Abstract
The extraction conditions applied in destructive analysis of Hibiscus sabdariffa L. (Hs) matrices before the measurement of antioxidant compounds may reduce their activities during isolation and purification due to decomposition. Hence, near infrared spectroscopic system was applied to determine these compounds. The calibration models developed by using partial least squares regression (PLSR) with 48 Hs samples and 24 unknown samples were used to confirm the robustness of the developed model. The results of calibration models effectively allowed the determination of the concentrations of total phenol content (TPC), total flavonoid content (TFC), total antioxidant content (TAC), ferric reducing antioxidant power (FRAP), and 1,1-diphenyl-2-picrylhydrazyl (DPPH) with high correlation coefficients (0.85–0.91) and small standard error of cross-validation (RMSECV) ranged from 0.08 to 0.33. The prediction capacity (RPD) for TPC, TFC, TAC, FRAP, and DPPH ranged from 3.30 to 8.25, thus demonstrating that the near infrared spectroscopic (NIRS) equations developed were applicable to unknown samples. This study demonstrates that NIRS can be considered as a fast tool for the nondestructive determination of antioxidant compounds (phenolics and flavonoids) and antioxidant activity of the Hs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hibiscus sabdariffa L. (Hs), also known in Sudan as Karkade, is grown in various parts of the country, mostly Kordofan and Darfur States, where large quantities are produced both for local utilization and for export. The dried Hs flowers are used for ice cream, jams, tea, syrup, beverage, and Sudanese traditional fermented sorghum drink hulu-mur. Nowadays, focus on plant study has raised around the world, and a huge body of data has been collected to demonstrate the vast potential of medicinal plants used in traditional systems (Uprety et al. 2012; Mahomoodally 2013). H. sabdariffa L. is rich in bioactive such as organic acids, phenols, flavonoids, and anthocyanins (Da-Costa-Rocha et al. 2014; Borrás-Linares et al. 2015), and they are well known as having high scientific and therapeutic interest (Sharma 2014).
Among the phytochemicals found in fruit, phenolic compounds has been reported to be the key polyphenols (Rhodes 1996), which are well known to be effective antioxidants and natural antagonists of plant pathogens (Prenesti et al. 2007). Indeed, the antioxidant activity of phenolic compounds is mainly due to their redox properties which allow them to act as reducing factors, hydrogen donors, singlet oxygen quenchers, and metal chelators (Rice-Evans et al. 1997). Commonly, total phenols are quantified using the Folin–Ciocalteu method (Singleton et al. 1999), while total flavonoid contents are determined by the colorimetric aluminum chloride method.
Various methods for measuring the antioxidant activity in plant extracts have been employed. On the other hand, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay is considered a rapid and easy method to assess the scavenging activity of antioxidants, since the radical compound is stable and does not have to be produced as in other radical scavenging methods (Sharma and Bhat 2009; Tabart et al. 2009). Ferric reducing antioxidant power (FRAP) is an additional simple and robust method of antioxidant assay method (Benzie and Strain 1999). The chemical analysis methods were applied to determine the specific antioxidant substances and antioxidant capacity up to now. The extraction methods applied before the determination represent a source of variations among laboratories and in some cases are not reliable (Gökmen et al. 2009). Moreover plant matrices may raise the problems, since the activity capacities of antioxidant substances may decrease during their separation and purification due to decomposition (Çelik et al. 2010).
Currently, the food processors have developed models using multivariate and chemical analysis results for predicting the concentrations of specific chemical compounds with chemometric methods (Corbella and Cozzolino 2006; Woodcock et al. 2009). Near infrared spectroscopy (NIRS) has been applied as rapid and low-cost technique to determine the quality of various foods and agricultural crops (Chen et al. 2015; Mata Sánchez et al. 2015; Pan et al. 2015; Shao et al. 2015). Additionally, it has the advantage of a minimum sample preparation required before measurements (González-Martín et al. 2011; Chen et al. 2012a, b). Escuredo et al. (2013) reported the feasibility of NIRS for prediction of antioxidant activity in honey. However, few data were available for prediction of antioxidant activity of H. sabdariffa using NIRS technology; thus, the aim of the study was to build calibration equations using the NIRS technique to measure antioxidant compounds and antioxidant activity of Sudanese Karkade (H. sabdariffa).
Materials and Methods
Chemicals and Hs Samples
All reagents were of analytical grade. Folin–Ciocalteu’s phenol reagent, gallic acid, ascorbic acid, sodium carbonate anhydrous, ferric chloride hexahydrate, sodium nitrite, sodium hydroxide, quercetin, aluminum chloride hexahydrate, trichloroacetic acid, ferric chlorid, potassium ferricynaide, HPLC grade methanol, dipotassium phosphate from (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), and 1,1-diphenyl-2-picrylhydrazyl (Sigma, St. Louis, MO, USA). H. sabdariffa samples were collected from various parts of Sudan, mainly from Geneina, Al-Fashir, Nyala, Kaduqli, Al-Ubayyid, and Omdurman (Fig. 1). Hs samples were milled to fine powder by a small electric mill prior to analysis. After this process, the samples are sieved with a 500-μm mesh, and these sieved powder samples were used for further study.
The aqueous extracts of Hs were prepared with double-distilled water at a mass ratio of calyx powder to water of 1:50 and sonicated (continuous, 20 kHz) for 20 min at 25 °C. Then, the extract was centrifuged for 10 min at 3000 rpm and filtered through Whatman No. 1 and after that stored in air tight containers for further investigation.
Antioxidant Compounds
Total Flavonoid Content
The total flavonoid content (TFC) in each Hs sample was measured by the following method of Zhishen et al. (1999). One milliliter of diluted (1:10) Hs extract was mixed with 4 mL of distilled water. At the baseline, 0.3 mL of NaNO2 (1:20) was added. After 5 min, 0.3 mL of AlCl3 (1:10) was added, followed by the addition of 2 mL of NaOH (1 M) 6 min later. The volume was increased to 10 mL by adding 2.4 mL distilled water followed by thorough mixing via a vortex mixture to ensure adequate mixing and the absorbance was read at 510 nm. A calibration curve was created using a standard solution of quercetin (0–100 μg/mL; R 2 = 0.98). The mean of three readings was used and expressed as milligrams of quercetin equivalents (QE) / 100 g of Hs.
Total Phenolic Content
Standard Folin–Ciocalteu method followed by Noor et al. (2014) with slight modification was used to determine the total phenolic contents (TPC). One milliliter of diluted Hs (1:10) was added to 5 mL of 0.2 N Folin–Ciocalteu reagent and mixed well for 10 min by using vortex mixture. Sodium carbonate 2 mL (75 g/L) was added and incubated for 2 h. Absorbance of reaction mixture was measured at 760 nm. Gallic acid (0–200 μg/mL) was used for standard curve (R 2 = 0.99). The collected data were presented in milligramgs of gallic acid equivalents (GAL) / 100 g of Hs.
Antioxidant Activity Measurements
DPPH Radical Scavenging Activity
DPPH radical scavenging activity (RSA%) was measured by method of Noor et al. (2014) with slight modification. One milliliter of diluted Hs (1:10) solution and DPPH 0.02 mg/mL prepared in methanol were mixed in 2:1 proportion, respectively. Reaction mixture was incubated at room temperature for 15 min followed by measurement of absorbance at 517 nm against 2 mL of diluted Hibiscus solution mixed with 1 mL of water as blank. Two milliliters of DPPH solution mixed with 5 mL methanol was used as negative control (Ac), while BHT was used as positive control. Each experiment was performed in triplicates. Inhibition% of DPPH radical was determined by following formula:
Ferric Reducing Antioxidant Power
Ferric reducing antioxidant power (FRAP) of Hs was determined by following the method of Saxena et al. (2010) with some modifications. A 2.5 mL of diluted Hs (1:10) solution was mixed with 2.5 mL of 0.2 M phosphate buffer (pH 6.6). Above reaction mixture was mixed with 2.5 mL of potassium ferricyanide (1 %) and incubated for 20 min at 50 C. Trichloroacetic acid (10 %) 2.5 mL was added, followed by thorough mixing via vortex mixture. Reaction mixture thus obtained was centrifuged for 10 min at 3000 rpm. Supernatant 2.5 mL was mixed with 2.5 mL of double-distilled water and 0.5 mL of FeCl3 (0.1 %) solution. Absorbance was measured at 700 nm. Ascorbic acid (0–10 μg/mL) was used as reference standard (R 2 = 0.96).
Total Antioxidant Content
The total antioxidant content (TAC) expressed as ascorbic acid equivalent antioxidant content (AEAC) was measured using the method of Saxena et al. (2010) with some modifications. A 3 mL of diluted Hs (1:10) solution was mixed with 1.5 mL of 0.02 mg/mL of DPPH solution that was prepared in methanol. The mixture was incubated at room temperature for 15 min, and the absorbance was measured at 517 nm using a spectrophotometer. The blank used was 3 mL of Hs solution mixed with 1.5 mL of water. Ascorbic acid standard solutions (0–10 μg/mL) prepared in double-distilled water were used to plot the calibration curve. Measurements were performed in triplicate, and the mean value was expressed as milligrams of AEAC / 100 g of Hs.
Spectra Collection
Based on our previous work on Hs (Xiaowei et al. 2014), the NIR spectra were obtained in the reflectance mode using the AntarisTM II Near-infrared spectrophotometer (Thermo Electron Co., USA) with an integrating sphere. Each spectrum was the average of 32 scanning spectra. The range of spectra was from 10,000 to 4000 cm−1, and the data were measured in every 3.856 cm−1, which resulted in 1557 variables.
A sample accessory holder was used to collect spectra of Hs powders. It was a standard sample cup specifically designed by Thermo Electron Co. About 2 g of Hs sample was placed in the sample cup. Each Hs sample was collected in triplicate after rotating the cup at 120°. The mean of the triplicate spectra, which were obtained from the identical sample, was used in further analysis. The relative humidity and temperature of the laboratory were kept at 60 % and 25 °C, correspondingly.
Spectral Preprocessing and Partial Least Squares Regression
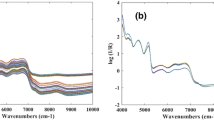
The obtained NIR spectra contained background information and noises, besides the sample information and noises as depicted in Fig. 2a. It is very important to preprocess spectra before model building. To this end, various models including standard normal variate transformation (SNV), multiplicative scatter correction (MSC), mean centering (MC), and baseline correction have been applied (Chen et al. 2012a, b; Fan et al. 2015). Indeed, SNV is a mathematical transformation technique of the log (1/R) spectra which is applied to eliminate slope difference and to correct for scatter effects. On this technique, each spectrum is corrected separately by first centering the spectral values, and then the centered spectrum is scaled by the standard deviation computed from the individual spectral values (Chen et al. 2008). MSC is another essential method for the correction of scatter light, on the basis of different particle sizes and technique used to compensate for additive and/or multiplicative effects in spectra (Helland et al. 1995). In addition, MC is used to estimate the average spectrum of the data set and to deduct the average from each spectrum. Finally, baseline correction, also known as detrending is applied on spectra to eliminate the effects of baseline shift and curvi-linearity (Luypaert et al. 2003).
Since the number of the wavelengths in the NIR spectra were greatly larger than the number of Hs samples in the data set, it was essential to apply multivariate statistical methods to validate extract information from the data set. The ultimate purpose was to build a statistical model that permits the prediction of the antioxidant compounds and antioxidant activity of Hs samples using NIR spectra. Partial least square regression (PLSR) was used to develop the calibration model from the threshold spectra with the reference chemical values (TPC, TFC, TAC, FRAP, and DPPH%). Statistically, PLSR method helps to simplify the correlation between X-data (spectral data) and Y-data (reference chemical data) by assuring that all latent variables are arranged on the basis of their relevance for predicting Y (Wold et al. 2001; Nicolaï et al. 2007). A cross-validation method was applied in model validation with as several validation subsets as there were samples involved in the calibration model (leave-one-out method). The robustness of the regression models was evaluated using the correlation coefficient in calibration (R c) and prediction (R p), the root mean square errors assessed by cross-validation (RMSECV). The ratio of the standard deviation of the response variable to the SEP called the ratio of performance to standard deviation (RPD) and provides a standardization of the SEP (Nicolaï et al. 2007; Wu et al. 2012). In general, a good model should have high correlation coefficients besides low RMSECV and root mean standard error of prediction (RMSEP) (Kamruzzaman et al. 2013; He et al. 2014). Moreover, a higher RPD value always reveals a better capability for prediction (Williams and Sobering 1996).
Software
PLSR was performed using Matlab Version 7.1 (Mathworks Inc., Natick, MA, USA) for Windows XP. Result Software (Antaris II System, Thermo Electron Co., USA) was used for obtaining NIR spectral data. The Student’s t test was performed using Origin Pro version 9.0 (OriginLab Corp. Northampton, USA).
Results and Discussions
Antioxidant Activity
Oxidation of biological molecules induce a variety of pathological events such as atherogensis, carcinogenesis, and aging (Finkel and Holbrook 2000). Therefore, supplementation of natural antioxidants such as phenolics, flavonoids, and anthocyanins has received much attention. That is why the determination of antioxidant activity of the functional food such as Hs and their active components has become important. The values of TPC ranged 146.97–148.94 mg/100 g and 25.70–27.50 mg/100 g for TFC. As shown in Table 1, the descriptive statistics confirmed that the Sudanese Hs slightly varied in antioxidant activity; this could be due to different calyx sources and types (dark red, red). We can conclude that the Sudanese Hs extracts contain important antioxidant substances, exhibiting considerable antioxidant capacity which may be used as a natural source of compounds with these properties. Currently, it is complicated to compare different studies because of the diverse doses and different extraction procedures used. Moreover, a wide range of extraction conditions has been used (Mohd-Esa et al. 2010; Ramirez-Rodrigues et al. 2011; Sindi et al. 2014).
Hs have different antioxidant substances, which indicate that antioxidant capacity cannot be measure using only one method (Sáyago-Ayerdi et al. 2007). Thus, in this study, different methods were used to measure the antioxidant activity of Hs extract. The reference antioxidant activity values of Hs were measured using the reference chemical data described in Table 1. The values of antioxidant activities measured using the radical scavenging activity (DPPH%) were in the range of 70.49–74.11 %, TAC 24.50–26.54 mg/100 g, and FRAP range of 137.86–140.61 mMFe2+/100 g. DPPH% of well-recognized synthetic antioxidant (BHT) used as standard in this study was 91.8 %. The main constituents of Hs related in the context of its antioxidant activity are anthocyanins, phenolics, flavonoids, and organic acid such as ascorbic acid (Umar et al. 2009; Sindi et al. 2014).
Comparison of Preprocessing Methods
In order to improve spectral features and to further build a robust prediction model, numerous data preprocessing methods were implemented, including SNV, MSC, MC, and baseline correction. PLS was performed to evaluate the preprocessed spectra. As seen in Table 2, the processed NIR spectra with SNV method and MSC were found to have the good ability in both calibration and validation and were obtained higher R c, R p, and RPD for TFC, TAC, FRAP, and DPPH% compared to other preprocessing techniques used. The preprocessed data by SNV can reflect more information corresponding to TFC, TAC, FRAP, and DPPH%, whereas the MSC spectra were more appropriate to determine the TPC in Hs powder. This could explain that TPC and other parameters measured were associated to different useful information in the spectra (Huang et al. 2011). These preprocessing techniques have advantages in correcting scatter light as presented in Fig. 2b for SNV and Fig. 2c for MSC. This finding is in good agreement with the literature where SNV and MSC were found to have better performance for extraction information related to antioxidants and antioxidant activity (Escuredo et al. 2013; Moncada et al. 2013).
Calibration and Validation of NIRS Models
Calibration of Models
In this study, the 72 Hs samples were divided arbitrarily into two subsets. The first subsets named the calibration set were used to build model, and the second named the prediction set was used to test the performance of model. Table 1 shows the reference chemical results and sample numbers both in calibration and external validation sets. As seen from Table 1, the scope of reference chemical results of each measurement in the calibration set roughly covers the scope in the prediction set, and their standard deviations between the two sets are no significant differences. As a result, the classification of the samples is appropriate both in the calibration and in the prediction sets. Generally, the degree to which the calibration best adjusts the data set was measured by the highest R c and the lowest and RMSECV (Wu et al. 2002). The results obtained in Table 2 indicate the ability of the calibration models to measure the antioxidant compounds and antioxidant activity in Hs samples. Excellent equations were developed for the determination of antioxidant substances and antioxidant activities in Hs. The R c values were 0.84, 0.85, 0.91, 0.91, and 0.87 for TPC, TFC, TAC, FRAP, and DPPH, respectively. Furthermore, acceptable values for RMSECV were obtained. The statistical parameters obtained exhibited a good calibration adjustment for all parameters measured.
Internal Validation (Prediction)
Cross-validation was applied for the evaluation of the models obtained. The group of calibration samples was assigned into a series of sub-groups for their calibration. By means of the data set, seven series were generated, and of these, six were chosen for the calibration group and one for the prediction group. The method was reiterated, as numerous times as there are sets, in order for all to pass through the calibration group and the prediction group. With this process, we validate the models used and proved its prediction abilities. The correlation of the values acquired from chemical analysis with respect to those predicted by NIR for TPC, TFC, TAC, DPPH, and FRAP are shown in Fig. 3. From the results of RMSEP and SEPC (RMSEP-Bias), we can conclude that the calibration model for parameters measured is robust, allowing the determination of these parameters in Hs of different compositions with excellent results.
Prediction Capability of the Model
The prediction capability of the model acquired was assessed with the ratio performance deviation (RPD) as described in Table 2. In this study, RPD values were computed as association between the standard deviation of the chemical method (SD) and the SEPC encountered in the NIRS model. RPD between 1.5 and 2 means that the model can discriminate low from high values of the response variable; a value between 2 and 2.5 shows that coarse quantitative predictions are possible, and a value between 2.5 and 3 or above corresponds to good and excellent prediction, respectively (Nicolaï et al. 2007). The RPD values obtained in this work were 8.25, 3.37, 4.19, 5.10, and 3.30 for TFC, TPC, TAC, DPPH, and FRAP, respectively. The high level RPD indicates that the NIRS equations obtained were excellent for prediction of antioxidant compounds and antioxidant activity in Hs.
External Validation
Hs samples (24) with a diverse composition and NIR spectral information were tested to examine the robustness of the method obtained (Table 3). The process was as follows: spectra were obtained three times and the spectral average was taken. Subsequently, the calibration equations acquired were used, and the predicted values were compared with chemical analysis data. The NIRS technique and the reference data concerning the TPC, TFC, TAC, DPPH%, and FRAP were compared with those of samples not related to the calibration model using the Student’s t test for paired values. The significance acquired were 0.28 for DPPH% and 0.80 for TAC as shown in Table 3. The significance level (P) of tested parameters was higher than 0.05; consequently, there were no differences between the results acquired. The mean residuals were between 0.04 for TFC and for 0.20 TAC. In general, the different analytical methods used for measurements of antioxidant compounds and antioxidant activity provide significantly comparable results.
Conclusions
NIR technique is applicable to the medicinal and tea industries; it is a very useful tool for the fast measurement of bioactive in Hs. In addition, NIR shows itself as a technique with a great potential for the quantification of antioxidant activity of Hs with minimal treatment. Excellent calibration and validation models were developed for TPC, TFC, and antioxidant activities, with a high ability of prediction (RPD > 3).
References
Benzie IFF, and Strain JJ (1999) [2] Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods in Enzymology. P. Lester, Academic Press. Volume 299: 15-27
Borrás-Linares I, Fernández-Arroyo S, Arráez-Roman D, Palmeros-Suárez PA, Del Val-Díaz R, Andrade-Gonzáles I, Fernández-Gutiérrez A, Gómez-Leyva JF, Segura-Carretero A (2015) Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Ind Crop Prod 69:385–394
Çelik SE, Özyürek M, Güçlü K, Apak R (2010) Determination of antioxidants by a novel on-line HPLC-cupric reducing antioxidant capacity (CUPRAC) assay with post-column detection. Anal Chim Acta 674(1):79–88
Chen Q, Zhao J, Liu M, Cai J, Liu J (2008) Determination of total polyphenols content in green tea using FT-NIR spectroscopy and different PLS algorithms. J Pharm Biomed Anal 46(3):568–573
Chen L, Wang J, Ye Z, Zhao J, Xue X, Heyden YV, Sun Q (2012a) Classification of Chinese honeys according to their floral origin by near infrared spectroscopy. Food Chem 135(2):338–342
Chen Q, Ding J, Cai J, Zhao J (2012b) Rapid measurement of total acid content (TAC) in vinegar using near infrared spectroscopy based on efficient variables selection algorithm and nonlinear regression tools. Food Chem 135(2):590–595
Chen Q, Zhang D, Pan W, Ouyang Q, Li H, Urmila K, Zhao J (2015) Recent developments of green analytical techniques in analysis of tea’s quality and nutrition. Trends Food Sci Technol 43(1):63–82
Corbella E, Cozzolino D (2006) Classification of the floral origin of Uruguayan honeys by chemical and physical characteristics combined with chemometrics. LWT–Food Sci Technol 39(5):534–539
Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M (2014) Hibiscus sabdariffa L.—a phytochemical and pharmacological review. Food Chem 165:424–443
Escuredo O, Carmen Seijo M, Salvador J, Inmaculada González-Martín M (2013) Near infrared spectroscopy for prediction of antioxidant compounds in the honey. Food Chem 141(4):3409–3414
Fan S, Huang W, Guo Z, Zhang B, and Zhao C (2015) Prediction of soluble solids content and firmness of pears using hyperspectral reflectance imaging. Food Analytical Methods: 1-11
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408(6809):239–247
Gökmen V, Serpen A, Fogliano V (2009) Direct measurement of the total antioxidant capacity of foods: the ‘QUENCHER’ approach. Trends Food Sci Technol 20(6–7):278–288
González-Martín M, Severiano-Pérez P, Revilla I, Vivar-Quintana A, Hernández-Hierro J, González-Pérez C, Lobos-Ortega I (2011) Prediction of sensory attributes of cheese by near-infrared spectroscopy. Food Chem 127(1):256–263
He H-J, Sun D-W, Wu D (2014) Rapid and real-time prediction of lactic acid bacteria (LAB) in farmed salmon flesh using near-infrared (NIR) hyperspectral imaging combined with chemometric analysis. Food Res Intl 62:476–483
Helland IS, Næs T, Isaksson T (1995) Related versions of the multiplicative scatter correction method for preprocessing spectroscopic data. Chemom Intell Lab Syst 29(2):233–241
Huang L, Wu D, Jin H, Zhang J, He Y, Lou C (2011) Internal quality determination of fruit with bumpy surface using visible and near infrared spectroscopy and chemometrics: a case study with mulberry fruit. Biosyst Eng 109(4):377–384
Kamruzzaman M, ElMasry G, Sun D-W, Allen P (2013) Non-destructive assessment of instrumental and sensory tenderness of lamb meat using NIR hyperspectral imaging. Food Chem 141(1):389–396
Luypaert J, Zhang MH, Massart DL (2003) Feasibility study for the use of near infrared spectroscopy in the qualitative and quantitative analysis of green tea, Camellia sinensis (L.). Anal Chim Acta 478(2):303–312
Mahomoodally MF (2013) Traditional medicines in Africa: an appraisal of ten potent African medicinal plants. Evid Based Complement Alternat Med 2013
Mata Sánchez J, Pérez Jiménez JA, Díaz Villanueva MJ, Serrano A, Núñez N, López Giménez J (2015) Assessment of near infrared spectroscopy for energetic characterization of olive byproducts. Renew Energy 74:599–605
Mohd-Esa N, Hern FS, Ismail A, Yee CL (2010) Antioxidant activity in different parts of roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds. Food Chem 122(4):1055–1060
Moncada GW, González Martín MI, Escuredo O, Fischer S, Míguez M (2013) Multivariate calibration by near infrared spectroscopy for the determination of the vitamin E and the antioxidant properties of quinoa. Talanta 116:65–70
Nicolaï BM, Beullens K, Bobelyn E, Peirs A, Saeys W, Theron KI, Lammertyn J (2007) Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: a review. Postharvest Biol Technol 46(2):99–118
Noor N, Sarfraz RA, Ali S, Shahid M (2014) Antitumour and antioxidant potential of some selected Pakistani honeys. Food Chem 143:362–366
Pan L, Lu R, Zhu Q, McGrath JM, Tu K (2015) Measurement of moisture, soluble solids, sucrose content and mechanical properties in sugar beet using portable visible and near-infrared spectroscopy. Postharvest Biol Technol 102:42–50
Prenesti E, Berto S, Daniele PG, Toso S (2007) Antioxidant power quantification of decoction and cold infusions of Hibiscus sabdariffa flowers. Food Chem 100(2):433–438
Ramirez‐Rodrigues MM, Plaza ML, Azeredo A, Balaban MO, Marshall MR (2011) Physicochemical and phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa. J Food Sci 76(3):C428–C435
Rhodes M (1996) Physiologically-active compounds in plant foods: an overview. Proc Nutr Soc 55(1B):371–384
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2(4):152–159
Saxena S, Gautam S, Sharma A (2010) Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem 118(2):391–397
Sáyago-Ayerdi SG, Arranz S, Serrano J, Goñi I (2007) Dietary fiber content and associated antioxidant compounds in roselle flower (Hibiscus sabdariffa L.) beverage. J Agric Food Chem 55(19):7886–7890
Shao Y, Xie C, Jiang L, Shi J, Zhu J, He Y (2015) Discrimination of tomatoes bred by spaceflight mutagenesis using visible/near infrared spectroscopy and chemometrics. Spectrochim Acta, Part A 140:431–436
Sharma R (2014) Chapter 59—Polyphenols in health and disease: practice and mechanisms of benefits. Polyphenols in human health and disease. R. R. Watson, V. R. Preedy and S. Zibadi. San Diego, Academic Press: 757-778
Sharma OP, Bhat TK (2009) DPPH antioxidant assay revisited. Food Chem 113(4):1202–1205
Sindi HA, Marshall LJ, Morgan MR (2014) Comparative chemical and biochemical analysis of extracts of Hibiscus sabdariffa. Food Chem 164:23–29
Singleton V, Orthofer LR, and Lamuela-Raventós RM (1999) [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology. P. Lester, Academic Press. Volume 299: 152-178
Tabart J, Kevers C, Pincemail J, Defraigne J-O, Dommes J (2009) Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem 113(4):1226–1233
Umar I, Maryoms N, Daikwo E, Gidado A, Buratai L, Igbokwe I, Ibrahim M (2009) The effect of aqueous extracts of Hibiscus sabdariffa (sorrel) calyces on heamatological profile and organ pathological changes in Trypanasoma congolense-infected rat. Afr J Tradis Complement Alternat Med 6(4)
Uprety Y, Asselin H, Dhakal A, Julien N (2012) Traditional use of medicinal plants in the boreal forest of Canada: review and perspectives. J Ethnobiol Ethnomed 8(7):14
Williams PC, and Sobering D (1996) How do we do it: a brief summary of the methods we use in developing near infrared calibrations. Near infrared spectroscopy: The future waves: 185-188.
Wold S, Sjöström M, Eriksson L (2001) PLS-regression: a basic tool of chemometrics. Chemom Intell Lab Syst 58(2):109–130
Woodcock T, Downey G, O’Donnell CP (2009) Near infrared spectral fingerprinting for confirmation of claimed PDO provenance of honey. Food Chem 114(2):742–746
Wu JG, Shi C, Zhang X (2002) Estimating the amino acid composition in milled rice by near-infrared reflectance spectroscopy. Field Crops Res 75(1):1–7
Wu D, Nie P, He Y, Bao Y (2012) Determination of calcium content in powdered milk using near and mid-infrared spectroscopy with variable selection and chemometrics. Food Bioproc Technol 5(4):1402–1410
Xiaowei H, Xiaobo Z, Jiewen Z, Jiyong S, Xiaolei Z, Holmes M (2014) Measurement of total anthocyanins content in flowering tea using near infrared spectroscopy combined with ant colony optimization models. Food Chem 164:536–543
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64(4):555–559
Acknowledgments
Authors acknowledged the National Science and Technology support program (2015BAD17B04), the Jiangsu Province Science Fund for Distinguished Young Scholars (BK20130010), and New Century Excellent Talents in University (NCET-11-0986).
Conflict of Interest
Haroon Elrasheid Tahir declares that he has no conflict of interest. Zou Xiaobo declares that he has no conflict of interest. Shi Jiyong declares that he has no conflict of interest. Abdalbasit A. Mariod declares that he has no conflict of interest. Tchabo Wiliam declares that he has no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Informed Consent
Informed consent is not applicable in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tahir, H.E., Xiaobo, Z., Jiyong, S. et al. Rapid Determination of Antioxidant Compounds and Antioxidant Activity of Sudanese Karkade (Hibiscus sabdariffa L.) Using Near Infrared Spectroscopy. Food Anal. Methods 9, 1228–1236 (2016). https://doi.org/10.1007/s12161-015-0299-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-015-0299-z