Abstract
Manganese is an important metal for human health, being absolutely necessary for development, metabolism, and the antioxidant system. Nevertheless, excessive exposure or intake may lead to a condition known as manganism, a neurodegenerative disorder that causes dopaminergic neuronal death and parkinsonian-like symptoms. Hence, Mn has a paradoxal effect in animals, a Janus-faced metal. Extensive work has been carried out to understand Mn-induced neurotoxicity and to find an effective treatment. This review focuses on the requirement for Mn in human health as well as the diseases associated with excessive exposure to this metal.
Please cite as: Met. Ions Life Sci. 13 (2013) 199–227
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- dopamine
- essentiality
- manganese
- manganese enzymes
- manganism
- mitochondria
- neurodegenerative diseases
- parkinson’s disease-related genes
- treatment
1 Introduction
Manganese, a group 7 metal in the periodic table, is the twelfth most abundant element in the earth’s crust. It exists in a number of chemical and physical forms in the atmosphere’s particulate matter and in water [1]. Mn does not occur naturally in a pure state, and is found as both inorganic and organic compounds, the inorganic form being the most common. Because the Mn outer electron shell can donate up to 7 electrons, it can occur in 11 different oxidation states, varying from −3 to +7 [2]. In living tissue, Mn has been found as Mn2+, Mn3+, and possibly as Mn4+, while Mn5+, Mn6+, Mn7+, and other complexes of Mn at lower oxidation states, are not observed in biological materials [3,4].
The versatile chemical properties of Mn have enabled its industrial usage in iron and steel production, manufacture of dry cell batteries, production of potassium permanganate and other chemicals, as oxidant in the production of hydroquinone, manufacture of glass and ceramics, textile bleaching, as an oxidizing agent for electrode coating in welding rods, adhesives, paint, matches and fireworks, and tanning of leather. Organic compounds of Mn are also present in fuel additive, methylcyclopentadienyl manganese tricarbonyl (MMT) as well as in several fungicides. Moreover, considering that Mn is a paramagnetic metal, namely that it has unpaired electrons in its outer d shell, it can also be detected with magnetic resonance imaging (MRI), positron emission tomography (PET), and single-photon emission computed tomography (SPECT) [1,5]. These techniques allow for the tracking of Mn dynamics repeatedly in the same subject in vivo [1,6]. Mn can also interact with fluorophore fura-2, by quenching and increasing its fluorescence, representing a new methodological approach for in vitro kinetic studies. Thus, given its ubiquitous nature and widespread use in both industrial and non-industrial processes, several health organizations have expressed concern about the potential health effects of occupational/environmental Mn exposure.
Mn is an essential element for humans, animals, and plants; it is required for growth, development, and maintenance of health. Routes of Mn exposure are mainly through dietary intake, dermal absorption, and inhalation. Accordingly, the primary source of Mn intoxication in humans is due to occupational exposure as in miners, smelters, welders, and workers in dry-cell battery factories [7–10], in which significant neurological dysfunction has been associated with Mn exposure [11,12]. Indeed, epidemiological studies of industrial workers have suggested a relationship between elevated environmental Mn exposure and an increased risk for parkinsonian disturbances [13–17], an association that has also been supported by numerous laboratory studies [18–24]. While the exact mechanisms underlying the neurotoxic effects of Mn remain unclear, these studies collectively suggest that elevated environmental exposures to Mn may be sufficient to exacerbate the emergence of neurological diseases [23,24]. Thus, in the next sections of this chapter we will discuss some details concerning Mn in health and disease.
1.1 Manganese Essentiality
Mn is an essential nutrient necessary for a variety of metabolic functions including those involved in normal human development, activation of certain metalloenzymes, energy metabolism, immunological system function, nervous system function, reproductive hormone function, and in antioxidant enzymes that protect cells from damage due to free radicals [25,26]. Mn also plays an essential role in regulation of cellular energy, bone and connective tissue growth, and blood clotting. Mn is an important cofactor for a variety of enzymes, including those involved in neurotransmitter synthesis and metabolism [27]. Indeed, in the mammalian brain, small amounts of Mn are required for brain development, cellular homeostasis, and for the activity of multiple enzymes [28–30]. Additionally, Mn is believed to be involved in the stellate process production in astrocytes, as well as in the metabolism of brain glutamate to glutamine, a step carried out by glutamine synthetase (GS).
Taking into account the variety of enzymatic processes which require Mn, an inadequate daily supply of the metal is associated with a variety of health repercussions, ranging from generalized growth impairment, birth defects, reduced fertility, and impaired bone formation, to altered metabolisms of lipids, proteins, and carbohydrates [31,32]. However, few occurrences of Mn deficiencies have been reported in humans, with symptoms including dermatitis, slowed growth of hair and nails, decreased serum cholesterol levels, decreased levels of clotting proteins, increased serum calcium and phosphorus concentrations, and increased alkaline phosphatase activity [25,33,34]. In addition, several human diseases have been reported to be associated to low blood Mn concentrations, including epilepsy, Mseleni disease, Down’s syndrome, osteoporosis, and Perthest disease [35], nevertheless, the role of Mn deficiency in these diseases remains unclear. In general, highly severe deficiencies in dietary Mn supply are necessary to observe clinical symptoms [36,37].
The U.S. Food and Drug Administration (U.S. FDA) suggests a Reference Daily Intake (RDI) for Mn at 2 mg/day for adults (Federal Register 2007, 72 FR 62149), although there is no consensus regarding the safe and adequate levels of this nutrient for various age groups. This recommended dosage is based upon the U.S. National Research Council’s (NRC), which established estimated safe and adequate dietary intake (ESADDI) of 2–5 mg/day for adults [38]. Additionally, it is known that Mn essentiality in humans varies depending of the life-stage and of the sex [39]. Accordingly, it is suggested by the National Academy of Sciences (NAAS) that an adequate intake of Mn is 2.3 mg/day for adult men and 1.8 mg/day for adult women [39,40]. The difference is accounted for by differential Mn absorption in men versus women [41]; it has been attributed to lower serum ferritin concentrations in men as compared to women [39,41]. Lactating or pregnant women are also thought to have increased Mn requirement [39]. Moreover, life-stages are also known to influence dietary Mn requirements. Accordingly, in newborns (less than six months of age) adequate Mn intake is defined as 3 μg/day; at seven to twelve months of age, adequate Mn intake increases to 600 μg/day [39]. In children one to three years of age, adequate Mn intake approximates 1.2 mg/day, and in children four to eight years of age, the adequate Mn intake increases to 1.5 mg/day.
1.2 Manganese Pharmacokinetics
Intricate regulation of Mn absorption and tissue specific accumulation is crucial for the proper regulation of the activity of Mn-dependent enzymes. Thus, understanding Mn’s essentiality and toxicity in the brain requires knowledge of its regulation in the periphery. Three major factors have been postulated to modulate plasma Mn levels. First, given that the main source of Mn is diet, tight regulation of gastrointestinal absorption of Mn is crucial. Second, following Mn absorption and a concomitant increase in plasma Mn levels, transport of Mn to target organs, including the liver, is necessary to prevent Mn-induced toxicity in the periphery. Finally, Mn must be eliminated from the plasma via shuttling to bile [42]. Thus, homeostatic controls tightly restrict Mn absorption and regulate Mn excretion to maintain stable tissue levels despite fluctuations in daily Mn dietary exposure. However, exposure to high Mn concentrations, as might occur in occupational settings, may overwhelm homeostatic controls and results in elevations in tissue Mn concentrations. Accordingly, both pulmonary uptake and particulate transport via the olfactory bulb [42,43] can lead to deposition of Mn within the striatum and cerebellum and inflammation of the nasal epithelium [44].
It is generally accepted that Fe has a strong influence on Mn homeostasis, since both metals share binding and uptake via the transferrin (Tf) transporter and the divalent metal transporter-1 (DMT-1) (see more details in Section 2 of this chapter). It is known that Mn ions (Mn3+) bind at the same location as ferric ions (Fe3+) on the large glycoprotein molecule mucin, which is known to stabilize the ions, preventing precipitation in the lumen of the gastrointestinal tract [45]. Moreover, both metals are known to have an affinity for the intercellular metal binding molecule mobilferrin [46].
Absorption of metal ions into enterocytes is known to take place via transmembrane transporters. Thus, during Fe deficiency the number of transporters in enterocyte membranes is increased in order to maximize Fe absorption [47]. This will inevitably result in increased Mn absorption, particularly in the absence of Fe. Indeed, in rodent models, Fe deficiency is associated with increased Mn absorption across the gastrointestinal tract, as well as increased Mn deposition in the brain [48,49]. Moreover, the absorption of Mn by the gastrointestinal tract is highly dependent upon the quantity of ingested Mn and net accumulated levels in the plasma. While Mn is transported by simple diffusion in the large intestine, Mn is absorbed by active transport in the small intestine [42]. In contrast, Mn excretion into bile is driven by concentration gradients leading to its flow from liver to bile [50].
About 3–5% of dietary Mn is absorbed in the gastrointestinal tract as Mn2+ and Mn4+ [29]. Mn2+ is oxidized to Mn3+ by liver and plasma ceruloplasmin and transported through the blood [51,52]. Mn tends to form tight complexes with other ligands [4]. Accordingly, a variety of plasma proteins or ligands have been implicated as specific Mn carrier proteins, including transglutaminase, beta-globulin, albumin, and Tf [53,54]. As a result, its free plasma and tissue concentrations tend to be extremely low [55].
Intracellular Mn2+ is sequestered in the mitochondria of the brain and liver via the Ca2+ uniporter [56,57]. Thus, mitochondria are the primary pool of intracellular Mn; however, nuclei have also been implied (remains debatable) to preferentially accumulate this metal [21,58,59]. In addition, it was recently shown that Mn2+ may induce fragmentation of the Golgi apparatus, indicating a specific role of this compartment in maintaining Mn homeostasis [60]. The Golgi harbors the Ca2+/Mn2+-ATPases of the secretory pathway (SPCAs) [61], which possesses a high-affinity Mn2+ transport capacity [62]. This is also supported by in vivo studies reporting that brain areas with high SPCA expression also show enhanced Mn2+ accumulation upon continuous systemic MnCl2 infusion in mice [63], and by the observation that a gain-of-function mutation in SPCA, which specifically enhances Golgi Mn2+ transport, improves survival of Mn2+-exposed cells [64]. Thus, failure of efficient Mn2+ detoxification by saturating the SPCA-mediated removal via the Golgi may result in enhanced Mn2+ accumulation in the mitochondria, thereby causing mitochondrial impairment [60].
Mn enters the brain from the blood either across the cerebral capillaries and/or the cerebrospinal fluid (CSF). At normal plasma concentrations, Mn appears to enter into the CNS primarily across the capillary endothelium, whereas at high plasma concentrations, transport across the choroid plexus appears to predominate [65,66], consistent with observations on the rapid appearance and persistent elevation of Mn in this organ [67]. Indeed, radioactive Mn injected into the blood stream is concentrated in the choroid plexus within 1 hour after injection. Three days post-injection it is localized at the dentate gyrus and CA3 of the hippocampus [68].
The concentration of Mn in the brain varies across brain regions. The highest Mn levels are found in the globus pallidus in humans and in the hypothalamus in rats [28,69–75]. Spectroscopy in rats has demonstrated that mitochondria in the basal ganglia accumulate the highest amount of Mn [76,77]. Differential metal transporter expression patterns and diffusion constants for Mn in various brain regions must explain, at least in part, the asymmetry in Mn accumulation across brain regions [78]. The preferential accumulation of Mn in basal ganglia is often associated with a clinical disorder referred to as manganism, which is characterized by a set of extrapyramidal symptoms resembling idiopathic Parkinson’s disease (IPD) (see more details in Section 3 of this chapter). However, further characterization of the absorption and elimination kinetics, as well as Mn uptake and export pathways is necessary to better understand the basis of differential Mn accumulation across different brain regions.
The physiological half-life of Mn in the adult rat and primate brain is approximately 51 to 74 days [52,55,73,79]. The main excretion mechanism for Mn depends on normal liver function. Indeed, blood Mn concentrations are increased during the active phase of acute hepatitis as well as in post hepatic cirrhosis, and a significant correlation exists between blood Mn and the activities of liver enzymes in patients with hepatitis and cirrhosis [80,81]. Corroborating these observations, MRI has consistently shown signal hyperintensity in the globus pallidus in cirrhotic patients [82]. Furthermore, direct measurements in pallidal samples obtained from the autopsies of cirrhotic patients revealed several-fold increases in Mn concentrations, and histopathologic evaluations showed Alzheimer’s type II astrocytosis [83]. The disorder is characterized as hepatic encephalopathy, and it is associated with cognitive, psychiatric, and motor impairments, all of which are known to be also associated with manganism [84].
From physiologically based pharmacokinetic models, it has been proposed that 54Mn clearance from the body follows biphasic elimination, with a short “fast” elimination phase (with half-times of around a few days) followed by a longer “slow” elimination phase. This elimination behavior was consistently observed with all exposure routes. Thus, the availability of tracer studies for multiple exposure routes permitted a comparison of dose route differences in elimination. Accordingly, the faster clearance in monkeys and humans occurred from oral exposure, whereas the slowest clearance occurred following intravenous (iv) administration [85].
1.3 Manganese Biochemistry and Physiology
As pointed out above, Mn is an essential nutrient necessary for a variety of functions. Accordingly, it acts as an activator of the gluconeogenic enzymes pyruvate carboxylase and isocitrate dehydrogenase, is involved in protecting mitochondrial membranes through superoxide dismutase, and it activates glycosyl transferase, which is involved in mucopolysaccharide synthesis [86], just to name a few. Mn it is also a cofactor for many other enzymes, such as transferases, hydrolases, lyases, and integrins. Nevertheless, additional investigation is needed to identify the complete set of Mn-dependent enzymes in mammalian systems as for many of these activities, Mn is not the only metal that can act as a cofactor; iron, magnesium or copper are readily able to substitute it. In addition, the majority of Mn-dependent enzymes are found in bacteria and plants and only a few have been systematically studied in mammalians.
Although Cu and Mg can substitute for Mn as a cofactor for some enzymes, there is a subset of enzymes with roles in neuron and/or glial that are strictly dependent upon the presence of Mn. These discrete Mn-binding proteins (manganoproteins) include glutamine synthetase, superoxide dismutase 2 (SOD2), arginase, pyruvate decarboxylase, and serine/threonine phosphatase [87–89].
Glutamine synthetase is the most abundant manganoprotein; it is predominantly expressed in astrocytes, where it converts glutamate to glutamine. Because GS contains four Mn ions per octamer [90], Mn has been proposed to regulate GS activity. In fact, insufficient Mn increases glutamate trafficking, glutamatergic signaling, and excitotoxicity [91]. Furthermore, it has been proposed that the increased susceptibility to seizures observed in individuals with Mn deficiency may be due to diminished GS levels and/or activity [92].
Arginase regulates elimination of ammonia from the body by converting L-arginine, synthesized from ammonia, to L-ornithine and urea as part of the urea cycle. Moreover, in the brain, L-arginine is converted to nitric oxide by neuronal nitric oxide synthetase. Proper regulation of arginase promotes neuronal survival by impairing nitric oxide signaling [93,94].
Pyruvate carboxylase is an essential enzyme required for glucose metabolism that interacts with Mn to generate oxaloacetate, a precursor of the tricarboxylic acid (TCA) cycle [95]. Interestingly, in the brain, pyruvate carboxylase is predominantly expressed in astrocytes [58,96]. Protein phosphatase-1 is essential for glycogen metabolism, cell progression, regulation synthesis, and release of neurotrophins, which promote neuronal survival and synaptic membrane receptors and channels [97].
Finally, SOD2 is a mitochondrial enzyme that detoxifies superoxide anions through the formation of hydrogen peroxide. Although the concentration of Mn within neurons is low (<10−5 M), their high mitochondrial energy demands is correlated with a propensity for increased SOD2 in neurons compared to glia [28,42]. Furthermore, loss of SOD2 activity increases the susceptibility to mitochondrial inhibitor induced toxicity and causes oxidative stress [98].
Thus, Mn deficiencies, although not frequently reported in humans, may result in several biochemical and structural defects [35,99,100]. Accordingly, taking into account the Mn-dependent enzymatic processes, it is clear that inadequate daily supply of this metal may be associated with a variety of health repercussions [31,32]. In contrast, excessive levels of Mn can accumulate in the brain and lead to neurotoxicity. Because the area of the CNS comprising the basal ganglia is very complex and its function is dependent upon the precise function and balance of several neurotransmitters, it is not surprising that symptoms of manganism often overlap with IPD. Indeed, evidence exists to support that persistent exposure to Mn may predispose an individual to acquire dystonic movements associated with PD (more details concerning Mn toxicity are provided in Section 3 of this chapter).
2 Manganese Transport
Considering the delicate relationship between essentiality and toxicity, Mn homeostasis is vital for the optimal functioning of any organism. Although some research has focused on mechanisms associated with the transport of Mn across the blood-brain barrier (BBB), the exact identity of the carrier(s) involved in Mn trafficking into the brain remains somewhat controversial. Over the past decades, active transport [65] and facilitated diffusion [51,66] mechanisms have been described. More recently, Mn transport has been ascribed to high affinity metal transporters of Ca and Fe.
Several of these transporters include DMT-1, which belongs to the family of natural resistance-associated macrophage protein (NRAMP) [47,101]; ZIP8, a member of the solute carrier-39 [102]; Tf receptor (TfR) [51,103], which is known to be responsible for Fe3+ uptake; voltage-regulated [104] and store-operated Ca2+ channels [105]; and the ionotropic glutamate receptor Ca2+ channels [106]. It was also observed that distribution after intravenous injection of 54Mn is readily transported into the brain as the free ion [66]. Moreover, studies have also demonstrated that a Mn-citrate complex could be transported either by a monocarboxylate transporter (MCT) [107], organic anion transporter polypeptide (OATP) or ATP-binding cassette (ABC) superfamilies [108].
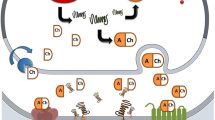
Leak-pathways for Mn also likely exist, especially in areas lacking intact BBB, namely the circumventricular organs [109] (Figure 1). While the relative contribution of each of these transporters and/or mechanism(s) remains unknown, it is likely that optimal tissue Mn concentrations are maintained by the involvement of all of them. As pointed out above, in plasma more than 80% of Mn2+ is bound to α2-macroglobulin and albumin. However, these complexes (Mn2+-albumin or α2-macroglobulin) may not take part in the transport across BBB (see Figure 1).
Mechanism of Mn transport across the BBB under physiological Mn exposure levels. Transporters and relevant manganese oxidation states associated with Mn transport are demonstrated. Mn bound to albumin is excluded from passing the BBB given its size. Arrow size depicts the relative importance of each of the transporters in this process, bolder arrows representing more prominent transport mechanisms. Please refer to the discussion for additional details. Since it has yet to be determined whether ZIP8 functions to transport Mn across the BBB, the process has been annotated with a question mark.
TRPM7, which is ubiquitously expressed in vertebrates and functions as an active Ca2+-selective transporter and a serine/threonine protein kinase, is a putative Mn transporter. The kinase activity is important for its metal transport function. Specifically, the transporter operates by regulating intracellular Ca2+ levels and Mg2+ homeostasis through the creation of an inward current, thus contributing to the establishment of a cellular membrane potential. Physiological levels of Mg2+ and Ca2+ are necessary for maintaining the permeability of TRPM7 to Mn2+, Co2+, and Ni2+ [110]. Finally, the homomeric purinoreceptors, including P2X and P2Y, have been invoked to transport Mn. These receptors are ATP-dependent and ubiquitously expressed on endothelial cells [111–113]. Nevertheless, purinoreceptors have a relatively lower affinity for Mn than for the other divalent metals they transport (Ca > Mg > Ba > Mn) [110].
Although non-protein-bound Mn enters the brain more rapidly than Tf-bound Mn [65,66], the question remains as to which form represents the predominant mechanism of transport in situ. Analyses of transport mechanisms based on tracer techniques employing bolus injections of Mn into the circulation cannot be easily interpreted. Thus, while tracer studies represent a sensitive technique for quantifying Mn transport, it must be noted that the information derived from such studies does not necessarily reflect the chemically active or functional forms in which Mn exists and is transported in vivo. This is due to the saturation of blood ligands for Mn and the likelihood that Mn in the free form exists at concentrations in excess to those expected under physiological conditions. Thus, transport kinetics under such conditions may not necessarily mimic physiological conditions. In the next subsections, we briefly discuss some aspects related to Mn transport in biological systems.
2.1 Manganese Uptake in Relation to Oxidative State
Mn can cross the BBB and blood-cerebrospinal fluid barrier (BCB) through several carriers (see Figure 1) and in different oxidation states [42]. Indeed, emerging reports have indicated that Mn2+ can be transported via DMT-1, the divalent metal/bicarbonate ion symporters ZIP8 and ZIP14, various calcium channels, the solute carrier-39 (SLC39) family of zinc transporters, park9/ATP13A2, and the Mg transporter hip14. Accordingly, DMT-1 belongs to a large family of metal transporters, which are responsible for the transport of divalent metals ions, including Mn, Fe, Cu, and Cd [114]. Thus, DMT-1 is involved in Mn accumulation in the brain. DMT-1 works as hydrogen ion symporter, transporting one hydrogen ion and one divalent cation in the same direction. This protein is responsible also for exporting the Mn2+, which is released into the cytoplasm [115]. Alternatively, Mn2+ ions may be directly transported from the blood stream by crossing the cellular membrane through voltage-regulated or glutamate-activated ionic channels, which are usually responsible for the transport of Ca2+ into the cell [116]. Finally, emerging experimental data has indicated that huntingtin-interacting protein 14 and 14L (Hip14, Hip14L) mediates transport of Mn2+ and other divalent metals (Mg2+, Sr2+, Ni2+, Ca2+, Ba2+, Zn2+) across cell membranes [117,118]. Alternatively, Mn3+ entry via the TfR, which mediates Fe3+ uptake, is also considered [88].
2.2 Cellular Manganese Uptake
A critical regulator of brain Mn levels is the DMT-1 (also referred to as the DCT, or divalent cation transporter) which is known to shuttle both Mn2+ and Fe2+ ions, as well as other divalent metals. This transporter belongs to the NRAMP gene family [47,101]. Disruption of the orthologous DMT-1 gene in the rat or mouse, results in significantly lower tissue levels and uptake of Mn and Fe in the brain [119–121]. Consistent with a role for DMT-1 in brain Mn uptake, nasal Mn absorption is also significantly attenuated in b/b rats, and olfactory DMT-1 protein levels are significantly elevated in Fe-deficient rats [122].
Notably, a recent study has shown that DMT-1 contributes to neurodegeneration in an experimental model of PD [123]. These authors observed increased expression of a specific DMT-1 isoform (DMT-1/Nramp2/Slc11a2) in the substantia nigra of PD patients. Moreover, the authors also showed that the administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, a dopaminergic toxin used in experimental models of Parkinson’s disease) increased DMT-1 expression in the ventral mesencephalon of mice, which was concomitant with Fe accumulation, oxidative stress, and dopaminergic cell loss [123]. Additionally, DMT-1-mediated metal transport across rat brain endothelial cells in culture has been reported to be pH-, temperature-, and Fe-dependent [110,124,125].
The TfR is the major cellular receptor for Tf-bound Fe, but because Tf can also bind trivalent Mn, TfR can also mediate Mn3+ transport by endocytosis. Mn3+ internalized through the endocytic pathway must be released from Tf and reduced to Mn2+, which is transported through DMT-1 to the cytosol. The TfR is an active transporter that is pH- and Fe-dependent [110]. Both in vivo and in vitro studies have reported that Mn is efficiently transported via the TfR. For example, a spontaneous mutation in a murine gene linked to the TfR, referred to as hypotransferrinemic, results in a drastic serum TfR deficiency, impairs Mn transport, and disrupts Fe deposition [126,127].
Additional cellular Mn transporters include the Mn-citrate transporters and the Mn-bicarbonate symporters. Indeed, a small fraction of Mn has been found in the plasma as citrate complex. Crossgrove and Yokel have demonstrated that a Mn-citrate tridentate complex with a non-coordinated central carboxylate moiety is a probable substrate for the anion transporter or a monocarboxylate transporter [107]. Moreover, the members of the organic anion transporter polypeptide or ATP-binding cassette superfamilies may transport Mn-citrate complexes [108]. The Mn-bicarbonate symporters, ZIP8 and ZIP14, have also been identified as members of the solute carrier-39, and are expressed on brain capillaries [102], although it has yet to be determined whether these proteins are functional at the BBB [1,128]. Nevertheless, these symporters utilize a HCO −3 gradient as the driving force for Mn uptake across the plasma membrane.
Gitler and colleagues have recently reported that the park9 gene responsible for early-onset Parkinsonism also transports Mn [118]. The park9 gene encodes a putative P-type transmembrane ATPase (ATP13A2) protein. Although the exact function of park9 is unknown, it is generally thought to be a shuttle for cations, including Mn across the cell membrane. Biochemical studies have demonstrated that the highest and lowest park9 mRNA levels are localized within the substantia nigra and cerebellum, respectively [129]. Mn transport via voltage-regulated channels [104], store-operated channels [105], ionotropic glutamate receptor channels [106] (all Ca2+ channels), and choline transporters [130] has also been described.
Another possible mechanism for Mn transport involves dopamine transporters (DAT). It is believed that DAT facilitates Mn transport into dopaminergic striatal neurons and that Mn accumulates in the globus pallidus via axonal transport [131,132]. As a result, blockage of the DAT in the striatum would attenuate Mn accumulation in striatal neurons and would cause decreased Mn concentrations in the globus pallidus [132] (see Figure 2). Nevertheless, while the tissue specific expression of each of the aforementioned Mn transporters is yet to be determined, it is likely that optimal tissue Mn levels are maintained through the involvement of all the above and likely other unknown Mn transporters.
2.3 Cellular Manganese Efflux
Little information exists on the putative extracellular transport mechanisms of Mn. In the intestine ferroportin-1 (Fpn) is located at the basal membrane and exports Fe to the circulation [133,134]. Yin and colleagues [135] have implicated Fpn as a Mn exporter. Using an inducible HEK293T cell model, they showed that Fpn expression reduced Mn-induced toxicity and decreased Mn accumulation. In addition, Mn was also able to increase Fpn levels [136]. From these studies, it was concluded that Fpn participates in Mn efflux (see Figure 2). In accordance to previous data, it was recently shown that Mn is a substrate for Fpn, and that this export process is inhibited by a low extracellular pH and by incubation in a high K+ medium, indicating the involvement of transmembrane ion gradients in Fpn-mediated Mn transport [137].
Interestingly, Fpn is expressed in tissues involved in both Fe and Mn homeostasis, including the developing and mature reticuloendothelial system, the duodenum, liver, and the pregnant uterus [138,139]. Fpn has also been identified in cells of the central nervous system including those of the BBB, choroid plexus, neurons, oligodendrocytes, astrocytes, and retina [133,140]. Nevertheless, it has yet to be determined whether Mn shares Fe exporters, such as Fpn, in this process. Moreover, the role of Fpn in Mn homeostasis remains to be elucidated in each of the neural cell types.
3 Manganism. A Neurodegenerative Disease
Manganism (also referred to as locura manganica) has been known for 150 years and it is caused by the preferential accumulation of Mn in brain areas rich in dopaminergic (DAergic) neurons (caudate nucleus, putamen, globus pallidus, substantia nigra, and subthalamic nuclei) [79,141,142]. Mn can readily oxidize catecholamines, including dopamine (DA), altering homeostasis in these areas [70]. Perturbation in striatal DA levels likely explains the biphasic syndrome experienced by patients with manganism. An initial phase of increased DA production is associated with psychotic episodes commonly observed in psychiatric patients [143]. As Mn poisoning progresses, catecholamine levels decrease, most likely due to the loss of nigrostriatal DAergic neurons and, consequently, the parkinsonian-like symptoms ensue [1,13,70]. Hence, in early stages of manganism, upon cessation of Mn exposure, the symptoms might be reversed, whilst in patients with motor disturbances, manganism is irreversible [144].
4 Symptoms and Sensitive Populations
The initial stages of manganism are characterized by psychiatric symptoms, including emotional liability, mania, compulsive or aggressive behavior, irritability, reduced response speed, hallucinations, feeding and sex disturbances, intellectual deficits, humor changes, sex dysfunctions, as well as mild motor impairment. In established manganism cases, the classic extrapyramidal symptoms (motor symptoms) become prominent and include mask-like face, limb rigidity, mild tremors, gait disturbance, cock-like walk, slurred speech, excessive salivation and sweating, and a disturbance of balance, all of which are also observed in IPD [144–147].
Considering the routes and sources of exposure, affected populations commonly include welders, miners, and people that work in a Mn-polluted environment [147–149]; infants fed with Mn-containing formulas [109]; patients with hepatic encephalopathy [150,151], and subjects fed with parenteral nutrition [152,153]. In addition, subjects that have genetic pre-disposition have been recently considered as sensitive populations, as described in Section 8.
5 Manganism versus Parkinson’s Disease
Idiopathic Parkinson’s disease is a progressive neurodegenerative disorder with a slow onset, and compared with the familial forms of the disease, it is associated with advanced age (>55 years of age). The four cardinal signs of IPD are tremor at rest, bradykinesia (hypokinesia and akinesia), rigidity and postural instability [154–156]. The disease is characterized by loss of neurons [154] in the substantia nigra pars compacta (SNpc) and decreased DA levels in the caudate and putamen [155].
Excessive brain Mn levels can also represent a risk factor for IPD [16,145,147–149]. Case-controlled studies have revealed a strong correlation between Mn-exposed populations and increased susceptibility to PD [16,145,147–149]. Manganism and PD share in their etiology common cellular mechanisms such as preferential accumulation of Mn within mitochondria resulting in oxidative stress [21,157] and selective DAergic neurotoxicity [1,158,159]. Parkinsonism in welders versus non-welders is clinically distinguishable only by the age of onset (46 versus 63 years, respectively) [149,160]. Furthermore, the prevalence of PD is higher among welders versus age-standardized individuals in the general population [149,160]. However, direct evidence that Mn exposure in welders is responsible for this increased prevalence has not been reported yet.
Manganism commonly occurs in response to acute Mn exposures, whereas PD likely reflects long-term exposure to relatively low Mn exposures [145,158]. Manganism features less frequent kinetic tremor, or no tremor versus patients with PD [129,147–149]. Acute high Mn exposures also lead to dystonias and a “cock-walk” with symptoms becoming progressive and irreversible [147,149]. In addition to affecting the basal ganglia, manganism is also known to affect other brain regions, including the cortex and hypothalamus and at the morphological level leading to neuronal loss and reactive gliosis in the globus pallidus and substantia nigra pars reticulata (SNpr) in the absence of Lewy bodies, which are a hallmark of PD [147,149,161,162]. Furthermore, in manganism, damage to the striatum (caudate nucleus and putamen) and subthalamic nucleus may occur, while the SNpc is generally spared whilst PD is predominantly characterized by neuronal loss in the SNpc [161].
PD and manganism are analogous in several mechanistic ways. Mn-induced neurotoxicity involves mitochondrial dysfunction, increase in endoplasmic reticulum stress factors and oxidative stress, as also observed in PD patients studied post-mortem [145,148,158,161]. Mn can cause the increase in fibril formation by α-synuclein, thus inducing neuronal death. The α-synuclein aggregates, named Lewy bodies, are one of the hallmarks of PD. Furthermore, there are several genetic factors associating both disorders, which will be described in detail below (Section 8).
6 Manganese in the Etiology of Other Neurodegenerative Disorders
6.1 Manganese and Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that affects motor neurons (MNs) in the spinal cord and the cortex [163]. ALS patients gradually loose MNs, and muscles weaken, ultimately leading to respiratory distress and death [163]. A small percentage of ALS cases are hereditary, due to mutations in the Cu/Zn superoxide dismutase (SOD1) gene. Expression of these mutant forms of SOD1 in animal models leads to ALS-like phenotypes [164]. Oxidative stress is proposed to be a central mechanism leading to Mn cell loss in ALS, other contributing mechanisms include excitotoxicity, astrocytosis, mitochondrial impairment, calcium/magnesium imbalance, and Mn toxicity [165–167]. Mn-SOD (SOD2), in contrast to its orthologue, SOD1, does not contribute to the genetic predisposition to ALS [168], but it may slow down ALS-like syndrome progression in mice, and its activity has been shown to be reduced in the serum and the CSF of ALS patients [168]. Mn levels were found to be lower in blood cells, but were significantly increased in the sera of ALS patients [169,170], supporting a role for Mn-mediated neurotoxicity in ALS.
6.2 Manganese and Alzheimer’s Disease
Heavy metals, especially the essential metal Zn and the non-essential metal Al, have been shown to play a role in amyloid-beta (Abeta) aggregation and toxicity, both of which are characteristics of Alzheimer’s disease (AD) [171]. In a recent study, Abeta precursor-like protein 1 (APLP1) was found to be the most up-regulated gene in the frontal cortex of monkeys (Cynomologous macaques) chronically exposed to Mn. This result was associated with cortical Mn accumulation [172], cortical neuron degeneration, and apoptotic marker expression [172], consistent with previous reports of cognitive impairment in those animals. Conversely, over-expression of Abeta in mice led to Mn accumulation in the brain, suggesting that Abeta could play a role in Mn homeostasis and toxicity [173]. Similar to ALS, despite Mn accumulation, SOD2 antioxidant activity is reduced in AD, likely contributing to oxidative stress [174]. Furthermore, Mn can also produce alterations related to AD without the senile plaque formation. In cases of chronic Mn exposure, neuronal degeneration in the globus pallidus is associated with the development of Alzheimer’s type II astrocytosis, in which cells typically exhibit enlarged, pale nuclei, margination of chromatin and, often, prominent nucleoli [175].
6.3 Manganese and Huntington’s Disease
Huntington’s disease (HD) is a progressive neurodegenerative disease with a prevalence of 5 in 10,000 people worldwide. HD is characterized by motor impairment, cognitive deterioration, emotional disturbance, and psychiatric deficits, caused by expansion in the glutamine-encoding triplet repeat by mutation in the HTT gene [175]. Environmental factors have also been suggested to contribute to the residual variation in age of onset, perhaps even more so than genetic factors [176]. In this context, metals such as Mn may be involved in modulating and interacting with HD. A study by Williams et al. [177] described a novel gene-environment interaction between expression of mutant HTT and Mn. Specifically, acute Mn exposure of cultured striatal cells unexpectedly decreased the vulnerability of mutant expressing cells (STHdh Q111/Q111) to Mn cytotoxicity compared to wild-type (STHdh Q7/Q7) [177]. Furthermore, total intracellular Mn levels following Mn exposure in STHdh Q7/Q7 and STHdh Q111/Q111 cells were significantly lower in mutant than wild-type cells [177,178]. Moreover, the mutant HTT–Mn interaction was corroborated in vivo using the YAC128Q mouse model of HD; these mice accumulated less Mn in the striatum than wild-type animals following subcutaneous Mn injections [179].
7 Molecular Mechanisms of Toxicity
The cellular, intracellular, and molecular mechanism(s) underlying Mn neurotoxicity are incompletely understood [21,109,141,142,158,161]. Nevertheless, it has been demonstrated that Mn affects numerous biological activities, dependent upon levels and routes of exposure, dosage, age of the exposed individual, and exposure duration [6,180,181]. Mn is known to induce increased oxidative stress, a well-established molecular mechanism of Mn-induced toxicity. Below, we discuss the main mechanisms that are believed to mediate Mn-induced neurodegeneration.
7.1 Dopamine Oxidation
Dopamine (DA) is one of the most abundant catecholamines within the brain, controlling locomotion, emotion, and neuroendocrine system. Chronic exposure to Mn has been shown to cause the degeneration of nigrostriatal DAergic neurons leading to symptoms that resemble PD [13,148,182]. However, Mn’s effects are dependent upon the experimental conditions, form of Mn (oxidation state), route of administration, and exposure duration [1,6,142,147,183]. Postnatal Mn exposure causes a decline in pre-synaptic DAergic functioning, reduced DA transporter expression, and DA uptake in the striatum, and a long-lasting decrease in DA efflux [184,185]. Conversely, in adult animal models, exposure to Mn inhibits DA neurotransmission and depletes striatal DA [29,144,183,186,187], thereby resulting in motor deficits [161].
Although it is generally accepted that free radicals play a key role in mediating Mn-induced DAergic neurodegeneration [188,189], the precise mechanism of Mn-induced neurotoxicity remains unknown. One hypothesis invokes the ability of Mn to enhance reactive oxygen species (ROS) generation, thus forming quinines [82,190,191]. Indeed, the Mn-catalyzed autoxidation of DA involves redox cycling of Mn2+ and Mn3+ in a reaction that generates ROS and DA-o-quinone, thereby leading to oxidative damage [82,190–192]. Thus, elevated rate of autoxidation of cytoplasmic DA induced by Mn may contribute to DAergic cell death secondary to the formation of cytotoxic quinones and ROS [190,191].
Mn-induced DA oxidation is a complex process involving several steps in which semiquinone, aminochrome intermediates, L-cysteine or copper and NADH are implicated [158,182,193]. Mechanisms underlying semiquinone and aminochrome-induced damage in the Mn-induced neurodegenerative process likely include: (i) NADH or NADPH depletion; (ii) inactivation of enzymes by oxidizing thiol groups or essential amino acids; (iii) formation of ROS; and (iv) lipid peroxidation. It is noteworthy that neither Mn2+ nor Mn3+ can generate hydroxyl radicals from hydrogen peroxide and/or superoxide via Fenton-type or Haber-Weiss-type reactions, while Mn2+ can scavenge and detoxify superoxide radicals [3,188].
7.2 Mitochondrial Dysfunction
As mentioned in Section 1.2, intracellular Mn preferentially accumulates in the mitochondria, mainly as Mn2+ via the Ca2+ uniporter [21,157,194–196]. Elevated intramitochondrial Mn interferes with oxidative respiration, leading to excessive production of ROS, and consequently, mitochondrial dysfunction [21,157,194–196]. The ability of Mn to enhance oxidative stress is due to the transition of its oxidative state +2 to +3, which increases its pro-oxidant capacity [192]. Superoxide produced in the mitochondrial electron transport chain may catalyze this transition through a set of reactions similar to those mediated by SOD and thus lead to the increased oxidant capacity of the metal [195]. Since Mn3+ has greater pro-oxidant potential than Mn2+, its production in the mitochondria may accentuate oxidative damage [197].
Moreover, Mn can directly impair mitochondrial function by inhibiting the mitochondrial electron transfer chain [21,88,198], resulting in a reduced ATP production, increased leakage of electrons, and increased O · −2 production [199]. Although, Mn3+ is more potent at inhibiting complex I [3,197], Mn2+ is the predominant species within cells and is largely bound to ATP [196,197]. Notably, Mn in biological media in any of the oxidation states will spontaneously generate Mn3+. Interestingly, even trace amounts of Mn3+ can cause formation of ROS [200]. The involvement of ROS in Mn-induced mitochondrial dysfunction is also supported by observations on the efficacy of antioxidants in attenuating its effects [201].
Mn also interferes with Ca2+ homeostasis in mitochondria by inhibiting its efflux [202,203]. Oxidative stress generated by high Mn concentrations leads to the induction and opening of the mitochondrial permeability transition (MPT) pore, a Ca2+-dependent process, resulting in increased solubility to protons, ions, and solutes, loss of the mitochondrial inner membrane potential (Δψm), impairment of oxidative phosphorylation, and ATP synthesis and mitochondrial swelling [202,204,205]. Indeed, Mn has been shown to decrease Δψm in a concentration-dependent manner, indicating that this Ca2+-dependent process likely mediates Mn neurotoxicity [204,206,207]. Apoptotic mechanisms secondary to changes in mitochondrial function have also been implicated in Mn-induced neurotoxicity. Ca2+-induced MPT opening leads to the activation of the Bcl-2 family of proteins, especially Bax/Bak, culminating with the release of cytochrome c (Cyt c) [208,209]. Cyt c activates, via ERK (extracellular signal-regulated kinases), the cysteine protease caspase-3, which mediates apoptosis, chromatin condensation and DNA fragmentation [210]. Consistent with these observations, Mn exposure has been shown to lead to ERK and caspase-3 activation in astrocytes [204]. Furthermore, DNA strand breakage at low Mn levels was reported, thereby reinforcing the mitochondrial role in mediating its neurotoxicity [211].
7.3 Astrocytosis
Astrocytes make up approximately 50% of the human brain volume [212] and assume many critical pathophysiological roles essential for normal neuronal activity, including glutamate uptake, glutamine release, K+ and H+ buffering, volume regulation and membrane–membrane mediated trophic cell signaling [92,180]. Unlike neurons, astrocytes concentrate Mn to levels at least 50-fold higher than the culture media, thus functioning as the major homeostatic regulators and storage site for Mn [213,214]. Primate models of Mn toxicity have shown astrocytic pathological alterations (Alzheimer type II) [17,22,151] and exposure of cultured astrocytes to pathophysiologically relevant concentrations of Mn led to a dose- and time-dependent cell swelling, which appears to be a consequence of oxidative stress and changes in MPT [215]. Increased accumulation of Mn in astrocytes has also been shown to alter glutamate homeostasis and elicit excitatory neurotoxicity [27]. For example, Mn decreases astrocytic glutamate uptake [180,181,216] and reduces the expression of the astrocytic glutamate:aspartate transporter (GLAST) [27,217,218], leading to increased extracellular glutamate levels. Additionally, expression of glutamine transporters was downregulated in Mn-exposed cultured astrocytes [219], contributing to the disruption of the glutamate-glutamine cycling in the brain.
The inhibition of the Na+/K+-ATPase by reactive oxygen species also likely contributes to Mn-induced astrocytic dysfunction [220]. Mn increases the uptake of the amino acid L-arginine, a substrate for the inducible form of nitric oxide synthase (iNOS), which can lead to ROS production as a consequence of nitric oxide production [221,222]. ROS have also been shown to directly interfere with glutamate uptake [223], possibly via oxidation of thiol groups in the transporter protein [220]. Thus, Mn accumulation in astrocytes has the potential to lead to oxidative damage in these cells as well as adverse effects on glutamate clearance from the extracellular space.
8 Genetic Susceptibility
Recently, the association of mutations of PD-related genes and manganism has been reported. DJ-1 (Park7), together with parkin (Park2) and Pink1 (Park6), form an E3 ubiquitin-ligase complex that is involved in α-synuclein degradation [224]. The physiological functions of these proteins involve protection against oxidative stress. Both mitochondrial dysfunction and oxidative stress can modulate the ubiquitin-proteasome pathway and have been implicated as causative factors for the abnormal accumulation of proteins in familial forms of PD [145]. Recessive inheritance of PARK 2 mutations may also cause increased environmental sensitivity to Mn exposure, as observed by Aboud et al. [225]. Using human induced pluripotent stem cells (hIPCs) derived from a subject at genetic risk by PARK 2 mutation, the authors found significant high reactive oxygen species levels and increased mitochondrial fragmentation after Mn exposure in vitro [225].
Mutations in parkin are associated with early onset of PD, associated with DAergic neurodegeneration, however, absent Lewy bodies formation [145]. The parkin gene encodes an E3 ubiquitin ligase, which has cytoprotective properties. Transient transfection with the parkin gene in SH-SY5Y cells inhibits Mn-induced cell death [226]. Exposure to welding fumes containing Mn led to decreased Park2 protein levels in DAergic brain areas in rodents [227]. Loss of function and/or decreased expression of parkin has been associated with overexpression of DMT-1 and linked to PD [123,227], as well as manganism [228]. Conversely, increased Parkin expression levels have been shown to attenuate Mn-induced neurotoxicity, likely by reducing its transport [226,228]. DJ-1 was also decreased in striatum of rats exposed chronically to welding fumes [227]. Mutations in dj-1 account for 1–2% of early-onset cases of PD [229]. The protein encoded by this gene is expressed in the brain, including neurons within the SNPc and striatum, areas primarily affected in PD [230]. DJ-1 expression has been localized to the matrix and intermembrane space of mitochondria [231] and it is thought to function as an antioxidant protein [232]. Dj-1-knockout mice exhibit increased mitochondrial free radical formation and inactivation of enzymes [232].
Leucine-rich repeat kinase 2 (LRRK2) or PARK8 is a cytoplasmic enzyme present in DAergic neurons. Mutations in this gene causing increased kinase activity lead to typical features of PD [233]. Kinases require the formation of an ATP-divalent metal cation complex, and Mg2+ typically participates in this catalysis. Recent studies in G2019S cells, where LRRK2 is mutated and shows increased enzyme activity, have demonstrated that Mn2+ can displace Mg2+ at the active site and increase the catalytic rate of the enzyme [234]. Because this mutation is present in 22–41% of PD cases, changes in the enzyme activity caused by Mn may result in a gain-of-function type mechanism of toxicity, leading to decreased cell survival [234].
Furthermore, mutations in a putative Mn exporter gene SLC30A10 have been recently described [235]. These mutations are associated with marked motor impairment, including a Parkinsonian-like syndrome. This inherited autosomal recessive mutation leads to hypermanganesemia, dystonia, polycythemia, and hepatic cirrhosis [236]. The hypermanganesemia associated with SLC30A10 mutation is extreme, with patients having whole blood Mn levels of 1200–6400 nmol/L, compared with normal whole blood Mn (<320 nmol/L) [236].
9 Treatment
Even though manganism has been studied for several years, treatment approaches are still controversial. Some authors have shown that levodopa, a standard treatment for PD, decreased Mn symptoms; however most of the studies do not indicate this drug as efficient [148,149]. Hence, other drugs have been tested in non-human animals and some of these treatments may be used in future clinical trials.
Antiinflammatory agents, such as indomethacin and para-aminosalicilic acid, reduced Mn-induced increase in oxidative stress (isoprostanes) and neuroinflammation (prostaglandin E2) [237–240]. Notably, indomethacin protected against progressive spine degeneration and dendritic damage in striatal medium spiny neurons of mice exposed to Mn [239,240]. This protection is probably mediated by the transcription factor NF-κB [241]. Using transgenic mice expressing a transcription factor fused to a green fluorescent protein (GFP), Moreno and coworkers showed that Mn exposure increased NF-κB reporter activity and nitric oxide synthase 2 (NOS2) expression in both microglia and astrocytes, and that these effects were prevented by supplementation with steroid 17β-estradiol [241]. Estrogen also decreased neuronal protein nitration in treated mice and inhibited apoptosis in striatal neurons co-cultured with Mn-treated astrocytes in vitro [241]. Furthermore, tamoxifen, an estrogen-related compound, effectively reversed glutamate transport inhibition in a Mn-induced model of glutamatergic deregulation, suggesting a potential therapeutic modality in neurodegenerative disorders characterized by altered glutamate homeostasis [242]. In agreement with this study, Xu et al. showed that the pretreatment of rats with the NMDA (N-methyl-D-aspartate) antagonist MK801 protected neurons from Mn-induced glutamate excitotoxicity [243]. Synthetic and natural antioxidants have been tested in Mn-induced neurotoxicity models. The chain breaking antioxidant Trolox concomitantly administered to Mn in developing pups showed neuroprotective effects by decreasing apoptotic activity, isoprostane levels and p38 phosphorylation [244]. Ebselen and diethyl-2-phenyl-2-tellurophenyl vinyl phosphonate are organochalcogens that also reversed the neurotoxic effects of this metal. Natural products such as Melissa officinalis extract and silymarin reduced oxidative stress associated to Mn exposure [245,246].
Several studies have addressed genetic factors that mediate Mn toxicity. Streifel and coworkers used mice lacking NOS, postulating that they would be protected from the neurotoxic effects of Mn [247]. They found that loss of NOS2 reduced NO-induced peroxynitrite formation, thus attenuating Mn-related peroxynitrite adduct formation in the striatal-pallidum and substantia nigra pars reticulata. These mice showed attenuated alterations in neurobehavioral function and neurochemistry in vivo and loss of NOS2 also prevented astrocyte-mediated neuronal apoptosis in vitro [247]. Expression of parkin, an E3 ubiquitin ligase also linked to PD, protects against Mn toxicity, as observed in SH-SY5Y cells [248]. Conversely, deletion of parkin leads to an increase in DMT-1 levels, thus causing increase in Mn uptake [248]. Furthermore, it was reported for yeast that expression of PARK9, a gene linked to PD, protected cells from Mn toxicity [118].
In C. elegans, Benedetto et al. observed that Mn-induced DAergic neurotoxicity requires the NADPH dual-oxidase BLI-3, suggesting that in vivo BLI-3 activity promotes the conversion of extracellular DA into toxic reactive species, which, in turn, can be taken up by DAT-1 in DAergic neurons, thus leading to oxidative stress and cell degeneration [248]. BLI-3 knockout or inhibition may represent a novel strategy for mitigating Mn neurotoxicity.
10 General Conclusions
Because of its paradoxal effects on human health, Mn exposure or intake has been studied for quite some time. Several mechanisms have been proposed for manganism, such as (i) dopamine oxidation; (ii) glial toxicity, particularly in astrocytes; (iii) oxidative stress; (iv) mitochondrial dysfunction; (v) alteration in the expression of PD-related genes. However, there are many questions that have yet to be clarified.
Treatment approaches have also been investigated, focusing on the mechanisms that were described in this chapter. Remarkably, although Mn intake is necessary for the normal functioning of the organism, it is necessary to regulate its environmental and occupational exposures, as once excessive exposure occurred, it may lead to neurological dysfunction for which effective treatment has yet to be developed.
- ABC:
-
ATP-binding cassette
- Abeta:
-
amyloid β
- AD:
-
Alzheimer’s Disease
- ALS:
-
amyotrophic lateral sclerosis
- APLP:
-
amyloid β precursor-like protein
- ATP:
-
adenosine 5′-triphosphate
- BBB:
-
blood-brain barrier
- BCB:
-
blood-cerebrospinal fluid barrier
- CA3:
-
cornu Ammonis, region 3
- CNS:
-
central nervous system
- CSF:
-
cerebrospinal fluid
- Cyt c :
-
cytochrome c
- DA:
-
dopamine
- DAT:
-
dopamine transporter type
- DMT:
-
divalent metal transporter
- ERK:
-
extracellular signal-regulated kinase
- ESADDI:
-
estimated safe and adequate dietary intake
- FDA:
-
Food and Drug Administration
- Fpn:
-
ferroportin
- GFD:
-
green fluorescent protein
- GLAST:
-
glutamate:aspartate transporter
- GS:
-
glutamine synthetase
- HD:
-
Huntington’s Disease
- Hip:
-
huntingtin-interacting protein
- hIPCs:
-
human induced pluripotent stem cells
- HTT:
-
huntingtin
- iNOS:
-
nitric oxide synthase (inducible form)
- IPD:
-
idiopatic Parkinson’s disease
- LRRK2:
-
leucine-rich repeat kinase 2
- MCT:
-
monocarboxylate transporter
- MMT:
-
methylcyclopentadienyl manganese tricarbonyl
- MNs:
-
motor neurons
- MPT:
-
mitochondrial transition pore
- MPTP:
-
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MRI:
-
magnetic resonance imaging
- NAAS:
-
National Academy of Sciences
- NADH:
-
nicotinamide adenine dinucleotide reduced
- NADPH:
-
nicotinamide adenine dinucleotide phosphate reduced
- NMDA:
-
N-methyl-D-aspartate
- NOS:
-
nitric oxide synthase
- NRAMP:
-
natural resistance-associated macrophage protein
- NRC:
-
National Research Council
- OATP:
-
organic anion transporter polypeptide
- PARK:
-
Parkinson protein
- PD:
-
Parkinson’s disease
- PET:
-
positron emission tomography
- RDI:
-
reference daily intake
- ROS:
-
reactive oxygen species
- SLC39:
-
solute carrier-39
- SNpc:
-
substantia nigra pars compacta
- SNpr:
-
substantia nigra pars reticulata
- SOD:
-
superoxide dismutase
- SPCA:
-
Ca2+/Mn2+ ATPase of the secretory pathway
- SPECT:
-
single-photon emission computed tomography
- TCA:
-
tricarboxylic acid
- Tf:
-
transferrin
- TfR:
-
transferrin receptor
- TRPM7:
-
transient receptor potential cation channel, subfamily M, member 7
References
M. Aschner, T. R. Guilarte, J. S. Schneider, W. Zheng, Toxicol. Appl. Pharmacol. 2007, 221, 131–147.
USEPA, Health Assessment Document for Manganese, EPA 600-83-013F, U.S.E.P. Agency, Editor, 1984, Washington, DC.
F. S. Archibald, C. Tyree, Arch. Biochem. Biophys. 1987, 256, 638–650.
C. L. Keen, in Proceedings of the Workshop on the Bioavailability and Oral Toxicity of Manganese, Ed S. Velazquez, US EPA, Environmental Criteria and Assessment Office, 1995, 3–11.
T. Inoue, T. Majid, R. G. Pautler, Rev. Neurosci. 2011, 22, 675–694.
M. C. Newland, Neurotoxicology 1999, 20, 415–432.
R. M. Bowler, S. Gysens, E. Diamond, S. Nakagawa, M. Drezgic, H. A. Roels, Neurotoxicology 2006, 27, 315–326.
Y. M. Jiang, X. A. Mo, F. Q. Du, X. Fu, X. Y. Zhu, H. Y. Gao, J. L. Xie, F. L. Liao, E. Pira, W. Zheng, J. Occup. Environ. Med. 2006, 48, 644–649.
J. E. Myers, M. L. Thompson, S. Ramushu, T. Young, M. F. Jeebhay, L. London, E. Esswein, K. Renton, A. Spies, A. Boulle, I. Naik, A. Iregren, D. J. Rees, Neurotoxicology 2003, 24, 885–894.
K. Ono, K. Komai, M. Yamada, J. Neurol. Sci. 2002, 199, 93–96.
D. G. Cook, S. Fahn, K. A. Brait, Arch. Neurol. 1974, 30, 59–64.
H. Roels, R. Lauwerys, J. P. Buchet, P. Genet, M. J. Sarhan, I. Hanotiau, M. de Fays, A. Bernard, D. Stanescu, Am. J. Ind. Med. 1987, 11, 307–327.
A. Barbeau, Neurotoxicology 1984, 5, 13–35.
F. M. Corrigan, L. Murray, C. L. Wyatt, R. F. Shore, Exp. Neurol. 1998, 150, 339–342.
J. M. Gorell, C. C. Johnson, B. A. Rybicki, E. L. Peterson, G. X. Kortsha, G. G. Brown, R. J. Richardson, Neurology 1997, 48, 650–658.
B. A. Rybicki, C. C. Johnson, J. Uman, J. M. Gorell, Mov. Disord. 1993, 8, 87–92.
M. Yamada, S. Ohno, I. Okayasu, R. Okeda, S. Hatakeyama, H. Watanabe, K. Ushio, H. Tsukagoshi, Acta Neuropathol. 1986, 70, 273–278.
R. Lucchini, L. Selis, D. Folli, P. Apostoli, A. Mutti, O. Vanoni, A. Iregren, L. Alessio, Scand. J. Work Environ. Health 1995, 21, 143–149.
H. A. Roels, P. Ghyselen, J. P. Buchet, E. Ceulemans, R. R. Lauwerys, Br. J. Ind. Med. 1992, 49, 25–34.
R. Lucchini, P. Apostoli, C. Perrone, D. Placidi, E. Albini, P. Migliorati, D. Mergler, M. P. Sassine, S. Palmi, L. Alessio, Neurotoxicology 1999, 20, 287–297.
C. E. Gavin, K. K. Gunter, T. E. Gunter, Neurotoxicology 1999, 20, 445–453.
C. W. Olanow, P. F. Good, H. Shinotoh, K. A. Hewitt, F. Vingerhoets, B. J. Snow, M. F. Beal, D. B. Calne, D. P. Perl, Neurology 1996, 46, 492–498.
R. H. Gwiazda, D. Lee, J. Sheridan, D. R. Smith, Neurotoxicology 2002, 23, 69–76.
R. Witholt, R. H. Gwiazda, D. R. Smith, Neurotoxicol. Teratol. 2000, 22, 851–861.
ATSDR (Agency of Toxic Substances and Disease Registry), Toxicological Profile for Manganese, U.S. Department of Health and Human Services Public Health Service, 2000.
IOM, Dietary Reference Intakes: Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc, Ed Institute of Medicine, National Academy Press, Washington DC, 2011.
K. M. Erikson, M. Aschner, Neurochem. Int. 2003, 43, 475–480.
J. R. Prohaska, Physiol. Rev. 1987, 67, 858–901.
D. G. Barceloux, J. Toxicol. Clin. Toxicol. 1999, 37, 293–307.
V. C. Culotta, M. Yang, T.V. O’Halloran, Biochim. Biophys. Acta 2006, 1763, 747–758.
C. L. Keen, J. L. Ensunsa, M. H. Watson, D. L. Baly, S. M. Donovan, M. H. Monaco, M. S. Clegg, Neurotoxicology 1999, 20, 213–223.
J. W. Finley, J. G. Penland, R. E. Pettit, C. D. Davis, J. Nutr. 2003, 133, 2849–2856.
C. L. Keen, S. Zidenberg-Cherr, Manganese Toxicity in Humans and Experimental Animals, in Manganese in Health and Disease, Ed D. J. Klimis-Tavantzis, CRC Press., Boca Raton, 1994, pp. 193–205.
J. W. Finley, C. D. Davis, Biofactors 1999, 10, 15–24.
J. A. Pennington, S. A. Schoen, Int. J. Vitam. Nutr. Res. 1996, 66, 350–362.
J. L. Greger, J. Nutr. 1998, 128, 368S–371S.
NAS, Panel on Micronutrients. 2001, available at www.nap.edu/books/0309072794/html/.
S. C. Sistrunk, M. K. Ross, N. M. Filipov, Environ. Toxicol. Pharmacol. 2007, 23, 286–296.
J. W. Finley, P. E. Johnson, L. K. Johnson, Am. J. Clin. Nutr. 1994, 60, 949–955.
M. Aschner, J. L. Aschner, Neurosci. Biobehav. Rev. 1991, 15, 333–340.
K. Thompson, R. M. Molina, T. Donaghey, J. E. Schwob, J. D. Brain, M. Wessling-Resnick, FASEB J. 2007, 21, 223–230.
D. C. Dorman, B. E. McManus, C. U. Parkinson, C. A. Manuel, A. M. McElveen, J. I. Everitt, Inhal. Toxicol. 2004, 16, 481–488.
J. J. Powell, R. Jugdaohsingh, R. P. Thompson, Proc. Nutr. Soc. 1999, 58, 147–153.
M. E. Conrad, J. N. Umbreit, E. G. Moore, C. R. Rodning, Blood 1992, 79, 244–247.
H. Gunshin, B. Mackenzie, U.V. Berger, Y. Gunshin, M.F. Romero, W.F. Boron, S. Nussberger, J. L. Gollan, M. A. Hediger, Nature 1997, 388, 482–488.
S. J. Garcia, K. Gellein, T. Syversen, M. Aschner, Toxicol. Sci. 2006, 92, 516–525.
V. A. Fitsanakis, N. Zhang, J. G. Anderson, K. M. Erikson, M. J. Avison, J. C. Gore, M. Aschner, Toxicol. Sci. 2008, 103, 116–124.
C. D. Klaassen, Toxicol. Appl. Pharmacol. 1974, 29, 458–468.
M. Aschner, M. Gannon, Brain Res. Bull. 1994, 33, 345–349.
A. Takeda, J. Sawashita, S. Okada, Brain Res. 1995, 695, 53–58.
A. C. Foradori, A. Bertinchamps, J. M. Gulibon, G. C. Cotzias, J. Gen. Physiol. 1967, 50, 2255–2266.
G. C. Cotzias, A. Bertinchamps, Clinical Experiences with Manganese, in Metal Binding in Medicine, Eds M. J. Seven, L. A. Johnson, Lippincott Co., Philadelphia, 1960, pp. 50–57.
G. C. Cotzias, K. Horiuchi, S. Fuenzalida, I. Mena, Neurology 1968, 18, 376–382.
T. E. Gunter, J. S. Puskin, Biophys. J. 1972, 12, 625–635.
J. J. Liccione, M. D. Maines, J. Pharmacol. Exp. Ther. 1988, 247, 156–161.
C. Zwingmann, D. Leibfritz, A. S. Hazell, J. Cereb. Blood Flow Metab. 2003, 23, 756–771.
K. Kalia, W. Jiang, W. Zheng, Neurotoxicology. 2008, 29, 466–470.
M. R. Sepulveda, F. Wuytack, A. M. Mata, J. Neurochem. 2012, 123, 824–836.
P. Vangheluwe, M. R. Sepulveda, L. Missiaen, L. Raeymaekers, F. Wuytack, J. Vanoevelen, Chem. Rev. 2009, 109, 4733–4759.
V. K. Ton, D. Mandal, C. Vahadji, R. Rao, J. Biol. Chem. 2002, 277, 6422–6427.
M. R. Sepulveda, T. Dresselaers, P. Vangheluwe, W. Everaerts, U. Himmelreich, A. M. Mata, F. Wuytack, Contrast Media Mol. Imaging. 2012, 7, 426–434.
S. Mukhopadhyay, A. D. Linstedt, Proc. Natl. Acad. Sci. USA 2011, 108, 858–863.
V. A. Murphy, K. C. Wadhwani, Q. R. Smith, S. I. Rapoport, J. Neurochem. 1991, 57, 948–954.
O. Rabin, L. Hegedus, J. M. Bourre, Q. R. Smith, J. Neurochem. 1993, 61, 509–517.
R. E. London, G. Toney, S. A. Gabel, A. Funk, Brain Res. Bull. 1989, 23, 229–235.
A. Takeda, T. Akiyama, J. Sawashita, S. Okada, Brain Res. 1994, 640, 341–344.
M. Yukawa, K. Amano, M. Suzuki-Yasumoto, M. Terai, Arch. Environ. Health 1980, 35, 36–44.
E. Bonilla, E. Salazar, J. J. Villasmil, R. Villalobos, Neurochem. Res. 1982, 7, 221–227.
E. D. Bird, A. H. Anton, B. Bullock, Neurotoxicology 1984, 5, 59–65.
V. J. Bush, T. P. Moyer, K. P. Batts, J. E. Parisi, Clin. Chem. 1995, 41, 284–294.
D. C. Dorman, M. F. Struve, R. A. James, B. E. McManus, M. W. Marshall, B. A. Wong, Toxicol. Sci. 2001, 60, 242–251.
A. St-Pierre, L. Normandin, G. Carrier, G. Kennedy, R. Butterworth, J. Zayed, Inhal. Toxicol. 2001, 13, 623–632.
A. Tracqui, J. Tayot, P. Kintz, G. Alves, M. A. Bosque, P. Mangin, Forensic Sci. Int. 1995, 76, 199–203.
M. Morello, A. Canini, P. Mattioli, R. P. Sorge, A. Alimonti, B. Bocca, G. Forte, A. Martorana, G. Bernardi, G. Sancesario, Neurotoxicology 2008, 29, 60–72.
J. J. Liccione, M. D. Maines, J. Pharmacol. Exp. Ther. 1989, 248, 222–228.
A. Nong, J. G. Teeguarden, H. J. Clewell, 3rd, D. C. Dorman, M. E. Andersen, J. Toxicol. Environ. Health A 2008, 71, 413–426.
M. C. Newland, C. Cox, R. Hamada, G. Oberdorster, B. Weiss, Fundam. Appl. Toxicol. 1987, 9, 314–328.
J. Versieck, F. Barbier, A. Speecke, J. Hoste, Clin. Chem. 1974, 20, 1141–1145.
L. Spahr, R. F. Butterworth, S. Fontaine, L. Bui, G. Therrien, P. C. Milette, L. H. Lebrun, J. Zayed, A. Leblanc, G. Pomier-Layrargues, Hepatology 1996, 24, 1116–1120.
R. F. Butterworth, L. Spahr, S. Fontaine, G. P. Layrargues, Metab. Brain Dis. 1995, 10, 259–267.
K. Weissenborn, C. Ehrenheim, A. Hori, S. Kubicka, M. P. Manns, Metab. Brain Dis. 1995, 10, 219–231.
A. S. Hazell, R. F. Butterworth, Proc. Soc. Exp. Biol. Med. 1999, 222, 99–112.
J. D. Schroeter, A. Nong, M. Yoon, M. D. Taylor, D. C. Dorman, M. E. Andersen, H. J. Clewell, 3rd, Toxicol. Sci. 2011, 120, 481–498.
S. H. Zlotkin, S. Atkinson, G. Lockitch, Clin. Perinatol. 1995, 22, 223–240.
A. B. Bowman, K. M. Erikson, M. Aschner, Manganese – The Two Faces of Essentiality and Neurotoxicity, in Metals and Neurodegeneration, Ed S. Huang, Research Signpost, Kerala, India, 2010.
A. Takeda, Brain Res. Brain Res. Rev. 2003, 41, 79–87.
D. W. Christianson, Prog. Biophys. Mol. Biol. 1997, 67, 217–252.
F. C. Wedler, R. B. Denman, Curr. Top. Cell Regul. 1984, 24, 153–169.
P. K. Maciejewski, D. L. Rothman, Neurochem. Int. 2008, 52, 809–825.
T. Eid, A. Williamson, T. S. Lee, O. A. Petroff, N. C. de Lanerolle, Epilepsia 2008, 49 Suppl. 2, 42–52.
D. E. Ash, J. D. Cox, D. W. Christianson, Met. Ions Biol. Syst. 2000, 37, 407–428.
A. G. Estevez, M. A. Sahawneh, P. S. Lange, N. Bae, M. Egea, R. R. Ratan, J. Neurosci. 2006, 26, 8512–8516.
A. S. Mildvan, M. C. Scrutton, M. F. Utter, J. Biol. Chem. 1966, 241, 3488–3498.
A. C. Yu, J. Drejer, L. Hertz, A. Schousboe, J. Neurochem. 1983, 41, 1484–1487.
N. M. Fong, T. C. Jensen, A. S. Shah, N. N. Parekh, A. R. Saltiel, M. J. Brady, J. Biol. Chem. 2000, 275, 35034–35039.
O. A. Andreassen, A. Dedeoglu, A. Friedlich, K. L. Ferrante, D. Hughes, C. Szabo, M. F. Beal, Exp. Neurol. 2001, 168, 419–424.
L. S. Hurley, C. L. Keen, Manganese, in Trace Elements in Human Health and Animal Nutrition, Vol. 1, 5th edn., Ed W. Mertz, Academic Press, San Diego, CA, 1987, pp. 185–222.
C. Keen, Nutritional and Toxicological Aspects of Manganese Intakes: An Overview, in Risk Assessment of Essential Elements, Eds W. Mertz, C. O. Abernathy, S. S. Olin, ILSI Press, Washington DC, 1994, pp. 221–235.
J. H. Freeland-Graves, C. Llanes, Models to Study Manganese Deficiency, in Manganese in Health and Disease, Ed D. J. Klimis-Tavantzis, CRC Press, Boca Raton, 1994.
M. D. Garrick, K. G. Dolan, C. Horbinski, A. J. Ghio, D. Higgins, M. Porubcin, E. G. Moore, L. N. Hainsworth, J. N. Umbreit, M. E. Conrad, L. Feng, A. Lis, J. A. Roth, S. Singleton, L. M. Garrick, BioMetals 2003, 16, 41–54.
L. He, K. Girijashanker, T. P. Dalton, J. Reed, H. Li, M. Soleimani, D. W. Nebert, Mol. Pharmacol. 2006, 70, 171–180.
L. Davidsson, B. Lonnerdal, B. Sandstrom, C. Kunz, C. L. Keen, J. Nutr. 1989, 119, 1461–1464.
C. M. Lucaciu, C. Dragu, L. Copaescu, V. V. Morariu, Biochim. Biophys. Acta 1997, 1328, 90–98.
A. Riccio, C. Mattei, R. E. Kelsell, A. D. Medhurst, A. R. Calver, A. D. Randall, J. B. Davis, C. D. Benham, M. N. Pangalos, J. Biol. Chem. 2002, 277, 12302–12309.
S. S. Kannurpatti, P. G. Joshi, N. B. Joshi, Neurochem. Res. 2000, 25, 1527–1536.
J. S. Crossgrove, R. A. Yokel, Neurotoxicology 2004, 25, 451–460.
J. S. Crossgrove, D. D. Allen, B. L. Bukaveckas, S. S. Rhineheimer, R. A. Yokel, Neurotoxicology 2003, 24, 3–13.
K. M. Erikson, K. Thompson, J. Aschner, M. Aschner, Pharmacol. Ther. 2007, 113, 369–377.
V. A. Fitsanakis, G. Piccola, A. P. Marreilha dos Santos, J. L. Aschner, M. Aschner, Hum. Exp. Toxicol. 2007, 26, 295–302.
Z. Xiang, G. Burnstock, Neuroreport 2005, 16, 903–907.
R. A. North, Physiol. Rev. 2002, 82, 1013–1067.
N. Tanaka, K. Kawasaki, N. Nejime, Y. Kubota, K. Nakamura, M. Kunitomo, K. Takahashi, M. Hashimoto, K. Shinozuka, J. Pharmacol. Sci. 2004, 95, 174–180.
C. Marzano, M. Pellei, F. Tisato, C. Santini, Anticancer Agents Med. Chem. 2009, 9, 185–211.
A. Sacher, A. Cohen, N. Nelson, J. Exp. Biol. 2001, 204, 1053–1061.
J. S. Crossgrove, R. A. Yokel, Neurotoxicology 2005, 26, 297–307.
A. Goytain, R. M. Hines, G. A. Quamme, J. Biol. Chem. 2008, 283, 33365–33374.
A. D. Gitler, A. Chesi, M. L. Geddie, K. E. Strathearn, S. Hamamichi, K. J. Hill, K. A. Caldwell, G. A. Caldwell, A. A. Cooper, J. C. Rochet, S. Lindquist, Nat. Genet. 2009, 41, 308–315.
A. C. Chua, E. H. Morgan, J. Comp. Physiol. B 1997, 167, 361–369.
M. D. Fleming, C. C. Trenor, 3rd, M. A. Su, D. Foernzler, D. R. Beier, W. F. Dietrich, N. C. Andrews, Nat. Genet. 1997, 16, 383–386.
M. D. Fleming, M. A. Romano, M. A. Su, L. M. Garrick, M. D. Garrick, N. C. Andrews, Proc. Natl. Acad. Sci. USA 1998, 95, 1148–1153.
K. Thompson, R. M. Molina, T. Donaghey, J. D. Brain, M. Wessling-Resnick, Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G640–644.
J. Salazar, N. Mena, S. Hunot, A. Prigent, D. Alvarez-Fischer, M. Arredondo, C. Duyckaerts, V. Sazdovitch, L. Zhao, L. M. Garrick, M. T. Nunez, M. D. Garrick, R. Raisman-Vozari, E. C. Hirsch, Proc. Natl. Acad. Sci. USA 2008, 105, 18578–18583.
V. A. Fitsanakis, G. Piccola, J. L. Aschner, M. Aschner, Neurotoxicology 2006, 27, 60–70.
V. A. Fitsanakis, G. Piccola, J. L. Aschner, M. Aschner, J. Neurosci. Res. 2005, 81, 235–243.
T. K. Dickinson, A. G. Devenyi, J. R. Connor, J. Lab. Clin. Med. 1996, 128, 270–278.
C. C. Trenor, 3rd, D. R. Campagna, V. M. Sellers, N. C. Andrews, M. D. Fleming, Blood 2000, 96, 1113–1118.
S. Potocki, M. Rowinska-Zyrek, D. Witkowska, M. Pyrkosz, A. Szebesczyk, K. Krzywoszynska, H. Kozlowski, Curr. Med. Chem. 2012, 19, 2738–2759.
A. Ramirez, A. Heimbach, J. Grundemann, B. Stiller, D. Hampshire, L. P. Cid, I. Goebel, A. F. Mubaidin, A. L. Wriekat, J. Roeper, A. Al-Din, A. M. Hillmer, M. Karsak, B. Liss, C. G. Woods, M. I. Behrens, C. Kubisch, Nat. Genet. 2006, 38, 1184–1191.
P. R. Lockman, K. E. Roder, D. D. Allen, J. Neurochem. 2001, 79, 588–594.
W. N. Sloot, J. B. Gramsbergen, Brain Res. 1994, 657, 124–132.
J. G. Anderson, P. T. Cooney, K. M. Erikson, Environ. Toxicol. Pharmacol. 2007, 23, 179–184.
L. J. Wu, A. G. Leenders, S. Cooperman, E. Meyron-Holtz, S. Smith, W. Land, R. Y. Tsai, U. V. Berger, Z. H. Sheng, T. A. Rouault, Brain Res. 2004, 1001, 108–117.
M. D. Garrick, Genes Nutr. 2011, 6, 45–54.
Z. Yin, H. Jiang, E. S. Lee, M. Ni, K. M. Erikson, D. Milatovic, A. B. Bowman, M. Aschner, J. Neurochem. 2010, 112, 1190–1198.
M. B. Troadec, D. M. Ward, E. Lo, J. Kaplan, I. De Domenico, Blood 2010, 116, 4657–4664.
M. S. Madejczyk, N. Ballatori, Biochim. Biophys. Acta 2012, 1818, 651–657.
S. Abboud, D. J. Haile, J. Biol. Chem. 2000, 275, 19906–19912.
A. S. Zhang, S. Xiong, H. Tsukamoto, C. A. Enns, Blood 2004, 103, 1509–1514.
P. Hahn, T. Dentchev, Y. Qian, T. Rouault, Z. L. Harris, J. L. Dunaief, Mol. Vis. 2004, 10, 598–607.
M. Aschner, D. C. Dorman, Toxicol. Rev. 2006, 25, 147–154.
M. Aschner, K. M. Erikson, D. C. Dorman, Crit. Rev. Toxicol. 2005, 35, 1–32.
G. Gianutsos, M. T. Murray, Neurotoxicology 1982, 3, 75–81.
D. B. Calne, N. S. Chu, C. C. Huang, C. S. Lu, W. Olanow, Neurology 1994, 44, 1583–1586.
A. Benedetto, C. Au, M. Aschner, Chem. Rev. 2009, 109, 4862–4884.
A. B. Bowman, G. F. Kwakye, E. Herrero Hernandez, M. Aschner, J. Trace Elem. Med. Biol. 2011, 191–203.
C. W. Olanow, Ann. N. Y. Acad. Sci. 2004, 1012, 209–223.
R. G. Lucchini, E. Albini, L. Benedetti, S. Borghesi, R. Coccaglio, E. C. Malara, G. Parrinello, S. Garattini, S. Resola, L. Alessio, Am. J. Ind. Med. 2007, 50, 788–800.
B. A. Racette, L. McGee-Minnich, S. M. Moerlein, J. W. Mink, T. O. Videen, J. S. Perlmutter, Neurology 2001, 56, 8–13.
J. A. Chalela, L. Bonillha, R. Neyens, A. Hays, Neurocrit. Care 2011, 14, 456–458.
A. Pentschew, F. F. Ebner, R. M. Kovatch, J. Neuropathol. Exp. Neurol. 1963, 22, 488–499.
D. B. Bertinet, M. Tinivella, F. A. Balzola, A. de Francesco, O. Davini, L. Rizzo, P. Massarenti, M. A. Leonardi, F. Balzola, J. Parenter. Enteral Nutr. 2000, 24, 223–227.
K. M. Hambidge, R. J. Sokol, S. J. Fidanza, M. A. Goodall, J. Parenter. Enteral Nutr. 1989, 13, 168–171.
J. Jankovic, J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376.
A. J. Lees, J. Hardy, T. Revesz, Lancet 2009, 373, 2055–2066.
E. Tolosa, G. Wenning, W. Poewe, Lancet Neurol. 2006, 5, 75–86.
C. E. Gavin, K. K. Gunter, T. E. Gunter, Toxicol. Appl. Pharmacol. 1992, 115, 1–5.
M. Aschner, K. M. Erikson, E. Herrero Hernandez, R. Tjalkens, Neuromolecular Med. 2009, 11, 252–266.
D. L. Stredrick, A. H. Stokes, T. J. Worst, W. M. Freeman, E. A. Johnson, L. H. Lash, M. Aschner, K. E. Vrana, Neurotoxicology 2004, 25, 543–553.
B. A. Racette, J. A. Antenor, L. McGee-Minnich, S. M. Moerlein, T. O. Videen, V. Kotagal, J. S. Perlmutter, Mov. Disord. 2005, 20, 492–496.
T. R. Guilarte, Environ. Health Perspect. 2010, 118, 1071–1080.
S. von Campenhausen, B. Bornschein, R. Wick, K. Botzel, C. Sampaio, W. Poewe, W. Oertel, U. Siebert, K. Berger, R. Dodel, Eur. Neuropsychopharmacol. 2005, 15, 473–490.
A. Eisen, S. Kim, B. Pant, Muscle Nerve 1992, 15, 219–224.
E. P. Pioro, H. Mitsumoto, Clin. Neurosci. 1995, 3, 375–385.
S. H. Appel, D. Beers, L. Siklos, J. I. Engelhardt, D. R. Mosier, Amyotroph. Lateral Scler. Other Motor Neuron. Disord. 2001, 2 Suppl. 1, S47–54.
Y. Kawahara, S. Kwak, Amyotroph. Lateral Scler. Other Motor Neuron. Disord. 2005, 6, 131–144.
Y. Li, J. C. Copin, L. F. Reola, B. Calagui, G. T. Gobbel, S. F. Chen, S. Sato, C. J. Epstein, P. H. Chan, Brain Res. 1998, 814, 164–170.
J. Tomkins, S. J. Banner, C. J. McDermott, P. J. Shaw, Neuroreport 2001, 12, 2319–2322.
E. Kapaki, C. Zournas, G. Kanias, T. Zambelis, A. Kakami, C. Papageorgiou, J. Neurol. Sci. 1997, 147, 171–175.
H. Nagata, S. Miyata, S. Nakamura, M. Kameyama, Y. Katsui, J. Neurol. Sci. 1985, 67, 173–178.
A. Gaeta, R. C. Hider, Br. J. Pharmacol. 2005, 146, 1041–1059.
L. A. Hazelwood, R. B. Free, D. M. Cabrera, M. Skinbjerg, D. R. Sibley, J. Biol. Chem. 2008, 283, 36441–36453.
C. J. Maynard, R. Cappai, I. Volitakis, R. A. Cherny, A. R. White, K. Beyreuther, C. L. Masters, A. I. Bush, Q. X. Li, J. Biol. Chem. 2002, 277, 44670–44676.
L. Esposito, J. Raber, L. Kekonius, F. Yan, G. Q. Yu, N. Bien-Ly, J. Puolivali, K. Scearce-Levie, E. Masliah, L. Mucke, J. Neurosci. 2006, 26, 5167–5179.
G. P. Bates, Nat. Rev. Genet. 2005, 6, 766–773.
N. S. Wexler, J. Lorimer, J. Porter, F. Gomez, C. Moskowitz, E. Shackell, K. Marder, G. Penchaszadeh, S. A. Roberts, J. Gayan, D. Brocklebank, S. S. Cherny, L. R. Cardon, J. Gray, S. R. Dlouhy, S. Wiktorski, M. E. Hodes, P. M. Conneally, J. B. Penney, J. Gusella, J. H. Cha, M. Irizarry, D. Rosas, S. Hersch, Z. Hollingsworth, M. MacDonald, A. B. Young, J. M. Andresen, D. E. Housman, M. M. De Young, E. Bonilla, T. Stillings, A. Negrette, S. R. Snodgrass, M. D. Martinez-Jaurrieta, M. A. Ramos-Arroyo, J. Bickham, J. S. Ramos, F. Marshall, I. Shoulson, G. J. Rey, A. Feigin, N. Arnheim, A. Acevedo-Cruz, L. Acosta, J. Alvir, K. Fischbeck, L. M. Thompson, A. Young, L. Dure, C. J. O’Brien, J. Paulsen, A. Brickman, D. Krch, S. Peery, P. Hogarth, D. S. Higgins, Jr., B. Landwehrmeyer, Proc. Natl. Acad. Sci. USA 2004, 101, 3498–3503.
B. B. Williams, D. Li, M. Wegrzynowicz, B. K. Vadodaria, J. G. Anderson, G. F. Kwakye, M. Aschner, K. M. Erikson, A. B. Bowman, J. Neurochem. 2010, 112, 227–237.
B. B. Williams, G. F. Kwakye, M. Wegrzynowicz, D. Li, M. Aschner, K. M. Erikson, A. B. Bowman, Toxicol. Sci. 2010, 117, 169–179.
M. Wegrzynowicz, H. K. Holt, D. B. Friedman, A. B. Bowman, J. Proteome Res. 2012, 11, 1118–1132.
M. Aschner, Environ. Health Perspect. 2000, 108 Suppl. 3, 429–432.
L. Normandin, A. S. Hazell, Metab. Brain Dis. 2002, 17, 375–387.
A. W. Dobson, K. M. Erikson, M. Aschner, Ann. N. Y. Acad. Sci. 2004, 1012, 115–128.
P. K. Pal, A. Samii, D. B. Calne, Neurotoxicology 1999, 20, 227–238.
C.C. Huang, Y.H. Weng, C.S. Lu, N.S. Chu, T.C. Yen, J. Neurol. 2003, 250, 1335–1339.
S. A. McDougall, C. M. Reichel, C. M. Farley, M. M. Flesher, T. Der-Ghazarian, A. M. Cortez, J. J. Wacan, C. E. Martinez, F. A. Varela, A. E. Butt, C. A. Crawford, Neuroscience 2008, 154, 848–860.
C. M. Reichel, J. J. Wacan, C. M. Farley, B. J. Stanley, C. A. Crawford, S. A. McDougall, Neurotoxicol. Teratol. 2006, 28, 323–332.
A. McNeill, D. Birchall, S. J. Hayflick, A. Gregory, J. F. Schenk, E. A. Zimmerman, H. Shang, H. Miyajima, P. F. Chinnery, Neurology 2008, 70, 1614–1619.
J. Donaldson, D. McGregor, F. LaBella, Can. J. Physiol. Pharmacol. 1982, 60, 1398–1405.
K. M. Erikson, D. C. Dorman, L. H. Lash, M. Aschner, Toxicol. Sci. 2007, 97, 459–466.
J. Donaldson, F. S. LaBella, D. Gesser, Neurotoxicology 1981, 2, 53–64.
R. Graumann, I. Paris, P. Martinez-Alvarado, P. Rumanque, C. Perez-Pastene, S. P. Cardenas, P. Marin, F. Diaz-Grez, R. Caviedes, P. Caviedes, J. Segura-Aguilar, Pol. J. Pharmacol. 2002, 54, 573–579.
S. H. Reaney, D. R. Smith, Toxicol. Appl. Pharmacol. 2005, 205, 271–281.
J. Segura-Aguilar, C. Lind, Chem. Biol. Interact. 1989, 72, 309–324.
B. Chance, J. Biol. Chem. 1965, 240, 2729–2748.
T. E. Gunter, C. E. Gavin, M. Aschner, K. K. Gunter, Neurotoxicology 2006, 27, 765–776.
T. E. Gunter, D. R. Pfeiffer, Am. J. Physiol. 1990, 258, C755–786.
S. F. Ali, H. M. Duhart, G. D. Newport, G. W. Lipe, W. Slikker, Jr., Neurodegeneration 1995, 4, 329–334.
S. Zhang, J. Fu, Z. Zhou, Toxicol. In Vitro 2004, 18, 71–77.
H. R. Scholte, J. Bioenerg. Biomembr. 1988, 20, 161–191.
D. HaMai, A. Campbell, S. C. Bondy, Free Radic. Biol. Med. 2001, 31, 763–768.
C. J. Chen, S. L. Liao, Exp. Neurol. 2002, 175, 216–225.
C. E. Gavin, K. K. Gunter, T. E. Gunter, Biochem. J. 1990, 266, 329–334.
F. Spadoni, A. Stefani, M. Morello, F. Lavaroni, P. Giacomini, G. Sancesario, Exp. Brain Res. 2000, 135, 544–551.
Z. Yin, J. L. Aschner, A. P. dos Santos, M. Aschner, Brain Res. 2008, 1203, 1–11.
M. Zoratti, I. Szabo, Biochim. Biophys. Acta 1995, 1241, 139–176.
K. V. Rao, M. D. Norenberg, J. Biol. Chem. 2004, 279, 32333–32338.
D. Milatovic, Z. Yin, R. C. Gupta, M. Sidoryk, J. Albrecht, J. L. Aschner, M. Aschner, Toxicol. Sci. 2007, 98, 198–205.
D. R. Green, J. C. Reed, Science 1998, 281, 1309–1312.
S. J. Korsmeyer, M. C. Wei, M. Saito, S. Weiler, K. J. Oh, P. H. Schlesinger, Cell Death Differ. 2000, 7, 1166–1173.
P. X. Petit, S. A. Susin, N. Zamzami, B. Mignotte, G. Kroemer, FEBS Lett. 1996, 396, 7–13.
E. A. Malecki, Brain Res. Bull. 2001, 55, 225–228.
M. K. Chen, J. S. Lee, J. L. McGlothan, E. Furukawa, R. J. Adams, M. Alexander, D. F. Wong, T. R. Guilarte, Neurotoxicology 2006, 27, 229–236.
M. Aschner, M. Gannon, H. K. Kimelberg, J. Neurochem. 1992, 58, 730–735.
M. Aschner, K. E. Vrana, W. Zheng, Neurotoxicology 1999, 20, 173–180.
K. V. Rama Rao, P. V. Reddy, A. S. Hazell, M. D. Norenberg, Neurotoxicology 2007, 28, 807–812.
A. S. Hazell, M. D. Norenberg, Neurochem. Res. 1997, 22, 1443–1447.
K. Erikson, M. Aschner, Neurotoxicology 2002, 23, 595–602.
K. M. Erikson, R. L. Suber, M. Aschner, Neurotoxicology 2002, 23, 281–288.
M. Sidoryk-Wegrzynowicz, E. Lee, J. Albrecht, M. Aschner, J. Neurochem. 2009, 110, 822–830.
T. D. Hexum, R. Fried, Neurochem. Res. 1979, 4, 73–82.
A. S. Hazell, M. D. Norenberg, Neurochem. Res. 1998, 23, 869–873.
A. S. Hazell, Neurochem. Int. 2002, 41, 271–277.
A. Volterra, Ren. Physiol. Biochem. 1994, 17, 165–167.
W. Zhou, J. B. Milder, C. R. Freed, J. Biol. Chem. 2008, 283, 9863–9870.
A. A. Aboud, A. M. Tidball, K. K. Kumar, M. D. Neely, K. C. Ess, K. M. Erikson, A. B. Bowman, Neurotoxicology 2012, 33, 1443–1449.
Y. Higashi, M. Asanuma, I. Miyazaki, N. Hattori, Y. Mizuno, N. Ogawa, J. Neurochem. 2004, 89, 1490–1497.
K. Sriram, G. X. Lin, A. M. Jefferson, J. R. Roberts, O. Wirth, Y. Hayashi, K. M. Krajnak, J. M. Soukup, A. J. Ghio, S. H. Reynolds, V. Castranova, A. E. Munson, J. M. Antonini, FASEB J. 2010, 24, 4989–5002.
J. A. Roth, S. Singleton, J. Feng, M. Garrick, P. N. Paradkar, J. Neurochem. 2010, 113, 454–464.
S. Hague, E. Rogaeva, D. Hernandez, C. Gulick, A. Singleton, M. Hanson, J. Johnson, R. Weiser, M. Gallardo, B. Ravina, K. Gwinn-Hardy, A. Crawley, P. H. St George-Hyslop, A. E. Lang, P. Heutink, V. Bonifati, J. Hardy, Ann. Neurol. 2003, 54, 271–274.
J. A. Olzmann, J. R. Bordelon, E. C. Muly, H. D. Rees, A. I. Levey, L. Li, L. S. Chin, J. Comp. Neurol. 2007, 500, 585–599.
R. M. Canet-Aviles, M. A. Wilson, D. W. Miller, R. Ahmad, C. McLendon, S. Bandyopadhyay, M. J. Baptista, D. Ringe, G. A. Petsko, M. R. Cookson, Proc. Natl. Acad. Sci. USA 2004, 101, 9103–9108.
T. Taira, Y. Saito, T. Niki, S. M. Iguchi-Ariga, K. Takahashi, H. Ariga, EMBO Rep. 2004, 5, 213–218.
C. Paisan-Ruiz, A. E. Lang, T. Kawarai, C. Sato, S. Salehi-Rad, G. K. Fisman, T. Al-Khairallah, P. St George-Hyslop, A. Singleton, E. Rogaeva, Neurology 2005, 65, 696–700.
J. P. Covy, B. I. Giasson, J. Neurochem. 2010, 115, 36–46.
M. R. Dewitt, P. Chen, M. Aschner, Biochem. Biophys. Res. Commun. 2013, 432, 1–4.
K. Tuschl, P. T. Clayton, S. M. Gospe, Jr., S. Gulab, S. Ibrahim, P. Singhi, R. Aulakh, R. T. Ribeiro, O. G. Barsottini, M. S. Zaki, M. L. Del Rosario, S. Dyack, V. Price, A. Rideout, K. Gordon, R. A. Wevers, W. K. Chong, P. B. Mills, Am. J. Hum. Genet. 2012, 90, 457–466.
A. P. Santos, R. L. Lucas, V. Andrade, M. L. Mateus, D. Milatovic, M. Aschner, M. C. Batoreu, Toxicol. Appl. Pharmacol. 2012, 258, 394–402.
D. Santos, D. Milatovic, V. Andrade, M. C. Batoreu, M. Aschner, A. P. Marreilha dos Santos, Toxicology 2012, 292, 90–98.
D. Milatovic, T. J. Montine, M. Aschner, Methods Mol. Biol. 2011, 758, 195–204.
D. Milatovic, R. C. Gupta, Y. Yu, S. Zaja-Milatovic, M. Aschner, Toxicol. Appl. Pharmacol. 2011, 256, 219–226.
J. A. Moreno, K. M. Streifel, K. A. Sullivan, W. H. Hanneman, R. B. Tjalkens, Toxicol. Sci. 2011, 122, 121–133.
E. Lee, M. Sidoryk-Wegrzynowicz, Z. Yin, A. Webb, D. S. Son, M. Aschner, Glia 2012, 60, 1024–1036.
Z. Xu, K. Jia, B. Xu, A. He, J. Li, Y. Deng, F. Zhang, Toxicol. Ind. Health 2010, 26, 55–60.
F. M. Cordova, A. S. Aguiar, Jr., T. V. Peres, M. W. Lopes, F. M. Goncalves, D. Z. Pedro, S. C. Lopes, C. Pilati, R. D. Prediger, M. Farina, K. M. Erikson, M. Aschner, R. B. Leal, Arch. Toxicol. 2013, 92, 638–644.
Y. Chtourou, K. Trabelsi, H. Fetoui, G. Mkannez, H. Kallel, N. Zeghal, Neurochem. Res. 2011, 36, 1546–1557.
E. N. Martins, N. T. Pessano, L. Leal, D. H. Roos, V. Folmer, G. O. Puntel, J. B. Rocha, M. Aschner, D. S. Avila, R. L. Puntel, Brain Res. Bull. 2012, 87, 74–79.
K. M. Streifel, J. A. Moreno, W. H. Hanneman, M. E. Legare, R. B. Tjalkens, Toxicol. Sci. 2012, 126, 183–192.
J. A. Roth, Neuromolecular Med. 2009, 11, 281–296.
A. Benedetto, C. Au, D. S. Avila, D. Milatovic, M. Aschner, PLoS Genet. 2010, 6, e1001084.
Acknowledgments
M.A. acknowledges funding by R01ES10563 and P30ES00267; D.S.A. acknowledges support by FAPERGS (ARD11/1673-7) and CNPq (Universal 476471/2011-7).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Avila, D.S., Puntel, R.L., Aschner, M. (2013). Manganese in Health and Disease. In: Sigel, A., Sigel, H., Sigel, R. (eds) Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences, vol 13. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7500-8_7
Download citation
DOI: https://doi.org/10.1007/978-94-007-7500-8_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7499-5
Online ISBN: 978-94-007-7500-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)