piperine (PIP), cells were exposed to 0.5 μg/mL TM for 2 h and evaluated for the expression of GRP78 and CHOP at 24 h post-tunicamycin exposure. Densitometry analysis were consistent with the results obtained from the MTT assay for both PIP and PA2 as demonstrated by decreased protein levels of GRP78 and CHOP (Figs. 3 and 5). The average percentage of decrease in GRP78 was 33% by using PIP at 500 nM concentration. PIP also diminished the ability of TM to induce CHOP by about 25% in comparison to TM-treated group. Similar to PIP, cells treated with PA2 showed decreased expression of GRP78 (by about 25%) and CHOP (by about 30%) in comparison to TM-treated group. The reference standard, 4PBA, decreased the expression of GRP78 (~15%), and CHOP (~70%) compared to TM-treated group (Fig. 2). PA1 and PA4 did not affect the expression of proteins tested (Table 2; Figs. 4 and 7). Intriguingly, the PA3 caused a paradoxical increase in the expression of CHOP by almost 69% using the concentration of 250 nM (Fig. 6).
Effect of piperine on tunicamycin-induced expression of apoptotic markers
To determine the mechanism(s) that underlie the protective effect of piperine against tunicamycin-induced ER stress and loss of cell viability, we evaluated its effect on the expression of apoptotic caspase-12 and caspase-3 at 24 h post-tunicamycin exposure. In correlation to our findings from MTT assay, pre-treatment with PIP at 500 nM caused a significant reduction (P < 0.05) in the protein levels of both apoptotic caspases induced in renal cells following exposure to tunicamycin (Fig. 8).
Effect of piperine (PIP) on tunicamycin (TM)-induced expression of cleaved caspase-12 and caspase-3 in NRK-52E cells as determined by western blotting (a) and quantified using densitometry (b, c). Values were normalized to β-actin and expressed as percentage of DMSO-treated control (mean ± SEM; n = 3). #P < 0.05 compared to DMSO-treated control group; *P < 0.05 compared to TM-treated group
Discussion
Piperine, the major ingredient of pepper species, is reported to possess multiple activities including antioxidant, neuroprotective, and anti-inflammatory effects (Zhang et al. 2015). Chemically, piperine is composed of a basic piperidine moiety that is connected to side chain through carbonylamide, a side chain with conjugated double bonds, and a methylenedioxyphenyl (MDP) ring. Based on its structure, any of its three components can be modified to evaluate their impact on the efficacy and potency of the parent compound piperine. A previous study conducted by Koul et al. has revealed that the piperidine moiety possess differential sensitivity for inhibition of CYP450 (Koul et al. 2000). On the other hand, the MDP ring in piperine, which could contribute to its activity, is a common component in many natural compounds. Therefore, in the current study, we focused on the modification of the piperidine moiety and evaluated its effect on the pharmacological activity against ER stress.
In this study, piperic acid (PA1) was prepared by the alkaline hydrolysis of piperine (PIP). Three amide piperine analogs (PA1 to PA4) were synthesized from PA1 and characterized as described in the “Materials and methods” section. The analogs were designed and prepared based on their similarity to the parent compound piperine and screened to evaluate their pharmacological activity against TM-induced ER stress and cell death in renal cells.
To establish an in vitro model of ER stress in renal cells, we chose tunicamycin (TM), an N-glycosylation inhibitor and a chemical inducer of ER stress (Schonthal 2012). Our choice to utilize TM was based on a previous study conducted by Peyrou et al., which compared the efficacy of TM with other chemical inducers of ER stress such as thapsigargin and oxidized dithiothreitol (ox-DTT) in four different renal cell lines—the porcine LLC-PK1, rat NRK-52E, canine MDCK, and human HEK-293 cells (Peyrou and Cribb 2007). Results from their study have indicated that tunicamycin is the most potent inducer of ER stress among other chemical inducers tested in the model.
We assessed the major hallmark proteins induced during ER stress response such as the ER chaperone GRP78 and the pro-apoptotic growth arrest and DNA damage-inducible protein 153 (CHOP/GADD153). GRP78 is an important resident chaperone that maintains cellular homeostasis by ensuring the proper folding of protein in the ER (Rovetta et al. 2012), whereas, CHOP is a pro-apoptotic protein, which is induced by all the three arms of UPR—ATF6, IRE1, and PERK pathways (Katsoulieris et al. 2010). Our results confirmed the ability of TM (at 0.5 μg/mL for 2 h) to induce ER stress (marked by the induction of GRP78 and CHOP), and cell death (evidenced by ~60% reduction in cell viability) in NRK-52E cells.
To compare the efficacy of piperine and its analogs to a known reference standard, we chose to use 4PBA, a well-known chemical chaperone. 4PBA is a nontoxic butyrate analog, which has the ability to enhance the protein folding capacity of ER and improve the trafficking of mutant proteins (Humeres et al. 2014). In our study, we found that 4PBA protects against TM-induced expression of both GRP78 and CHOP. However, a higher magnitude of reduction was observed on CHOP expression (Carlisle et al. 2014). Our findings are in line with a previous study in HK-2 cells by Carlisle et al., where 4PBA has been shown to prevent TM-induced CHOP expression without affecting the expression of GRP78 (Carlisle et al. 2014).
In this study, four piperine analogs (piperic acid and 3 amide piperine analogs) were synthesized and their structures were elucidated by pertinent spectroscopic techniques. Each analog was tested at two different concentrations (250 and 500 nM) for its pharmacological activity to relieve ER stress induced by TM in renal cells and compared against its parent compound piperine and reference standard 4PBA.
Based on the literature, all the prepared analogs were used in nanomolar (250 and 500 nM) concentrations, whereas the reference standard 4PBA was used in millimolar (1 and 2 mM) concentration. Although the effects of piperine were similar to that of 4PBA, its effects were mainly mediated through reduction in the expression of GRP78. In contrast, 4PBA appears to mediate its effects mainly through reduction in CHOP expression. Thus, future studies could examine the synergistic effects expected out of combination of 4PBA and piperine as a strategy to prevent ER stress in renal cells. Moreover, a previous study revealed the ability of piperine to downregulate the mRNA expression of GRP78 and the protein expression on XBP1 and ATF6 in the livers of HFD-fed mice (Jwa et al. 2012). Thus, future studies could also examine the effect of piperine and its analogs on the expression of the XBP-1 and ATF6 in NRK-52E cells exposed to TM.
The most potent compound in our study was piperine followed by the cyclohexylamino analog (PA2). Similar to piperine, PA2 decreased the expression of GRP78 and CHOP in NRK-52E cells. Thus, its effects could be potentially mediated through either PERK and/or ATF6 arms of the UPR. Further studies to investigate the effect of this analog on the expression of XBP1 and JNK (downstream targets of IRE1 arm) would help to unravel the mechanism(s) behind its protective effects against ER stress. Our studies with piperine in a model of DTT-induced ER stress in NRK-52E cells recapitulated the findings obtained from the model of tunicamycin-induced ER stress on GRP78 and CHOP expression (shown in ESM 2). Consistent with our findings, topical application of piperine and its cyclohexylamino analog for 4 weeks was shown to ameliorate vitiligo (Faas et al. 2008), a condition that is strongly associated with ER stress (Jeong et al. 2010; Toosi et al. 2012). Together, these findings indicate the therapeutic potential of piperine and its cyclohexylamino analog to act as “chemical chaperones” under the conditions of ER stress.
Unlike piperine, piperic acid (PA1) did not affect the expression of CHOP and GRP78. Intriguingly, treatment with diethylamino analog (PA3) caused a paradoxical increase in the expression of CHOP in renal cells. Thus, further studies are required to understand their impact on the CHOP expression and to determine whether its effects on CHOP are transient or permanent. Although treatment with pyrrolidinyl analog (PA4) did not alter the induction of ER stress markers GRP78 and CHOP in renal cells, it showed improvements in cell viability against tunicamycin-induced cytotoxicity.
Our studies to unravel the mechanisms underlying the chemical chaperone activity and cytoprotective effects of piperine indicate that pre-treatment with piperine decreases the induction of ER-associated caspase-12 and the executioner caspase-3 in renal cells subjected to tunicamycin. Although studies in cancer lines (Lin et al. 2013; Samykutty et al. 2013; Yaffe et al. 2015) have demonstrated piperine’s ability to induce apoptosis especially at high concentrations (in micromolar range), to our knowledge, this is the first study to demonstrate the anti-apoptotic effects of piperine (at nanomolar concentrations) in normal cells. The discrepancy in the pro-apoptotic vs. anti-apoptotic effects of piperine could be attributed to the neoplastic state of the cells (cancerous vs. non-cancerous) and the dose tested, i.e., anti-apoptotic at low (nanomolar) concentrations and pro-apoptotic at high (micromolar) concentrations.
Further studies with different side chains and amine substituents in the aromatic moiety are required to elucidate the structure–activity relationship (Ferreira et al. 2011) of these compounds. Nevertheless, based on our research findings, we were able to identify and propose the following.
-
1.
Piperine and its analogs are pharmacologically active at nanomolar (nM) concentrations.
-
2.
Similar to 4PBA, piperine (PIP) and its cyclohexylamino analog (PA2) can decrease the expression of GRP78 and CHOP in renal cells and attenuate ER stress.
-
3.
Opening of piperidine ring structure in piperine (diethylamino analog (PA3)) causes a paradoxical increase in CHOP expression, which might aggravate ER stress.
-
4.
Removal of the piperidine ring by hydrolysis of piperine (PIP) or replacement of six-membered ring with a five-membered ring (pyrrolidinyl analog (PA4)) appears to result in loss of pharmacological activity against ER stress.
-
5.
Compounds PIP, PA2, and PA4 also possess cytoprotective properties against ER stress-induced cell death.
-
6.
In terms of potency, piperine and its analogs (used in nanomolar concentrations) are about 4000 times more potent when compared to the reference standard 4PBA (used in millimolar concentration).
In conclusion, considering their high potency (used in nM), efficacy, and a wide margin of safety, piperine (PIP) and its cyclohexylamino analog (PA2) appear to be promising drug candidates for further investigation in vivo for prevention of ER stress and potential application to treat ER stress-related renal disorders in patients.
References
Brandizzi F, Frigerio L, Howell SH, Schafer P (2014) Endoplasmic reticulum-shape and function in stress translation. Front Plant Sci 5:425. doi:10.3389/fpls.2014.00425
Carlisle RE, Brimble E, Werner KE, Cruz GL, Ask K, Ingram AJ, Dickhout JG (2014) 4-Phenylbutyrate inhibits tunicamycin-induced acute kidney injury via CHOP/GADD153 repression. PLoS One 9:e84663. doi:10.1371/journal.pone.0084663
Cybulsky AV, Takano T, Papillon J, Bijian K (2005) Role of the endoplasmic reticulum unfolded protein response in glomerular epithelial cell injury. J Biol Chem 280:24396–24403. doi:10.1074/jbc.M500729200
Cybulsky AV, Takano T, Papillon J, Bijian K, Guillemette J, Kennedy CR (2009) Glomerular epithelial cell injury associated with mutant alpha-actinin-4. American journal of physiology renal physiology 297:F987–F995. doi:10.1152/ajprenal.00055.2009
Doucette CD, Greenshields AL, Liwski RS, Hoskin DW (2015) Piperine blocks interleukin-2-driven cell cycle progression in CTLL-2 T lymphocytes by inhibiting multiple signal transduction pathways. Toxicol Lett 234:1–12. doi:10.1016/j.toxlet.2015.01.020
Drawz P, Hostetter TH, Rosenberg ME (2015) Chapter 49—slowing progression of chronic kidney disease. In: Kimmel PL, Rosenberg ME (eds) Chronic renal disease. Academic Press, San Diego, pp 598–612. doi:10.1016/B978-0-12-411602-3.00049-4
Faas L, Venkatasamy R, Hider RC, Young AR, Soumyanath A (2008) In vivo evaluation of piperine and synthetic analogues as potential treatments for vitiligo using a sparsely pigmented mouse model. Br J Dermatol 158:941–950. doi:10.1111/j.1365-2133.2008.08464.x
Ferreira C et al (2011) Leishmanicidal effects of piperine, its derivatives, and analogues on Leishmania amazonensis. Phytochemistry 72:2155–2164. doi:10.1016/j.phytochem.2011.08.006
Fujii Y et al (2006) The effect of dexamethasone on defective nephrin transport caused by ER stress: a potential mechanism for the therapeutic action of glucocorticoids in the acquired glomerular diseases. Kidney Int 69:1350–1359. doi:10.1038/sj.ki.5000317
Greenshields AL et al (2015) Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett 357:129–140. doi:10.1016/j.canlet.2014.11.017
Henriquez FL et al (2005) Toxoplasma gondii dense granule protein 3 (GRA3) is a type I transmembrane protein that possesses a cytoplasmic dilysine (KKXX) endoplasmic reticulum (ER) retrieval motif. Parasitology 131:169–179
Humeres C et al (2014) 4-Phenylbutyric acid prevent cytotoxicity induced by thapsigargin in rat cardiac fibroblast. Toxicology In Vitro 28:1443–1448. doi:10.1016/j.tiv.2014.07.013
Inagi R (2010) Endoplasmic reticulum stress as a progression factor for kidney injury. Curr Opin Pharmacol 10:156–165. doi:10.1016/j.coph.2009.11.006
Inagi R et al (2005) Involvement of endoplasmic reticulum (ER) stress in podocyte injury induced by excessive protein accumulation. Kidney Int 68:2639–2650. doi:10.1111/j.1523-1755.2005.00736.x
Jeong KH, Shin MK, Uhm YK, Kim HJ, Chung JH, Lee MH (2010) Association of TXNDC5 gene polymorphisms and susceptibility to nonsegmental vitiligo in the Korean population. Br J Dermatol 162:759–764. doi:10.1111/j.1365-2133.2009.09574.x
Jwa H, Choi Y, Park UH, Um SJ, Yoon SK, Park T (2012) Piperine, an LXRalpha antagonist, protects against hepatic steatosis and improves insulin signaling in mice fed a high-fat diet. Biochem Pharmacol 84:1501–1510. doi:10.1016/j.bcp.2012.09.009
Katsoulieris E, Mabley JG, Samai M, Sharpe MA, Green IC, Chatterjee PK (2010) Lipotoxicity in renal proximal tubular cells: relationship between endoplasmic reticulum stress and oxidative stress pathways. Free Radic Biol Med 48:1654–1662. doi:10.1016/j.freeradbiomed.2010.03.021
Koul S et al (2000) Structure-activity relationship of piperine and its synthetic analogues for their inhibitory potentials of rat hepatic microsomal constitutive and inducible cytochrome P450 activities. Bioorg Med Chem 8:251–268
Kumar A, Sasmal D, Sharma N (2015) Immunomodulatory role of piperine in deltamethrin induced thymic apoptosis and altered immune functions. Environ Toxicol Pharmacol 39:504–514. doi:10.1016/j.etap.2014.12.021
Lin Y, Xu J, Liao H, Li L, Pan L (2013) Piperine induces apoptosis of lung cancer A549 cells via p53-dependent mitochondrial signaling pathway. Tumour Biol 35:3305-3310. doi:10.1007/s13277-013-1433-4
Markan S et al (2009) Up regulation of the GRP-78 and GADD-153 and down regulation of Bcl-2 proteins in primary glomerular diseases: a possible involvement of the ER stress pathway in glomerulonephritis. Mol Cell Biochem 324:131–138. doi:10.1007/s11010-008-9991-2
Meghwal M, Goswami TK (2013) Piper nigrum and piperine: an update. Phytotherapy research: PTR 27:1121–1130. doi:10.1002/ptr.4972
Nagelkerke A, Bussink J, Sweep FC, Span PN (2014) The unfolded protein response as a target for cancer therapy. Biochim Biophys Acta 1846:277–284. doi:10.1016/j.bbcan.2014.07.006
Peyrou M, Cribb AE (2007) Effect of endoplasmic reticulum stress preconditioning on cytotoxicity of clinically relevant nephrotoxins in renal cell lines. Toxicology In Vitro 21:878–886. doi:10.1016/j.tiv.2007.03.001
Rogers M, Goettsch C, Aikawa E (2013) Medial and intimal calcification in chronic kidney disease: stressing the contributions. J Am Heart Assoc 2:e000481. doi:10.1161/JAHA.113.000481
Rovetta F et al (2012) ER signaling regulation drives the switch between autophagy and apoptosis in NRK-52E cells exposed to cisplatin. Exp Cell Res 318:238–250. doi:10.1016/j.yexcr.2011.11.008
Samykutty A et al (2013) Piperine, a bioactive component of pepper spice exerts therapeutic effects on androgen dependent and androgen independent prostate cancer cells. PLoS One 8:e65889. doi:10.1371/journal.pone.0065889
Sangwan PL et al (2008) Piperine analogs as potent Staphylococcus aureus NorA efflux pump inhibitors. Bioorg Med Chem 16:9847–9857. doi:10.1016/j.bmc.2008.09.042
Schonthal AH (2012) Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica 2012:857516. doi:10.6064/2012/857516
Sovolyova N, Healy S, Samali A, Logue SE (2014) Stressed to death—mechanisms of ER stress-induced cell death. Biol Chem 395:1–13. doi:10.1515/hsz-2013-0174
Staehelin LA (1997) The plant ER: a dynamic organelle composed of a large number of discrete functional domains. The Plant journal: for cell and molecular biology 11:1151–1165
Toosi S, Orlow SJ, Manga P (2012) Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol 132:2601–2609. doi:10.1038/jid.2012.181
Venkatasamy R, Faas L, Young AR, Raman A, Hider RC (2004) Effects of piperine analogues on stimulation of melanocyte proliferation and melanocyte differentiation. Bioorg Med Chem 12:1905–1920. doi:10.1016/j.bmc.2004.01.036
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086. doi:10.1126/science.1209038
Wattanathorn J, Chonpathompikunlert P, Muchimapura S, Priprem A, Tankamnerdthai O (2008) Piperine, the potential functional food for mood and cognitive disorders. Food Chem Toxicol 46:3106–3110. doi:10.1016/j.fct.2008.06.014
Yaffe PB, Power Coombs MR, Doucette CD, Walsh M, Hoskin DW (2015) Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol Carcinog 54:1070–1085. doi:10.1002/mc.22176
Yoon YC, Kim SH, Kim MJ, Yang HJ, Rhyu MR, Park JH (2015) Piperine, a component of black pepper, decreases eugenol-induced cAMP and calcium levels in non-chemosensory 3T3-L1 cells. FEBS Open Bio 5:20–25. doi:10.1016/j.fob.2014.11.008
Zhang J et al (2015) Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int Immunopharmacol 24:50–58. doi:10.1016/j.intimp.2014.11.012
Zhuang A, Forbes JM (2014) Stress in the kidney is the road to pERdition: is endoplasmic reticulum stress a pathogenic mediator of diabetic nephropathy? J Endocrinol 222:R97–111. doi:10.1530/JOE-13-0517
Acknowledgments
This study was supported by the intramural grants (QUUG-CPH-CPH-13/14-2; QUST-CPH-SPR-12/13-5; QUSTCPH-FALL-13/14-6; QUST-CPH-SPR-13/14-8; QUST-CPH-FALL-14/15-5; QUST-CPH-SPR-14/15-14) awarded by the Office of Academic Research, Qatar University, Doha, Qatar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hammad, A.S., Ravindran, S., Khalil, A. et al. Structure–activity relationship of piperine and its synthetic amide analogs for therapeutic potential to prevent experimentally induced ER stress in vitro. Cell Stress and Chaperones 22, 417–428 (2017). https://doi.org/10.1007/s12192-017-0786-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-017-0786-9