Abstract
Fourier transform mid-infrared spectroscopy equipped with attenuated total reflectance (FT-IR–ATR) combined to partial least squares (PLS) regression was used for the quantification of total phenolic contents (TPCs) and antioxidant activities in 98 samples of ethanolic extract of propolis (EEP) from the southwest region of Paraná, Brazil. The Pearson’s correlation coefficients were applied, and results ranged from 0.96 to 0.88 and showed higher correlation coefficients among TPC and ferric-reducing antioxidant power (FRAP) followed by 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) ABTS and 2,2-diphenyl-1-picrylhydrazyl hydrate (DPPH). Calibration was performed using a Savitzky-Golay filter (15 pt) and first derivative as well as standard normal variate (SNV) and mean center correction pretreatments. The determination coefficient in the calibration models ranged from 0.95 to 0.87. The range error ratio (RER) indicates the quality of estimation of the models and the results obtained were 10.0, 8.11, 16.8, and 8.99 for TPC, DPPH, ABTS, and FRAP, respectively. Thus, the results obtained for calibration and prediction parameters indicated that the models for DPPH, FRAP, and TPC have a low predictive capacity which complicates the data modeling. However, the ABTS model is validated and can be used for quantification of antioxidant activity of new extracts of propolis, being useful as an alternative to rapid analysis, reducing waste generation and cost, and indicating that the mid-infrared spectroscopy associated with PLS regression can be used to predict ABTS radical scavenger.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Propolis is a resinous material produced by bees by mixing salivary enzymes (β-glucosidase), wax, pollen, and collected natural resins used to fill gaps and to seal parts of the hive (Silva-Carvalho et al. 2015; Daneshmand et al. 2015) and can be considered a nutraceutical and functional ingredient in food products (Salami et al. 2013; Pasupuleti et al. 2017). Almost all ancient civilizations used bee-derived products as resources in their medicine due to its pharmacological properties (Tiveron et al. 2016). Propolis has characteristics that are beneficial to human health, such as antioxidant (Oldoni et al. 2011; Calegari et al. 2017), anti-inflammatory (Luis-Villaroya et al. 2015), antifungal (Siqueira et al. 2015), antiviral, anticariogenic, and antibacterial properties (Bankova 2009). The chemical composition of propolis is very complex, varying according to the bee species, the seasonality, and the flora of the region. Because of the plant variability found in South America, the chemical composition of Brazilian propolis is completely different from the other parts of the world (Bankova 2009; Calegari et al. 2017; López et al. 2014).
In propolis, there are several bioactive compounds and it is possible to emphasize the presence of aromatic acids, esters, chalcones, phenolics, and terpenoids (Park et al. 2002; Soltani et al. 2017; Zabaiou et al. 2017; Al-Ghamdi et al. 2017). Among the phenolic compounds identified, the presence of chrysin, galangin, pinocembrin, pinostrobin, caffeic acid phenethyl ester (Vargas-Sánchez et al. 2015), caffeic acid, p-coumaric acid, acid benzoic (Schnitzler et al. 2010), and Artepilin C (Veiga et al. 2017) is common. Flavones, flavonols, flavanones, and dihydroflavonols are also present in samples of propolis, as reported by Popova et al. (2017). These bioactive compounds, mainly phenolic acids and flavonoids, act directly as collaborators of the pharmacological properties, contributing mainly to the antioxidant activity.
The compounds with antioxidant capacity are able to inhibit the oxidative stress caused by the presence of free radicals in the organism (Castro et al. 2014). Free radicals consist of reactive oxygen and nitrogen species generated by cellular metabolism, which are beneficial at moderate levels but at higher concentrations can damage tissues by oxidative stress caused by an imbalance between pro- and antioxidants, resulting in a loss of biological functions and homeostasis issues (Vasconcelos et al. 2007; Barbosa et al. 2010). The human body has several mechanisms to prevent oxidative stress by the neutralization of free radicals by antioxidant compounds, which are produced naturally in the body or supplied externally through food or supplements (Pham-Huy et al. 2008).
Generally, the methods for determining the chemistry and biological activities of propolis involve conventional techniques such as UV, TLC, GC, and HPLC (Silva et al. 2007; Alves and Kubota 2013; Castro et al. 2014; Morlock et al. 2014; Rufatto et al. 2017), which are very useful in the identification and quantification of various chemical compounds in propolis (Wu et al. 2008). For the analysis of the total phenolic content (TPC), the Folin–Ciocalteu colorimetric method has been used in different types of samples. This method is based on a chemical reduction of a reagent, a mixture of tungsten and molybdenum oxides yielding a bluish compound that is measured at 740 nm (Singleton et al. 1999). For antioxidant capacity determination, indirect measurements such as 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and ferric reducing antioxidant power (FRAP) assays have been used because of their capacity to scavenge species of radicals and reduce Fe 3+ to Fe 2+ with the production of species that absorb at 517, 734, and 595 nm, respectively (Jurd and Geissman 1956; Brand-Williams et al. 1995; Re et al. 1999). These methods become infeasible if numerous samples are required to be analyzed. As a result, the development of a faster and simpler analytical method for high-throughput screening of propolis material is necessary.
As an alternative to colorimetric methods, spectroscopic fingerprint coupled with multivariate regression analysis has been proposed herein. The vibrational spectroscopy Fourier transform mid-infrared spectroscopy (FT-MIR) is a technique that expresses a unique “fingerprint” for a complex system of different samples. When FT-IR is equipped with an attenuated total reflectance (ATR) device, it enables the acquisition of spectra of both liquid and solid samples in which a light is totally internally reflected and the sample interacts with the evanescent wave. Because of the small light, penetration depth is ideal for highly absorbing samples, such as aqueous solutions, and for surfaces and thin film measurements (Wilson and Tapp 1999; Grdadolnik 2002). This spectroscopic technique presents some advantages, such as versatility, low cost, efficiency, and speed, with minimal or no sample preparation and without the use of expensive reagents, making it an attractive alternative for traditional analytical methods (Rodriguez-Saona and Allendorf 2011).
Partial least squares (PLS) regression enables correlation between two matrices. PLS is useful when the number of predictors (i.e., spectral peaks) is much higher than the number of samples in the dataset. Studies related the use of FT-NIR for quantification of compounds in propolis (Cai et al. 2012; Xu et al. 2013; González-Martín et al. 2017; Revilla et al. 2017) but there are few studies (Wu et al. 2008) on propolis using FT-IR–ATR and PLS, and the studies on Brazilian propolis that show a complex chemical composition are still limited and scarce (Picoli et al. 2016; Tiveron et al. 2016). Taking this into account, the aim of this study was to apply and evaluate PLS and FT-IR–ATR spectroscopy to quantify the total phenolic composition and antioxidant activity in ethanolic extract of propolis (EEP) produced by Apis mellifera honey bees in the south of Brazil.
Materials and Methods
Chemical Reagents
The reagents 2,2-diphenyl-1-picrylhydrazyl hydrate (DPPH), gallic acid, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), 6-hydroxy-2,5,7,8 -tetramethylchroman-2-carboxylic acid (Trolox), 2,4,6-tripyridyl-s-triazine (TPTZ), Folin–Ciocalteu, and ethanol 99% were obtained from Sigma Co. (St. Louis, MO).
Samples
The samples of propolis (98 samples) were directly collected by beekeepers in south of Brazil, Parana state. The samples were collected mostly with a mesh and by using the scraping technique from different beekeepers. All samples were cleaned, crushed with N2, homogenized, and stored at (− 6 °C) until used in the laboratory. The ethanolic extracts of propolis (EEP) were prepared as described by Oldoni et al. (2015) . Twenty five milliliters of ethanol/water (80:20 v/v−1) was added to 2 g aliquot of sample, and extraction was subsequently carried out in a water bath at 70 °C for 45 min. The hydroalcoholic extract was filtered through Whatman grade No.4 filter paper, and the liquid filtrated was transferred to a volumetric flask.
Reference Analysis
Total Phenolic Content
The total phenolic content (TPC) present in EEP was determined by the Folin–Ciocalteu colorimetric method (Singleton et al. 1999). In a test tube, 0.5 mL of EEP (3.2 g L−1) and 2.5 mL of Folin–Ciocalteu (10 g L −1) reagent were added. After 5 min, 2.0 mL of a NaCO3 (40 g L−1) solution was added. The solutions were incubated at room temperature in the dark for 2 h, and subsequently, the absorbance was measured using a spectrophotometer (model UV–VIS Lambda 25, Perkin Elmer) at 740 nm. The gallic acid was used as a standard reference in concentrations ranging from 5 to 100 mg L−1, and the results were expressed in mg gallic acid equivalent (GAE) g−1 dry weight (DW). All measurements were performed in triplicate.
Antioxidant Activity Using 2,2-Diphenyl-1-Picrylhydrazyl Hydrate-Free Radical Scavenging Method
The antioxidant activity was performed through the DPPH method described by Brand-Williams et al. (1995); the technique is based on the reaction of EEP 0.5 mL (1.6 g L−1), 3 mL of ethanol P.A., and 0.3 mL of DPPH radical solution (0.5 mmol L−1) in ethanol P.A. After 45 min in the dark, the absorbance was measured in a spectrophotometer (UV–VIS model lambda 25, Perkin Elmer) at 517 nm. The calibration curve was constructed using Trolox as a standard in concentrations that ranged from 15 to 200 μmol L−1. The results were expressed in μmol of Trolox g−1 DW. All measurements were performed in triplicate.
Antioxidant Activity Using 2,2′-Azino-Bis (3-Ethylbenzothiazoline-6-Sulphonic Acid) ABTS. + Method
The determination of antioxidant activity was performed by the ABTS method according to Re et al. (1999). The ABTS radical was prepared from the reaction of 5 mL of ABTS solution (7.0 mmol L−1) with 88 μL of potassium persulfate (140 mmol L−1), incubated at 25 °C in the dark for 16 h. The radical was diluted in ethanol and resulted in an absorbance value of 0.700 ± 0.200 at 734 nm. Under dark conditions, a solution of reaction was prepared with 30 μL EEP (16 g L−1) and 3 mL of the radical solution. The calibration curve was constructed using Trolox as a standard in concentrations that ranged from 100 to 2000 μmol L−1. The results were expressed in μmol of Trolox g−1 DW. All measurements were performed in triplicate.
Antioxidant Activity Using the FRAP Method
Proposed by Benzie and Strain (1996), the FRAP reagent was prepared from the mixture of 25 mL of acetate buffer solution 300 mmol L−1at pH 3.6, 2.5 mL of TPTZ solution 10 mmol L−1, and 2.5 mL of an aqueous ferric chloride solution 20 mmol L−1.The method consisted of a mixture of 100 μL of the EEP (1.6 g L−1) with 3 mL of the FRAP reagent; the mixture was incubated in a water bath (37 °C) in the dark for 30 min and then the absorbance was measured in a spectrophotometer (model UV–VIS lambda 25, Perkin Elmer) at 595 nm. The calibration curve was constructed using ferrous sulfate as a standard in concentrations ranging from 200 to 2000 μmol L−1. The results were expressed in μmol of FeSO4 g−1 DW. All measurements were performed in triplicate.
Spectra Acquisition
The extracts were analyzed in a Fourier transform mid-infrared spectrometer (FT-IR) Frontier from Perkin Elmer. All MIR spectra were acquired between 4000 and 400 cm−1 using an optical resolution of 8 cm−1 and 32 accumulations. For the signal horizontal ATR, the index of refraction (η) of diamond was 2.4 and leads to a depth of penetration (Dp) of 1.66 μm, with data interval 1 cm−1 and scan speed 0.2 cm/s. Approximately 0.05 ml of sample was placed onto the ATR crystal, and after each analysis, the ATR crystal was washed with deionized water and dried with non-abrasive wipe.
Regional Spectral Data Preprocessing
In order to transform the data into a form suitable for analysis, data are often pre-treated, avoiding baseline drift, light scattering, and other factors during the process of sampling. In commonly used spectral pretreatment methods, first derivative (1D) can be used to correct the baseline drift and spectral rotation in the background of this system, Savitzky-Golay (SG) smoothing can filter out the high-frequency noise in the spectral data and effectively preserve the authenticity of the original signal, and standard normal variate (SNV) is a filter based on individual observation and is less sensitive to peculiarities in the raw data, mainly used for the scattering correction of spectra (Tan et al. 2018). This study adopted the SNV, 1D, SG smoothing, and their combination methods to preprocess the original spectral data.
Data Analysis
All samples were subjected to multivariate analysis by PLS. PLS-toolbox 5.8 of Matlab version 7.8.0.347 (MathWorks. Inc. USA) was used for the chemometric analysis. The 98 spectra were divided into two datasets: calibration set (69 samples) and test set (29 samples). In this work, the developed models were evaluated through root mean square error of cross-validation (RMSECV), root mean square error of prediction (RMSEP), ratio of performance to deviation (RPD), range error ratio (RER), and coefficient of determination (R2).
The RMSEP value can be obtained from Eq. (1):
where ỹ i is the predicted value for set sample i, y i the measured value for predicted sample I, and I p is the number of observations in the prediction set (Viegas et al. 2016).
The RMSECV was calculated as follows:
where ŷ i is the predicted value of the ith observation, y i the measured value of ith observation, and Ic is the number of observation in the calibration set (Viegas et al. 2016).
The coefficient of determination (R2) between the experimental and predicted values was calculated using Eq. (3), where n is the number of observations in the calibration and prediction sets; ŷ i , y i are the predicted and measured values of sample i in the calibration and prediction sets, and y̅ the mean of the reference measurement results for all samples in the calibration and prediction sets (Viegas et al. 2016).
The range error ratio (RER) can be determined using E q. (4):
being (y max − y min) the calibration range, and RMSEV the validation error (RMSECV or RMSEP). RER values above 10 are indicative of models with good estimate (Páscoa et al. 2013). The RPD is the relationship between the standard deviation of values measured by the conventional method and the standard validation error. For RPD values above 2, the calibration equations are considered good (Dal Zotto et al. 2008). In order to avoid overfitting in models, the choice of the number of PLS latent variables was based on the minimization of the error, calculated by means leave-one-out cross-validation (Laghi et al. 2011; Martins and Ferreira 2013).
Results and Discussion
The results obtained by the referenced methods for the total phenolic content, the antioxidant capacity (determined by FRAP, ABTS, and DPPH) of the propolis analyzed are shown in Table 1. The samples were divided into two datasets, that of calibration (69 samples) and that of external validation (29) and Table 1 shows the mean, minimum, maximum, standard deviations, and coefficient of variation for both groups. The observed range for total phenolic content (5.3 to 50.4 mg EAG g−1) and antioxidant activity evaluated by the DPPH method (11.68 at 275.2 μmol Trolox g−1) were similar to those reported by Tiveron et al. (2016) and Picoli et al. (2016) for propolis produced in the south of Brazil. With regard to antioxidant activity determined by the ABTS method, the results showed a wide range of scavenging capacity from 19.0 to 1077 μmol Trolox g−1, and the values obtained by the FRAP method ranged from 66.74 to 1164 μmol FeSO4 g−1.
Currently, there is a great variety of in vitro assays because it is unrealistic to expect a single assay to be able to determine the total antioxidant activity in the screening of numerous samples. It is well known that each assay has particularities, especially with respect to the radicals and reagents involved, so ideally, the choice should cover a mix of methods based in hydrogen atom transfer (HAT) and/or single electron transfer (SET), encompassing different antioxidant mechanisms. Thus, to ensure a better evaluation of the antioxidant activity of extracts of propolis, we used a combination of assays which cover different antioxidant mechanisms and radicals: TPC and FRAP assays are based in SET mechanism and DPPH and ABTS assays are based in both SET and HAT mechanisms.
The Pearson’s correlation coefficients (PCCs) among the studied antioxidant reference analysis are shown in Table 2, being a measure of the strength and direction of a linear relationship between two random variables (Zhou et al. 2016). In this paper, significant positive correlations (p ≤ 0.01) were observed between all variables, PCC ranged from 0.96 to 0.88, and higher correlation coefficients were obtained between TPC and FRAP, followed by ABTS and DPPH. Revilla et al. (2017) reported correlation coefficients lower than those observed in this study.
FT-IR–ATR Spectroscopy
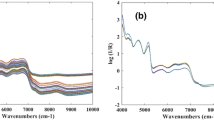
Figure 1 shows the IR spectra of the 98 ethanolic extracts of propolis obtained using Fourier transform middle infrared (FT-MIR) equipped with an attenuated total reflectance (ATR) device. In the range of 900–690 cm−1, bands can be seen related to the stretching and bending vibrations of C–H from aromatic rings, related to phenolic compounds (Soares 2002; Sousa et al. 2007). The range of 3000–2800 cm−1 is the location of bands connected with asymmetric and symmetric stretching modes of C–H: νas (CH3), νs (CH3), νas (CH2), and νs (CH2), arising from methyl and methylene groups. Two intense bands between 1300 and 1000 cm−1 represent C–O stretching and C–OH bending originated from alcohols, ethers, esters, and carboxylic acids, which are functions present mainly in phenolic acids and flavonoids (de Cardoso et al. 2017; Cao et al. 2017) which are found in extracts of propolis (Soltani et al. 2017; Bankova 2005; Menezes 2005; Banskota et al. 2001; Chang-Bravo et al. 2014).
The vibration of the carbonyl group arising from the stretching vibration of the carbonyl group and C=C from the stretching of aromatic rings are located at 1680–1630 cm−1 and 1680–1600 cm−1, respectively (Pavia et al. 2010). The same stretching was observed in extracts of red propolis Chang-Bravo et al. (2014). In the 3400 cm−1 region, a very intense band is present which represents the absorption of the OH functional group (Pavia et al. 2010), which represents alcohols. This band was already expected, since EEP is prepared with ethanol as described in samples in the “Materials and Methods” section.
Partial Least Square Regression Modeling
The calibration model was implemented with MIR spectra and reference analysis. The best prediction models were obtained using the Savitzky-Golay filter (15 pt) and first derivative as well as standard normal variate (SNV) and mean center preprocessing, and the statistical descriptors are shown in Table 3. The number of latent variables used for the construction of these models was determined from the cross-validation (CV) in order to avoid overfitting the data (Martins and Ferreira 2013).
For CV, the leave-one-out technique was applied; one sample was removed from the calibration model and the remaining samples were then used to predict the value corresponding to the removed sample. This process was repeated until all calibration samples were removed once (Silva et al. 2014). Results of CV showed higher RMSECV values than RMSEC obtained in calibration. The equations developed for the determinations of TPC, DPPH, ABTS, and FRAP showed high values for the R2 coefficient (0.95–0.87), indicating that the results of the calibration adjustment were good for these parameters.
The predictive capacity of the models was verified by means of external prediction. Figure 2 shows the correlation of the values obtained in the laboratory (reference value) with regard to those predicted by the FT-IR for TPC and antioxidant activity. The values of R2 closer to 1 indicate a higher probability that the FT-IR-predicted value (y-axis) is related to the reference analysis (x-axis) (Silva et al. 2014). As shown in Table 3, the best model was obtained for ABTS, in which the calibration and validation parameters were the best, with a R2cal of 0.95, lowest RMSEP, highest RER (16.8), and R2pred (0.74) and RPD above 2, suggesting a good practical utility (Fagan et al. 2007; Dal Zotto et al. 2008; Kapper et al. 2012).
The determination coefficients and RPD obtained for DPPH, FRAP, and TPC were lower than 0.72 and 2, respectively, suggesting a low correlation of these parameters with the calibration models, indicating a low predictive capacity. The estimated range and high RMSEP values obtained (Table 3) also support these observations. As suggested by Silva et al. (2014), the low prediction obtained for antioxidant activity models can be attributed to the fact that the range of data references used in this study was not representative for the respective models.
Another important parameter that impacts the quality of PLS models is the presence of outliers, which are as important as the determination of principal components employed in the model. The quality of the calibration set should ensure that samples produce a homogenous set by removing atypical values from the dataset. In this study, the leverage vs residuals graph (Fig. 3) was used for detection of outliers. The studentized residuals as a function of leverage for the ABTS, DPPH, FRAP, and TPC datasets showed that there were no influential values for the ABTS, FRAP, and TPC responses, because the studentized residuals values are between − 2.5 and + 2.5. The same case no. is verified for the DPPH response, because there exists a value (sample 1) considered influent close by − 3.5 for the studentized residuals.
In general, some samples may present values with some influence in ours PLS models. Our models were constructed to detect wide range values of ABTS, DPPH, FRAP, and TPC. However, some models were prejudiced to present extremes values.
Conclusions
Our primary objective in this work was to develop, for the first time, a reliability method using FT-IR–ATR spectroscopy for the determination of antioxidant activity of ethanolic extract of propolis. The results obtained for calibration and prediction parameters indicated that the ABTS model is validated and can be used for quantification of antioxidant activity of new extracts of propolis. The models for DPPH, FRAP, and TPC indicated a low predictive capacity which complicates the data modeling. However, for application purposes, further investigation is required to develop a mathematical model to control and predict the optimization parameters of the antioxidant activity. Green extraction techniques are a useful alternative to rapid analysis, reducing waste generation and cost.
References
Al-Ghamdi AA, Bayaqoob NIM, Rushdi AI, Alattal Y, Simoneit BRT, el-Mubarak AH, al-Mutlaq KF (2017) Chemical compositions and characteristics of organic compounds in propolis from Yemen. Saudi J Biol Sci 24(5):1094–1103. https://doi.org/10.1016/j.sjbs.2016.12.012
Alves E, Kubota EH (2013) Conteúdo de fenólicos, flavonoides totais e atividade antioxidante de amostras de própolis comerciais. Rev Ciências Farm Básica e Apl 34:37–41
Bankova V (2005) Recent trends and important developments in propolis research. Evid-Based Complement Alternat Med 2(1):29–32. https://doi.org/10.1093/ecam/neh059
Bankova V (2009) Chemical diversity of propolis makes it a valuable source of new biologically active compounds. J ApiProduct ApiMedical Sci 1(2):23–28. https://doi.org/10.3896/IBRA.4.01.2.01
Banskota AH, Tezuka Y, Kadota S (2001) Recent progress in pharmacological research of propolis. Phyther Res 15(7):561–571. https://doi.org/10.1002/ptr.1029
Barbosa KBF, Costa NMB, Alfenas RCG, et al (2010) Estresse oxidativo: Conceito, implicações e fatores modulatórios. Rev Nutr 23:629–643. https://doi.org/10.1159/000320546
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma ( FRAP ) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76. https://doi.org/10.1006/abio.1996.0292
Brand-Williams M, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Leb Technol 28(1):25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Cai R, Wang S, Meng Y et al (2012) Rapid quantification of flavonoids in propolis and previous study for classification of propolis from different origins by using near infrared spectroscopy. Anal Methods 4(8):2388. https://doi.org/10.1039/c2ay25184a
Calegari MA, Prasniewski A, Silva C et al (2017) Propolis from southwest of Parana produced by selected bees: influence of seasonality and food supplementation on antioxidant activity and phenolic profile. An Acad Bras Cienc 89(1):45–55. https://doi.org/10.1590/0001-3765201620160499
Cao J, Peng LQ, Du LJ, Zhang QD, Xu JJ (2017) Ultrasound-assisted ionic liquid-based micellar extraction combined with microcrystalline cellulose as sorbent in dispersive microextraction for the determination of phenolic compounds in propolis. Anal Chim Acta 963:24–32. https://doi.org/10.1016/j.aca.2017.01.063
Castro C, Mura F, Valenzuela G, Figueroa C, Salinas R, Zuñiga MC, Torres JL, Fuguet E, Delporte C (2014) Identification of phenolic compounds by HPLC-ESI-MS / MS and antioxidant activity from Chilean propolis. FRIN 64:873–879. https://doi.org/10.1016/j.foodres.2014.08.050
Chang-Bravo L, López-Córdoba A, Martino M (2014) Biopolymeric matrices made of carrageenan and corn starch for the antioxidant extracts delivery of Cuban red propolis and yerba mate. React Funct Polym 85:11–19. https://doi.org/10.1016/j.reactfunctpolym.2014.09.025
Dal Zotto R, De Marchi M, Cecchinato A et al (2008) Reproducibility and repeatability of measures of milk coagulation properties and predictive ability of mid-infrared reflectance spectroscopy. J Dairy Sci 91(10):4103–4112. https://doi.org/10.3168/jds.2007-0772
Daneshmand A, Sadeghi G, Karimi A et al (2015) Evaluating complementary effects of ethanol extract of propolis with the probiotic on growth performance, immune response and serum metabolites in male broiler chickens. Livest Sci 178:195–201. https://doi.org/10.1016/j.livsci.2015.04.012
de Cardoso EO, Conti BJ, Santiago KB et al (2017) Phenolic compounds alone or in combination may be involved in propolis effects on human monocytes. J Pharm Pharmacol 69(1):99–108. https://doi.org/10.1111/jphp.12660
Fagan CC, Everard C, Donnell CPO et al (2007) Evaluating mid-infrared spectroscopy as a new technique for predicting sensory texture attributes of processed cheese. J Dairy Sci 90(3):1122–1132. https://doi.org/10.3168/jds.S0022-0302(07)71598-9
González-Martín MI, Revilla I, Vivar-Quintana AM, Betances Salcedo EV (2017) Pesticide residues in propolis from Spain and Chile. An approach using near infrared spectroscopy. Talanta 165:533–539. https://doi.org/10.1016/j.talanta.2016.12.061
Grdadolnik J (2002) ATR-FTIR spectroscopy: its advantages and limitations. Acta Chim Slov 49:631–642
Jurd L, Geissman TA (1956) Absorption spectra of metal complexes of flavonoid compounds. J Org Chem 88(7):1405–1421. https://doi.org/10.1021/cr00089a017
Kapper C, Klont RE, Verdonk JMAJ, Williams PC, Urlings HAP (2012) Prediction of pork quality with near infrared spectroscopy (NIRS) 2. Feasibility and robustness of NIRS measurements under production plant conditions. Meat Sci 91(3):300–305. https://doi.org/10.1016/j.meatsci.2012.02.006
Laghi L, Versari A, Parpinello GP et al (2011) FTIR spectroscopy and direct orthogonal signal correction preprocessing applied to selected phenolic compounds in red wines. Food Anal Methods 4(4):619–625. https://doi.org/10.1007/s12161-011-9240-2
López BG, Morgado E, Eberlin MN, Sawaya ACHF (2014) Phytochemical markers of different types of red propolis. Food Chem 146:174–180. https://doi.org/10.1016/j.foodchem.2013.09.063
Luis-Villaroya A, Espina L, Garcia-Gonzalo D et al (2015) Bioactive properties of a propolis-based dietary supplement and its use in combination with mild heat for apple juice preservation. Int J Food Microbiol 205:90–97. https://doi.org/10.1016/j.ijfoodmicro.2015.03.020
Martins JPA, Ferreira MMC (2013) Qsar modeling: a new open source computational package to generate and validate Qsar models. Quim Nova 36(4):554–U250. https://doi.org/10.1590/S0100-40422013000400013
Menezes H (2005) Própolis : Uma Revisão Dos Recentes Estudos. Arq Inst Biol 72:405–411
Morlock GE, Ristivojevic P, Chernetsova ES (2014) Combined multivariate data analysis of high-performance thin-layer chromatography fingerprints and direct analysis in real time mass spectra for profiling of natural products like propolis. J Chromatogr A 1328:104–112. https://doi.org/10.1016/j.chroma.2013.12.053
Pavia DL, Lampman GM, Kritz GS, et al (2010) Introduction to Spectroscopy, 4th edn. Brooks Cole: Washington
Pham-Huy LA, He H, Pham-Huy C (2008) Free Radicals, Antioxidants in Disease and Health. Int J Biomed Sci 4:89–96. https://doi.org/10.1073/pnas.0804252105
Oldoni TLC, Cabral ISR, D’Arce MABR et al (2011) Isolation and analysis of bioactive isoflavonoids and chalcone from a new type of Brazilian propolis. Sep Purif Technol 77(2):208–213. https://doi.org/10.1016/j.seppur.2010.12.007
Oldoni TLC, Oliveira SC, Andolfatto S et al (2015) Chemical characterization and optimization of the extraction process of bioactive compounds from propolis produced by selected bees. J Braz Chem Soc 26:2054–2062. https://doi.org/10.5935/0103-5053.20150186
Park YK, Alencar SM, Aguiar CL (2002) Botanical origin and chemical composition of Brazilian propolis. J Agric Food Chem 50(9):2502–2056. https://doi.org/10.1021/jf011432b
Páscoa RNMJ, Magalhães LM, Lopes JA (2013) FT-NIR spectroscopy as a tool for valorization of spent coffee grounds: application to assessment of antioxidant properties. Food Res Int 51(2):579–586. https://doi.org/10.1016/j.foodres.2013.01.035
Pasupuleti VR, Sammugam L, Ramesh N, Gan SH (2017) Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. Oxidative Med Cell Longev 2017:1–21. https://doi.org/10.1155/2017/1259510
Picoli T, Peter CM, Hoffmann JF et al (2016) Caracterização química e ação antibacteriana de extrato de própolis marrom da região sul do Brasil. Rev Bras Med Vet 38:365–371
Popova M, Trusheva B, Bankova V (2017) Content of biologically active compounds in Bulgarian propolis : a basis for its standardization. Bulg Chem Commun 49:115–120
Re R, Pellegrini N, Proteggente a et al (1999) Antioxidant activity applying an improved Abts radical cation decolorization assay. Free Radic Biol Med 26(9-10):1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Revilla I, Vivar-Quintana AM, González-Martín I, Escuredo O, Seijo C (2017) The potential of near infrared spectroscopy for determining the phenolic, antioxidant, color and bactericide characteristics of raw propolis. Microchem J 134:211–217. https://doi.org/10.1016/j.microc.2017.06.006
Rodriguez-Saona LE, Allendorf ME (2011) Use of FTIR for rapid authentication and detection of adulteration of food. Annu Rev Food Sci Technol 2(1):467–483. https://doi.org/10.1146/annurev-food-022510-133750
Rufatto LC, dos Santos DA, Marinho F, Henriques JAP, Roesch Ely M, Moura S (2017) Red propolis: chemical composition and pharmacological activity. Asian Pac J Trop Biomed 7(7):591–598. https://doi.org/10.1016/j.apjtb.2017.06.009
Salami A, Seydi E, Pourahmad J (2013) Use of nutraceuticals for prevention and treatment of cancer. Iran J Pharm Res 12:219–220
Schnitzler P, Neuner A, Nolkemper S, Zundel C, Nowack H, Sensch KH, Reichling J (2010) Antiviral activity and mode of action of propolis extracts and selected compounds. Paul Phyther Res 24(S1):S20–S28. https://doi.org/10.1002/ptr.2868
Silva BB, Rosalen PL, Cury JA, Ikegaki M, Souza VC, Esteves A, Alencar SM (2007) Chemical composition and botanical origin of red propolis, a new type of Brazilian propolis. Evid-Based Complement Altern Med 5(3):313–316. https://doi.org/10.1093/ecam/nem059
Silva SD, Feliciano RP, Boas LV, Bronze MR (2014) Application of FTIR-ATR to Moscatel dessert wines for prediction of total phenolic and flavonoid contents and antioxidant capacity. Food Chem 150:489–493. https://doi.org/10.1016/j.foodchem.2013.11.028
Silva-carvalho R, Baltazar F, Almeida-aguiar C (2015) Propolis : a complex natural product with a plethora of biological activities that can be explored for drug development. Evid-Based Complement Altern Med 2015:206439. https://doi.org/10.1155/2015/206439
Singleton VL, Orthofer R, Lamuela-Raventόs RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Siqueira ABS, de Rodriguez LRNA, Santos RKB et al (2015) Antifungal activity of propolis against Candida species isolated from cases of chronic periodontitis. Braz Oral Res 29(1):1–6. https://doi.org/10.1590/1807-3107BOR-2015.vol29.0083
Soares SE (2002) Ácidos Fenólicos como antioxidantes. Rev Nutr 15(1):71–81. https://doi.org/10.1590/S1415-52732002000100008
Soltani EK, Cerezuela R, Charef N et al (2017) Algerian propolis extracts: chemical composition, bactericidal activity and in vitro effects on gilthead seabream innate immune responses. Fish Shellfish Immunol 62:57–67. https://doi.org/10.1016/j.fsi.2017.01.009
Sousa CMDM, Silva HRE, Vieira GM et al (2007) Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quim Nova 30(2):351–355. https://doi.org/10.1590/S0100-40422007000200021
Tan W, Sun L, Yang F, Che W, Ye D, Zhang D, Zou B (2018) Study on bruising degree classification of apples using hyperspectral imaging and GS-SVM. Optik (Stuttg) 154:581–592. https://doi.org/10.1016/j.ijleo.2017.10.090
Tiveron AP, Rosalen PL, Franchin M, Lacerda RCC, Bueno-Silva B, Benso B, Denny C, Ikegaki M, Alencar SM (2016) Chemical characterization and antioxidant, antimicrobial, and anti-inflammatory activities of south Brazilian organic propolis. PLoS One 11(11):1–18. https://doi.org/10.1371/journal.pone.0165588
Vargas-Sánchez RD, Mendoza-Wilson AM, Torrescano-Urrutia GR, Sánchez-Escalante A (2015) Antiradical potential of phenolic compounds fingerprints of propolis extracts : DFT approach. Comput Theor Chem 1066:7–13. https://doi.org/10.1016/j.comptc.2015.05.003
Vasconcelos SML, Goulart MOF, Moura JBF, et al (2007) Espécies reativas de oxigênio e de nitrogênio, antioxidantes e marcadores de dano oxidativo em sangue humano: Principais métodos analíticos para sua determinação. Quím Nova 30:1323–1338. https://doi.org/10.1590/S0100-40422007000500046
Veiga RS, Mendonça S, Mendes PB et al (2017) Artepillin C and phenolic compounds responsible for antimicrobial and antioxidant activity of green propolis and Baccharis dracunculifolia DC. J Appl Microbiol 122(1):911–920. https://doi.org/10.1111/ijlh.12426
Viegas TR, Mata ALML, Duarte MML, Lima KMG (2016) Determination of quality attributes in wax jambu fruit using NIRS and PLS. Food Chem 190:1–4. https://doi.org/10.1016/j.foodchem.2015.05.063
Wilson RH, Tapp HS (1999) Mid-infrared spectroscopy for food analysis: recent new applications and relevant developments in sample presentation methods. TrAC - Trends Anal Chem 18(2):85–93. https://doi.org/10.1016/S0165-9936(98)00107-1
Wu Y-W, Sun S-Q, Zhao J, Li Y, Zhou Q (2008) Rapid discrimination of extracts of Chinese propolis and poplar buds by FT-IR and 2D IR correlation spectroscopy. J Mol Struct 883–884:48–54. https://doi.org/10.1016/j.molstruc.2007.12.009
Xu L, Yan S, Cai C, Yu X (2013) Untargeted detection and quantitative analysis of poplar balata ( PB ) in Chinese propolis by FT-NIR spectroscopy and chemometrics. Food Chem 141:4132–4137. https://doi.org/10.1016/j.foodchem.2013.07.013
Zabaiou N, Fouache A, Trousson A, Baron S, Zellagui A, Lahouel M, Lobaccaro JMA (2017) Biological properties of propolis extracts: something new from an ancient product. Chem Phys Lipids 207(Pt B):214–222. https://doi.org/10.1016/j.chemphyslip.2017.04.005
Zhou H, Deng Z, Xia Y, Fu M (2016) A new sampling method in particle filter based on Pearson correlation coefficient. Neurocomputing 216:208–215. https://doi.org/10.1016/j.neucom.2016.07.036
Acknowledgements
The authors acknowledge fellowship from Federal Technological University of Parana (UTFPR) and Central de Analises.
Funding
This study was funded by Federal Technological University of Paraná (UTFPR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
C. da Silva declares that she has no conflict of interest. A. Prasniewski declares that she has no conflict of interest. M. A. Calegari declares that he has no conflit of interest. V. A. de Lima declares that he has no conflict of interest. T. L. C. Oldoni declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
da Silva, C., Prasniewski, A., Calegari, M.A. et al. Determination of Total Phenolic Compounds and Antioxidant Activity of Ethanolic Extracts of Propolis Using ATR–FT-IR Spectroscopy and Chemometrics. Food Anal. Methods 11, 2013–2021 (2018). https://doi.org/10.1007/s12161-018-1161-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1161-x