Abstract
Fruits contain nutrients, minerals, trace elements, vitamins, and phytochemicals that are important in terms of human health. When fruits are consumed in a balanced diet, they have a positive effect with regard to reducing disease risk. As a result of heavy metal contamination of the environment, they can be a potential source of toxic elements so it is important to determine the toxic, essential, and trace elements in fresh fruit and commercial fruit products such as fruit juice, marmalade. This study aims to determine the concentrations of Al, B, Ca, Cu, Fe, K, Mg, Mn, Na, Zn, P, and S in tropical fruits (tamarind (Tamarindus indica), star fruit (Averrhoa carambola), golden berry (Physalis peruviana), kumquat (Citrus japonica), dragon fruit (Hylocereus undatus), passion fruit (Passiflora edulis)) by using inductively coupled plasma optical emission spectrometry (ICP-OES) after different microwave acid digestion procedures. The efficiencies of digestion in tropical fruit samples were investigated and compared. Six milliliters of HNO3 + 2 mL of H2O2, 6 mL of HNO3 + 2 mL of HCl, 8 mL of HNO3, 8 mL of HNO3 + 4 mL of H2O2 (30%), 8 mL of HNO3 + 4 mL of HCl, and 12 mL of HNO3 were used for microwave acid digestion procedures. Microwave digestion procedures were applied for the analysis of seventy-two tropical fruit samples purchased in triplicate for each tropical fruit and major, toxic, and minor element contents of tropical fruits were determined. The parameters of the merit evaluated were validated according to limits of detection (LOD) and limits of quantification (LOQ), linearity, recovery, and precision. The highest results of LOD and LOQ were found for Ca, K, Na, Mg, and S. The recovery percentages are in the range from 71.01 to 117.31% in tropical fruits. The digestion efficiency was correlated with the residual carbon content, which was determined by total organic carbon analyzer (TOC). Six milliliters of HNO3 + 2 mL of HCl digestion method was chosen as the effective digestion method because of the lowest residual carbon contents and the accuracy results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fruits are beneficial foods for human health because they have minerals, antioxidants, vitamins, and essential fatty acids. The use of tropical fruits has rapidly increased in recent years. The chemical composition of these fruits is important, owing to their toxicological and nutritional properties. For this reason, it is necessary to determine organic and inorganic contents in fruits (Sa et al. 2019).
Golden berry (Physalis peruviana L.) is a yellow-orange fleshed berry of great commercial interest on account of nutritional value and bioactive compound content. As well as golden berry is consumed as fresh product, its commercial products such as juice and marmalades are also consumed (Ballesteros-Vivas et al. 2019). Multi-element concentrations were investigated in P. peruviana, P. geminiflora, and E. insignis species (Moreda-Piñeiro et al. 2018). Golden berry has medicinal properties, and it is rich in vitamins (vitamin A and C) and minerals (iron, phosphorus, alkaloids, flavonoids, and carotenoids) (Marchioretto et al. 2020).
Passion fruit has antioxidant and anti-inflammatory activities. P. edulis’s fruit juice decreases blood pressure in hypertension patients. Carotenoids, vitamins, soluble fiber, polysaccharides, and minerals have been found in passion fruit. Minerals which are in passion fruit help to regulate enzyme metabolism, muscular, and neurological activity (Novaes et al. 2017).
Tamarindus indica L. (tamarind) belongs to the Caesalpiniaceae family. Tamarindus indica fruit has hypolipemic and antioxidant, anti-inflammatory, antimicrobial, cytotoxic activities against gastrointestinal spasms. In addition, Thai traditional medicine confirms Tamarindus indica fruit as digestive, carminative, laxative, expectorant, and blood tonic (Escalona-Arranz et al. 2010).
Dragon fruit (Hylocereus undatus) grows on the Hylocereus cactus (Sa et al. 2019). It is also rich in antioxidant properties, potassium, protein, fiber, sodium, and calcium. The dragon fruit supports the digestive process, prevents colon cancer and diabetes, neutralizes toxic substances such as heavy metal, decreases cholesterol levels and high blood pressure, and treats asthma and cough (Ruzainah et al. 2009).
Kumquat (Genus citrus) belongs to the family Rutaceae. Citrus fruits have important compounds such as antioxidant, flavonoid, minerals, and vitamins A and C. Citrus fruits are useful fruits which have some bioactivities such as antiviral, anti-cancer, and anti-inflammatory. In addition, kumquat prevents cardiovascular diseases. Thus, consumption of kumquat is important for human health and human nutrition (Young et al. 2019).
Averrhoa carambola L. is classified in the Oxalidaceae family. While Averrhoa carambola L. fruit has pharmacologically active, Averrhoa carambola L.’s leaves have anti-inflammatory activity. In addition, Averrhoa carambola L. treats disease such as eczema, inappetence, headache, coughing, and vomiting, as well as Averrhoa carambola L. has antioxidant activities (Liang et al. 2020).
There are bibliographic references analyzed by using acid mixtures for further determination of multielement contents in coffee and milk powder samples (Castro et al. 2009; Bizzi et al. 2011a; b). Three acid digestion methods were evaluated for traditional medicine samples by using HNO3–HClO4, HNO3, and HNO3–HCl (Uddin et al. 2016). This acid mixture could also be an alternative for tropical fruit digestion for further multielement content determination.
Microwave-assisted sample digestion has become an important routine method for further analysis of inorganic and organic matrices as the advantages include high sample throughput, limited to no loss of volatile species and very low contamination levels [https://lab-training.com/2014/01/19/benefits-of-microwave-digestion-over-open-acid-digestions/].
Inductively coupled plasma optical emission spectrometry (ICP-OES), also known as inductively coupled plasma-atomic emission spectrometry (ICP-AES), is well suited for such applications because it is highly sensitive to trace-level concentrations and small changes in concentration (Brennan et al. 2009) and can simultaneously detect multiple elements. ICP OES technique provides good quantitative multielement capability, wide linear dynamic ranges, high sensitivity, low detection limits, and speed (Sa et al. 2019). Hence, ICP-OES can, in principle, provide useful elemental information for surface species conjugated on AuNPs. The advantages of the ICP include high temperature, long residence times, presence of no or few molecular species, few ionization interferences, and being optically thin. Developments in inductively coupled plasma optical emission spectrometry (ICP-OES) continue, including instrumentation. The practice of ICP-OES invariably involves the comparison of the unknown to standards via a calibration curve (Sneddon and Vincent 2008). ICP-OES is used for all the matrices of environmental samples especially for high-matrix samples. Only analytical grade reagents can be sufficient. If the elements do not need lower detection limit that inductively coupled plasma mass spectrometry (ICP-MS) delivers. It can be disadvantage for ICP-OES [https://www.thermofisher.com].
There is no study analyzed by ICP-OES in the literature to determine the elemental content of the tropical fruits used in this study so the study is unique in this regard. In this study, tamarind (Tamarindus indica), golden berry (Physalis peruviana), kumquat (Citrus japonica), dragon fruit (Hylocereus undatus), star fruit (Averrhoa carambola), and passion fruit (Passiflora edulis) were bought from a local market of Tekirdağ/Turkey. The elemental contents of these tropical fruits were determined. Moreover, efficiency of different digestion procedures was investigated.
Experimental
Reagent and Solutions
All solutions were prepared from high-purity analytical reagents and ultra-pure water with specific resistivity of 18.2 MΩ cm (Milli-Q, Millipore, USA). Concentrated HNO3 (14 mol L−1, 65% w/w) and H2O2 (9.8 mol L−1, 30% w/w) and concentrated HCl (12 mol L−1, 37% w/w) (Merck, Germany) were used for all sample digestion.
The multielement analytical curve was prepared from monoelement solutions of the analytes Al, B, Cu, Fe, Mn, and Zn (Merck, Germany) in the concentrations of 0–1000 μg/L. The following concentrations of standard calibration solutions were applied in the preparation of the analytical curves: 0–300 μg/mL (Ca, K); 0–100 μg/mL (Mg, P); 0–25 μg/mL (Na); and 0–10 μg/mL (S). The solutions were prepared in 6 mL of HNO3 + 2 mL of H2O2, 6 mL of HNO3 + 2 mL of HCl, 8 mL of HNO3, 8 mL of HNO3 + 4 mL of H2O2, 8 mL of HNO3 + 4 mL of HCl, and 12 mL of HNO3 medium.
Instrumentation
An inductively coupled plasma optical emission spectrometer (Spectro-Spectroblue, Analytical Instruments GmbH, Kleve, Germany) was used for analyses. The instrumental operating parameters were as follows: 1.4 kW of Rf power, 1.0 L/min of nebulizer gas flow, 12 L/min of plasma-Ar flow, and 1.0 L/min of auxiliary gas flow. Digestion of the samples was carried out in a microwave oven (MARS 6, CEM Corp., Matthews, NC, USA) equipped with EasyPrep Plus extra-high-pressure Teflon TFM vessels were used in the acid digestions of the samples. The microwave oven was operated in a temperature-controlled mode. Residual carbon content was determined by using a total organic carbon analyzer (TOC-L CPH/CPN, Shimadzu Corporation, Kyoto, Japan).
Sample Collection
Sample collection is a very important section in the experiment. Samples of tamarind (Tamarindus indica), star fruit (Averrhoa carambola), golden berry (Physalis peruviana), kumquat (Citrus japonica), dragon fruit (Hylocereus undatus), and passion fruit (Passiflora edulis) were purchased in triplicate from four different local markets in Tekirdağ/Turkey (December 2019). Total n = 72 different tropical fruit samples (tamarind n = 12, passion fruit n = 12, star fruit n = 12, dragon fruit n = 12, kumquat n = 12, golden berry n = 12) were analyzed to determine element contents.
Sample Preparation Procedure
All parts of the samples were taken so that samples were homogeneous. Samples were ground in the grinder (RETSCH Knife Mill Grindomix GM200, Fisher Scientific, USA) and were dried on hot plate at 60 °C until constant mass, for approximately 48 h. The dried samples were weighed. Dry weight was taken into account.
Microwave Digestion
Similar microwave digestion methods have been reported in the literature (Mketo et al. 2015; Mohammed et al. 2017; Chaves et al. 2010). Five hundred milligrams of real samples in reaction vessels is directly added to each flasks. Eight milliliters of a freshly prepared mixture of concentrated HNO3–H2O2 (6:2, v/v), HNO3–HCl (6:2, v/v), and HNO3 (8 mL) and 12 mL of a freshly prepared mixture of concentrated HNO3–H2O2 (8:4, v/v); HNO3–HCl (8:4, v/v); and HNO3 (12 mL). In the heating program’s first step, the temperature was linearly raised to 120 °C in 5 min with a maximum power of 1000 W. The temperature was kept at 120 °C for 2 min in the second step. The third step comprises rising the temperature linearly to 210 °C in 10 min, and the temperature was kept at 210 °C for 15 min in the fourth step. After the end of the heating program, vessels were cooled down to room temperature. The oven was kept in 1600 W in all steps. When the heating program finished, the vessels were cooled down for 15 min. Digested samples were completed to 25.0 mL with ultrapure water. Blanks were prepared in the same way.
Recovery
Five hundred milligram tropical fruit samples were weighed. Five hundred and 625 μL of 10 μg/mL standard solutions were added and then digested with 6 mL of HNO3 + 2 mL of H2O2, 6 mL of HNO3 + 2 mL of HCl, 8 mL of HNO3, 8 mL of HNO3 + 4 mL of H2O2, 8 mL of HNO3 + 4 mL of HCl, and 12 mL of HNO3. Values of recovery % were calculated for each element. Three replicates were analyzed. The LOD and LOQ of values were determined by using calibration standards. LOD and LOQ were calculated to be 3 s/S and 10 s/S, respectively, where S is the slope of the calibration curve and s is the standard deviation of the intercept of the regression equation.
Residual Carbon Content
The carbon mass is calculated by comparison to a National Institute of Standards and Technology external calibration standard, potassium hydrogen phthalate, C8H5KO4. Standards were prepared from reagent grade potassium hydrogen phthalate in ultra-pure water. Standards were calibrated according to the manufacturer’s instructions. Calibration curve of potassium hydrogen phthalate (C8H5KO4) was linear over the concentration range of 0–100 μg/mL. Samples were digested by six different microwave digestion methods and then samples were filtered and were diluted 100 times.
Statistical Analysis
The statistical important differences between major and minor element concentrations of tropical fruits by using different digestion procedures were calculated by ANOVA (one way). The different results were obtained in the statistical evaluation and the data are given as the mean ± standard deviation. Pearson’s correlation coefficients (r) were used for relationships between concentrations of major and minor elements in tropical fruits. Analysis of variance (ANOVA) followed by the Mann-Whitney U test was used for calculating statistically significant differences. Relationships between the concentrations of the same elements in different microwave digestion methods were assessed by using Mann-Whitney U test.
Results and Discussion
Tamarind (Tamarindus indica), star fruit (Averrhoa carambola), golden berry (Physalis peruviana), kumquat (Citrus japonica), dragon fruit (Hylocereus undatus), and passion fruit (Passiflora edulis) were analyzed by using different microwave digestion procedures for determine multielement contents. These procedures were as follows: 6 mL of HNO3 + 2 mL of H2O2; 6 mL of HNO3 + 2 mL of HCl, 8 mL of HNO3, 8 mL of HNO3 + 4 mL of H2O2, 8 mL of HNO3 + 4 mL HCl, and 12 mL of HNO3. The efficiencies of digestion in tropical fruit samples were compared. Figure 1 shows the photo of tamarind samples after analysis with six different microwave digestion methods. The study compares the results for the 12 elements (Al, B, Ca, Cu, Fe, K, Mg, Mn, Na, Zn, P, and S) that presented measurable concentrations by ICP-OES and the 13 other elements (As, Bi, Cd, Co, Cr, Mo, Ni, Pb, Pt, Sb, Sn, Ti, and W) that showed concentrations below LOD, that is, they were investigated, but not determined in the samples.
The method was evaluated regarding linearity, LOD, LOQ, recovery, and relative standard deviation (%RSD). Table 1 shows values of LOD and LOQ. RSD were mostly found below 9%. The values of RSD were analyzed in the same day. Tables 2, 3, 4, 5, 6, and 7 show the major and minor element concentrations of tropical fruits. Three replicates (acid digests) were performed for each sample.
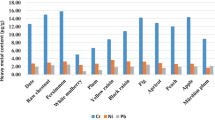
Recovery results of tropical fruits digested with 12 mL HNO3 are given in Table 9. Currently, in analytical procedures, recovery percentages in the range from 71.01 to 117.31 with precision of about 20% are accepted. On the other hand, depending on matrix complexity, this range can be extended from 50 to 120% with precision of 15% (Basilio de Caland et al. 2012). Considering the digestion efficiency obtained in this work, the proposed method presents accuracy and precision sufficient to be applied for the determination of major and minor element contents in tropical fruits. Figure 2 and Fig. 3 show graph of major and minor element contents (μg/g) in tropical fruits analyzed according to 6 mL HNO3–2 mL HCl digestion method. While the lowest value of LOD was obtained by 8 mL HNO3 + 4 mL H2O2, the highest value of LOQ was found by using 6 mL HNO3 + 2 mL H2O2.
The relation between concentrations of major and minor elements in all tropical fruits was determined according to the Pearson’s correlation coefficients (r) by results obtained 6 mL HNO3 + 2 mL HCl digestion method which was chosen as the best digestion method. As passion fruit has high positive (r) values more than other tropical fruits, so correlations between elements in passion fruit are given in Table 10. There is a very high positive correlation (r > 0.9) for concentrations of the following pairs of elements—B-Fe, B-Mn, B-P, Cu-Na, Fe-Mn, Fe-P, Mn-K, Zn-Mg, Zn-K, and Mg-K, Mg-S, K-P, K-S—and high negative correlation (r > − 0.9) for concentrations of the following pairs of elements: B-Na, Cu-Mn, Cu-Zn, Fe-Na, Fe-K, Fe-Ca, Fe-S, Mn-K, Mn-Ca, and Mn-S (Table 10). High correlations (r = 0.7–0.9) were found for concentrations of Fe-K, Mn-Zn, Mn-S, Zn-P, Na-Mg, P-S, B-K, B-Ca, Mn-Mg, Zn-Na, Na-S, and Mg-P. High negative correlations (− 0.7 < |r| < − 0.9) were found for concentrations of Al-K, Al-S, B-K, B-S, Zn-Na, Zn-Ca, Al-Na, Cu-Mg, Zn-K, Zn-S, and Mg-Ca in Table 10. Because of providing the highest correlation, 6 mL HNO3–2 mL HCl as digestion method was chosen.
Three different samples were taken for each fruit sample. One-way ANOVA was analyzed for the statistical evaluation of the results. There are significant statistical differences between major, minor, and toxic (Al) element concentrations analyzed by using different digestion methods in all tropical fruits (p < 0.001). The comparison of different microwave digestion methods showed statistically significant differences in results obtained with these six procedures. In statistical evaluation, it was found that the differences were important (p < 0.001) among types. Three stars indicate the statistical significance beyond the 0.001 in Tables 2, 3, 4, 5, 6, and 7. It shows that different processing methods caused these differences. Statistical differences between groups analyzed by different microwave digestion methods were analyzed by using the Mann-Whitney U test, and the results are given in Tables 2, 3, 4, 5, 6, and 7. In statistical evaluation, it was found that statistical differences between some groups were not significant at 0.05 level, while differences among the other groups were mostly significant (p < 0.05) according to Mann-Whitney U test.
The digestion efficiencies of methods were evaluated by determination of RCC (residual carbon content) in the final digests. The RCC values of digested tropical fruit samples that were found to be between 28 and 77, 13 and 25, 36 and 69, 15 and 90, 13 and 92, and 19 and 85 g/kg for golden berry, tamarind, dragon fruit, passion fruit, kumquat, and star fruit, respectively. Table 8 shows residual carbon contents (g/kg) in tropical fruits. Six milliliter HNO3 + 2 mL HCl was found the lowest carbon contents, generally. The lowest contents of residual carbon were found for mostly digested samples and confirmed the high efficiency of the proposed sample digestion procedure, using oxidant mixture and closed-vessel microwave oven. The oxidant mixture of 6 mL HNO3 + 2 mL HCl presents a high oxidizing power, increasing the pressure and the temperature inside the closed-vessels during the sample digestion (Tables 9, 10 and 11).
Major Element Contents in Tropical Fruits
The concentrations (μg/g) of Ca, Mn, K, P, Na, Zn, Fe, S, and Mg in golden berry (Physalis peruviana) are given in Table 2 . Major element concentrations investigated in golden berry decreased in the following order: K > P > Mg > S > Na > Ca > Zn > Mn > Fe for 8 mL HNO3, 6 mL HNO3 + 2 mL H2O2, 6 mL HNO3 + 2 mL HCl, 12 mL HNO3, and 8 mL HNO3 + 4 mL HCl. K > P > Mg > S > Na > Ca > Zn > Fe > Mn for 8 mL HNO3+ 4 mL H2O2.
Table 3 shows the concentrations (μg/mL) of K, P, Mg, S, Na, Zn, Ca, and Cu in passion fruit (Passiflora edulis). Major element concentrations investigated in passion fruit decreased in the following order: K > P > Mg > S > Na > Zn > Ca > Cu for all digestion methods.
The concentrations (μg/g) of K, Mg, P, S, Ca, Na, B, and Zn in tamarind (Tamarindus indica) are presented in Table 4. Major element concentrations investigated in tamarind decreased in the following order: K > Mg > P > S > Ca > Na > B > Zn for 8 mL HNO3, 6 mL HNO3 + 2 mL H2O2, and 6 mL HNO3 + 2 mL HCl. K > Mg > P > Ca > S > Na > B > Zn for 12 mL HNO3, K > Mg > P > S > Na > Ca > B > Zn for 8 mL HNO3 + 4 mL H2O2, and K > Mg > P > Ca > S > B > Na > Zn for 8 mL HNO3 + 4 mL HCl.
Table 5 shows the concentrations (μg/g) of K, P, Mg, S, Ca, Na, Mn, Zn, and B in dragon fruit (Hylocereus undatus). Major element concentrations investigated in dragon fruit decreased in the following order: K > P > Mg > S > Ca > Na > Mn > Zn > B for 8 mL HNO3, 6 mL HNO3 + 2 mL H2O2, 12 mL HNO3, 8 mL HNO3 + 4 mL H2O2, and 8 mL HNO3 + 4 mL HCl. K > P > Mg > S > Ca > Mn > Na > Zn > B for 6 mL HNO3 + 2 mL HCl.
The concentrations (μg/g) of K, P, S, Mg, Ca, Na, and B in Kumquat (Citrus japonica) are presented in Table 6. Major element concentrations investigated in kumquat decreased in the following order: K > P > S > Mg > Ca > Na > B for 8 mL HNO3, 6 mL HNO3 + 2 mL H2O2, 6 mL HNO3 + 2 mL HCl, and 8 mL HNO3 + 4 mL HCl. K > P > S > Ca > Mg > Na > B for 12 mL HNO3 and 8 mL HNO3 + 4 mL H2O2..
Table 7 shows the concentrations (μg/g) of K, P, Mg, S, Na, Zn, Ca, and Mn in star fruit (Averrhoa carambola). Major element concentrations investigated in star fruit decreased in the following order: K > P > Mg > S > Na > Zn > Ca > Mn for 8 mL HNO3, 6 mL HNO3 + 2 mL H2O2, 6 mL HNO3 + 2 mL HCl, 12 mL HNO3, and 8 mL HNO3 + 4 mL HCl. K > P> Mg > S> Na > Ca >Zn > Mn for 8 mL HNO3 + 4 mL H2O2.
K was the highest concentration for all digestion methods in all tropical fruits, but the minimum concentrations were different from each other in all the tropical fruits. In all digestion methods, Cu was the lowest concentration for passion fruit, the minimum concentration was observed for Zn in tamarind, B was the lowest concentration in dragon fruit and kumquat, and Mn was the minimum concentration in star fruit. For golden berry, Fe was the minimum concentration in all digestion methods except for 8 mL HNO3 + 4 mL H2O2. But Mn was the lowest concentration for 8 mL HNO3 + 4 mL H2O2..
Some differences were observed in major element contents analyzed by using different digestion methods in tropical fruits, but the highest concentrations were generally found for 6 mL HNO3 + 2 mL HCl and 8 mL HNO3+ 4 mL HCl digestion methods.
Minor Element Contents in Tropical Fruits
The concentrations (μg/g) of Al, B, and Cu in golden berry (Physalis peruviana) are presented in Table 2 . Minor element concentrations investigated in golden berry decreased in the following order: B > Cu > Al except for 6 mL HNO3 + 2 mL HCl and 8 mL HNO3. B > Al > Cu for 8 mL HNO3 and Al > B > Cu for 6 mL HNO3 + 2 mL HCl.
Table 3 shows the concentrations (μg/g) of Mn, B, Fe, and Al in passion fruit (Passiflora edulis). Minor element concentrations investigated in passion fruit decreased in the following order: B > Mn > Fe > Al for 6 mL HNO3 + 2 mL H2O2, 12 mL HNO3, and 8 mL HNO3 + 4 mL H2O2, Mn > B > Fe > Al for 8 mL HNO3 and 8 mL HNO3 + 4 mL HCl, and Al > Mn > B > Fe for 6 mL HNO3+ 2 mL HCl.
The concentrations (μg/g) of Al, Cu, Mn, and Fe in tamarind (Tamarindus indica) are presented in Table 4. Minor element concentrations investigated in tamarind decreased in the following order: Al > Cu > Mn > Fe for 8 mL HNO3, Cu > Mn > Fe > Al for 6 mL HNO3 + 2 mL H2O2, 12 mL HNO3, and 8 mL HNO3 + 4 mL HCl, Al > Mn > Cu > Fe for 6 mL HNO3 + 2 mL HCl, and Cu > Al > Mn > Fe for 8 mL HNO3 + 4 mL H2O2.
Table 5 shows the concentrations (μg/mL) of Fe, Cu, and Al in dragon fruit (Hylocereus undatus). Minor element concentrations investigated in dragon fruit decreased in the following order: Fe > Cu > Al except for 8 mL HNO3 + 4 mL HCl and Fe > Al > Cu for 8 mL HNO3 + 4 mL HCl.
The concentrations (μg/g) of Fe, Zn, Mn, Al, and Cu in kumquat (Citrus japonica) are presented in Table 6. Minor element concentrations investigated in kumquat decreased in the following order: Fe > Zn > Mn > Cu > Al for 8 mL HNO3, Zn > Cu > Fe > Mn > Al for 6 mL HNO3 + 2 mL H2O2, Al > Zn > Mn > Fe > Cu for 6 mL HNO3 + 2 mL HCl, Zn > Mn > Fe > Cu > Al for 12 mL HNO3 and 8 mL HNO3 + 4 mL HCl, and Zn > Mn > Fe > Al > Cu for 8 mL HNO3 + 4 mL H2O2..
Table 7 shows the concentrations (μg/g) of Fe, B, Cu, and Al in star fruit (Averrhoa carambola). Minor element concentrations investigated in star fruit decreased in the following order: Fe > B > Cu > Al except for 6 mL HNO3 and 2 mL H2O2 and B > Fe > Cu > Al for 6 mL HNO3 + 2 mL H2O2.
Some differences were observed in minor element contents analyzed by using different digestion methods in tropical fruits, but the highest concentrations were generally found for 6 mL HNO3 + 2 mL HCl.
Toxic Element Contents in Tropical Fruits
The highest Al concentrations were observed in toxic element contents analyzed by using 6 mL HNO3 + 2 mL HCl digestion method. The highest values of Al were found to be 12.44 and 14.99 μg/mL in kumquat and golden berry, respectively. The presence of Al in tropical fruits is important for human health. If we expose to high doses of Al, our nervous system can be damaged (Ayar et al. 2009). The limited value advised for Al intake is 24 μg/g by the Turkish Food Codex (60 kg body weight) (Ayar et al. 2009; Turkish Food Codex 2001). The concentrations of Al which is found in these fruits are acceptable level for human health.
The use of pressurized solvents (liquids at a high pressure and/or high temperature without reaching the subcritical point) or microwave energy were investigated to accelerate enzymatic hydrolysis processes of Brazil nut, golden berries, acai fruit, and heart of palm from Amazon region for multielement determinations by an ICP-MS. The target elements were Ca, Co, Cu, K, Mg, Ni, P, and Rb (Moreda-Piñeiro et al. 2018). While Cu and K concentrations in the samples were lower than this study, the concentrations obtained for Ca was higher than determined in comparison with the results of this study. On the other hand, P and Mg values were lower than this study except for 12 mL HNO3.
Arsenic (As), cadmium (Cd), chromium (Cr), lead (Pb), manganese (Mn), and nickel (Ni) concentrations in 21 fruit juices from 4 different brands in the Portuguese market were determined. This study showed that Mn levels in Passion fruit were lower than Anastácio et al. (Anastácio et al. 2018). Novaes et al. determined element concentrations in various passion fruits in Brazil by using diluted HNO3 by ICP-OES. Ca, Co, Cu, Fe, K, Mg, Mn, Na, and Zn concentrations ranged as follows: 43, 1.3, 10, 10, 20, 26.6, 0.33, 56.6, and 10 ppm. According to the results of this study, Ca and Fe concentrations in passion fruit were higher but K, Mg, Mn, Na, and Zn values were lower than Novaes et al. The concentrations obtained for Cu were similar to this study (Novaes et al. 2017).
The tamarind leaves’ compositions of two extracts employing gas chromatography/mass spectrometry (GC/MS), high-performance thin-layer chromatography-ultraviolet spectroscopy (HTLC-UV), and inductively coupled plasma optic emission spectrometry (ICP-OES) techniques were investigated. For tamarind leaves, Zn values were lower than this study except for 8 mL HNO3 + 4 mL HCl digestion method. Cu and Fe values were higher but Mn concentrations were lower than Arranz et al. Al values were higher than 12 mL HNO3, 8 mL HNO3 + 4 mL HCl, and 6 mL HNO3 + 2 mL H2O2, but Al concentrations were lower than the other digestion methods. First and second extraction method’s results were lower than this study for Cu, Fe, Mn, and Zn concentrations (Escalona-Arranz et al. 2010).
Hylocereus undatus has antioxidant activity and the fatty acid profile. Jerônimo et al. investigated lipid, moisture, protein, and element contents in dragon fruit. K, Mn, Cr, Na, and Ca concentrations were 3.090 mg, 2.230 mg, 1.250 mg, 0.140 mg, and 0.040 mg /100 g, respectively. K, Mn, Na, Ca, Al, Cu, Fe, Mg, Zn, and P concentrations determined for all digestion methods in dragon fruit in my study were lower than Jerônimo et al. (2015).
Narain et al. investigated the elemental levels of citrus fruits, and values of Ca, Mg, Na, and K (minimum-maximum in μg/g) ranged as follows K (95.13–270.4), Ca (10.57–75.29), Zn (0.466–1.611), and Mn (0.035–1.902). The concentrations of toxic elements (Pb, Cd, As, Al, Hg) were very low. In my study, the concentrations obtained for Zn, Mn, Ca, and K in kumquat were lower than Narain et al. (2001).
The physical, physico-chemical, and chemical characteristics at different stages of maturity as related to the apparent color variations in carambola fruit were determined. Fe, Ca, and P concentrations in this study were lower than Hong et al. (Young et al. 2019).
Table 11 shows recommend daily allowance (RDA) and dietary reference intake (DRI) results which are the levels of intake of essential nutrients considered to be adequate to meet the needs of practically all healthy adults [(https://www.lenntech.com/recommended-daily-intake.htm 2020), https://www.atsdr.cdc.gov/ToxProfiles/tp22-c1-b.pdf, (Hewlings and Kalman 2019), http://www.nap.edu/catalog/11537.html, (Lopez et al. 2002)]. Ca is an important element which is required for bones and bodily functions as well as provides enzyme activation. Ca decreases risk of mortality due to total stroke (Umesawa et al. 2006; Hays and Swenson 1985; Malhotra 1998; Murray et al. 2000). P is a main nutrition required for physiological functions. High P intake can cause renal calcification and vascular and renal tubular disease (Chang and Anderson 2017). Na is the one of most significant minerals due to electrolyte property, and Na is necessary for osmotic pressure and acid-base balance of body fluids. On the other hand, Na take part in normal nerve and muscle function (https://www.msdmanuals.com/home/hormonal-and-metabolic-disorders/electrolytebalance/overview-of-sodium-s-role-in-the-body 2019; William et al. 2015).
K is a significant element with regard to human health. Low P intake can lead to cardiovascular disease (Hays and Swenson 1985; Malhotra 1998; Murray et al. 2000). S is composed of cystine, cysteine, and methionine. For adequate S intake, it is necessary to eat food which is rich in protein (Malhotra 1998; Murray et al. 2000). Zn provides insulin activity, protein, and DNA synthesis. Zn controls and regulates immune responses and attacks cancerous cells, treats diarrhea, effects learning and memory, reduces risk of age-related chronic disease, and prevents pneumonia. Zn is found in beans, animal meats, nuts, fish, and other seafood and dairy products (Osredkar and Sustar 2011; Burjonrappa and Miller 2012; WHO Contributors 2007; https://www.medicinalnewstoday.com/articles/263176 2020).
The recommended dietary allowance (RDA) for Cu in normal healthy adults is 2 mg/day. While low Cu intake can lead to fatigue and anemia, high Cu intake cause liver and kidney diseases. Fe is required for blood production (National Research Council, Food Nutrition Board 1980). Iron is found in the red blood cells. Hemoglobin provides transport of O2 from the lungs to the tissues in our blood (Chandra 1990; Galan et al. 2005; Food and Nutrition Board, Institute of Medicine, National Academy of Science 2001). The established RDA for Fe is 8 mg/day and 18 mg/day for men and old women, respectively. Mn is an important element for human health. Mn also provides fat and carbohydrate metabolism, calcium absorption, and blood sugar regulation. The recommended dietary allowance (RDA) for Mn is 2.3 mg/day and 1.8 mg/day for adult males and females, respectively (Food and Nutrition Board, Institute of Medicine, National Academy of Science 2001; Roger 2011; Emsley 2001; Silva Avila et al. 2013; Henn et al. 2010). Major, minor, and toxic element concentrations were below the recommend daily allowance and dietary reference intake levels so the results show tropical fruits are not enough source for dietary intakes.
Conclusion
The concentrations of major and minor elements in tropical fruits were investigated. It was worked for the first time in tamarind (Tamarindus indica), golden berry (Physalis peruviana), kumquat (Citrus japonica), dragon fruit (Hylocereus undatus), passion fruit (Passiflora edulis), and star fruit (Averrhoa carambola) using different microwave digestion methods by ICP-OES. HNO3, HNO3/H2O2, and HNO3/HCl digestion procedures were successfully applied for further the determination multielement contents by ICP-OES. The efficiency of digestion was expressed by RCC. Six milliliters of HNO3 + 2 mL of HCl digestion method was preferred as the suitable digestion method because of the lowest residual carbon contents.
The highest values of Al were found in golden berry and kumquat. These concentrations are acceptable levels by Turkish Food Codex. Major and minor element concentrations were below the recommend daily allowance levels so the findings indicate tropical fruits are not good source of essential elements for contribution to dietary intakes.
References
Anastácio M, Marreilha dos Santos AP, Aschner M, Mateus L (2018) Determination of trace metals in fruit juices in the Portuguese market. Toxicol Rep 5:434–439. https://doi.org/10.1016/j.toxrep.2018.03.010
Ayar A, Sert D, Akın N (2009) The trace metal levels in milk and dairy products consumed in middle Anatolia-Turkey. Environ Monit Assess 152:1–12. https://doi.org/10.1007/s10661-008-0291-9
Ballesteros-Vivas D, Alvarez-Rivera G, Leon C, Morantes SJ, Ibanez E, Parada-Alfonso F, Cifuentes A, Valdes A (2019) Anti-proliferative bioactivity against HT-29 colon cancer cells of a with anolides-rich extract from golden berry (Physalis peruviana L.) calyx investigated by Foodomics. J Funct Foods. https://doi.org/10.1016/j.jff.2019.103567
Basilio de Caland L, Silveira ELC, Tubino M (2012) Determination of sodium, potassium, calcium and magnesium cations in biodiesel by ion chromatography. Anal Chim Acta 718:116–120. https://doi.org/10.1016/j.aca.2011.12.062
Bizzi CA, Flores EMM, Picoloto RS, Barin JS, Garcia EE, Nóbrega JA (2011a) Understanding the process of microwave-assisted digestion combining diluted nitric acid and oxygen as auxiliary reagent. Microchem J 99:193–196. https://doi.org/10.1016/j.microc.2011.05.002
Bizzi CA, Barin JS, Garcia EE, Nóbrega JA, Dressler VL, Flores EMM (2011b) Improvement of microwave-assisted digestion of milk powder with diluted nitric acid using oxygen as auxiliary reagent. Spectrochim Acta B At Spectrosc 66:394–398. https://doi.org/10.1016/j.sab.2011.04.013
Brennan RG, Rabb SA, Holden MJ, Winchester MR, Turk GC (2009) Potential primary measurement tool for the quantification of DNA. Anal Chem 81:3414–3420. https://doi.org/10.1021/ac802688x
Burjonrappa SC, Miller M (2012) Role of trace elements in parenteral nutrition support of the surgical neonate. J Pediatr Surg 47:760–771. https://doi.org/10.1016/j.jpedsurg.2012.01.015
Castro JT, Santos EC, Santos WPC, Costa LM, Korn M, Nóbrega JA, Korn MGA (2009) A critical evaluation of digestion procedures for coffee samples using diluted nitric acid in closed vessels for inductively coupled plasma optical emission spectrometry. Talanta. 78:1378–1382. https://doi.org/10.1016/j.talanta.2009.02.030
Chandra RK (1990) Micro-nutrients and immune functions: an overview. Ann N Y Acad Sci 587:9–16. https://doi.org/10.1111/j.1749-6632.1990.tb00128.x
Chang AR, Anderson C (2017) Dietary phosphorus intake and the kidney. Annu Rev Nutr 37:321–346. https://doi.org/10.1146/annurev-nutr-071816-064607
Chaves ES, dos Santos EJ, Araujo RGO, Oliveira JV, Frescura VLA, Curtius AJ (2010) Metals and phosphorus determination in vegetable seeds used in the production of biodiesel by ICP OES and ICP-MS. Microchem J. https://doi.org/10.1016/j.microc.2010.01.021
Emsley J (2001) Manganese. In: Emsley J (ed) Nature’s building blocks an A-Z guide to the elements. Oxford University Press, Oxford, pp 249–253
Escalona-Arranz JC, Pérez-Rosés R, Jiménez IL, Rodríguez-Amado J, Argota-Coello H, Cañizares-Lay J, Morris-Quevedo HJ, Sierra-González G (2010) Chemical constituents of Tamarindus indica L. Leaves. Revista Cubana de Quimica 22:65–71
Food and Nutrition Board, Institute of Medicine, National Academy of Science (2001) Dietary reference intakes for vitamin a, vitamin K, arsenic, boron, chromium, copper, iodine, Iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academy Press, Washington, DC
Galan P, Viteri F, Bertrais S, Czernichow S, Faure H (2005) Serum concentrations of beta carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J Clin Nutr 59:1181–1190. https://doi.org/10.1038/sj.ejcn.1602230
Hays VW, Swenson MJ (1985) Minerals and bones. In: Dukes’ physiology of domestic animals, 10th edn. Cornell University Press, London, pp 449–466
Henn C, Ettinger AS, Schwartz J, TéllezRojo MM, Lamadrid-Figueroa H, Hernández-Avila M, Schnaas L, Amarasiriwardena C, Bellinger DC, Hu H, Wright RO (2010) Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology 21:433–439. https://doi.org/10.1097/EDE.0b013e3181df8e52
Hewlings S, Kalman D (2019) Sulfur in human health. EC Nutrition
https://www.lenntech.com/recommended-daily-intake.htm Accessed 17 Mar 2020
https://www.medicinalnewstoday.com/articles/263176 Accessed 17 Mar 2020
https://www.msdmanuals.com/home/hormonal-and-metabolic-disorders/electrolytebalance/overview-of-sodium-s-role-in-the-body. Accessed 30 Aug 2019
Jerônimo MC, Costa Orsine JV, Borges KK, Carvalho Garbi Novaes MR (2015) Chemical and physical-chemical properties, antioxidant activity and fatty acids profile of red pitaya [Hylocereus Undatus (Haw.) Britton & Rose] grown in Brazil. J Drug Metab Toxicol. https://doi.org/10.4172/2157-7609.1000188
Liang XM, Huang RB, Huang JC, Chen CX, Qin FZ, Liu AT, Tang XJ, Ning T, Nong HL (2020) Effect of an aqueous extract of Averrhoa carambola L. on endothelial function in rats with ventricular remodelling. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2019.109612
Lopez FF, Cabrera C, Lorenzo ML, Lopez MC (2002) Aluminium content of drinking waters, fruit juices and soft drinks:contribution to dietary intake. Sci Total Environ 292:205–213
Malhotra VK (1998) Biochemistry for students, 10th edn. Jaypee Brothers Medical Publishers (P) Ltd, New Delhi
Marchioretto LD, De Rossi A, Conte ED (2020) Chemical root pruning improves quality and nutrient uptake of cape gooseberry (Physalis peruviana) seedlings. Sci Hortic 261:108948. https://doi.org/10.1016/j.scienta.2019.108948
Mketo N, Nomngongo PN, Ngila JC (2015) A rapid microwave-assisted acid extraction method based on the use of diluted HNO3-H2O2 followed by ICP-MS analysis for simultaneous determination of trace elements in coal samples. Int J Environ Anal Chem 95:453–465. https://doi.org/10.1080/03067319.2015.1025226
Mohammed E, Mohammed T, Mohammed A (2017) Optimization of an acid digestion procedure for the determination of Hg, As, Sb, Pb and Cd in fish muscle tissue. MethodsX 4:513–523. https://doi.org/10.1016/j.mex.2017.11.006
Moreda-Piñeiro J, Sánchez-Piñero J, Mañana-López A, Turnes-Carou I, Alonso-Rodríguez E, López-Mahía P, Muniategui-Lorenzo S (2018) Multi-element determinations in foods from Amazon region by ICP-MS after enzymatic hydrolysis assisted by pressurisation and microwave energy. Microchem J 137:402–409. https://doi.org/10.1016/j.microc.2017.11.018
Murray RK, Granner DK, Mayes PA, Rodwell VW (2000) Harper’s biochemistry, 25th edn. McGraw-Hill, Health Profession Division, New York
Narain N, Bora PS, Holschuh HJ, Vasconcelos MAD (2001) Physical and chemical composition of carambola fruit (Averrhoa carambola L.) at three stages of maturity. J Food. https://doi.org/10.1080/11358120109487721
National Research Council, Food Nutrition Board (1980) Cupper. In: Recommended dietary allowances, Washington, DC, pp. 151–154
Novaes CG, Silva Romão IL, Santos BG, Ribeiro JP, Bezerra MA, Paranhos da Silva EG (2017) Screening of Passiflora L. mineral content using principal component analysis and Kohonen self-organizing maps. Food Chem. https://doi.org/10.1016/j.foodchem.2017.04.111
Osredkar J, Sustar N (2011) Copper and zinc, biological role and significance of copper/zinc imbalance. J Clin Toxicol s3. https://doi.org/10.4172/2161-0495.S3-001
Roger M (2011) The minerals you need. Safe Goods Publishing, USA
Ruzainah AJ, Ahmad Ridhwan BAR, Nor Zaini CM, Vasudevan R (2009) Proximate analysis of dragon fruit (Hylecereus polyhizus). Am J Appl Sci 6:1341–1346
Sa RR, Caldas JD, Santana DD, Lopes MV, Santos WNL, Korn MGA, Santos AD (2019) Multielementar/centesimal composition and determination of bioactive phenolics in dried fruits and capsules containing Goji berries (Lycium barbarum L.). Food Chem. https://doi.org/10.1016/j.foodchem.2018.05.124
Silva Avila D, Puntel RL, Aschner M (2013) Manganese in health and disease. In: Sigel A, Sigel H, Sigel RKO (eds) Interrelations between essential metal ions and human diseases, metal ions in life sciences, vol 13. Springer, Dordrecht, pp 199–227
Sneddon J, Vincent MD (2008) ICP-OES and ICP-MS for the determination of metals: application to oysters. Anal Lett 41:1291–1303. https://doi.org/10.1080/00032710802013991
Turkish Food Codex (2001) Communique on fermented milk, Official Gazette, 3 September, 24512, 96
Uddin AH, Khalid RS, Alaama M, Abdualkader AM, Kasmuri A, Abbas SA (2016) Comparative study of three digestion methods for elemental analysis in traditional medicine products using atomic absorption spectrometry. J Anal Sci Technol 7. https://doi.org/10.1186/s40543-016-0085-6
Umesawa M, Iso H, Date C, Yamamoto A, Toyoshima H, Watanabe Y, Kikuchi S, Koizumi A, Kondo T, Inaba Y, Tanabe N, Tamakoshi A (2006) Dietary intake of calcium in relation to mortality from cardiovascular disease: the JACC study. Stroke 37:20–26. https://doi.org/10.1161/01.STR.0000195155.21143.38
WHO Contributors (2007) The impact of zinc supplementation on childhood mortality and severe morbidity, Geneva, Switzerland, World Health Organization
William BF, David GE, Claudine TJ, William SW (2015) Dietary sodium and health: more than just blood pressure. J Am Coll Cardiol 65:1042–1050. https://doi.org/10.1016/j.jacc.2014.12.039
Young SH, Ji YC, Eun YN, In Min H, Naeem K, Nargis J, Kyong SK (2019) Determination of macro, micro and trace elements in citrus fruits by inductively coupled plasma-optical emission spectrometry (ICP-OES), ICP-mass spectrometry and direct mercury analyzer. J Sci Food Agric 99:1870–1879. https://doi.org/10.1002/jsfa.9382
Funding
This study was supported by the Research Fund of the University of Namik Kemal University (Project Number: NKUBAP. 00.GA.16.026).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ayca Karasakal declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by author.
Informed Consent
Informed consent is not applicable in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karasakal, A. Determination of Major, Minor, and Toxic Elements in Tropical Fruits by ICP-OES After Different Microwave Acid Digestion Methods. Food Anal. Methods 14, 344–360 (2021). https://doi.org/10.1007/s12161-020-01884-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01884-3