Abstract
A review on phthalate esters or phthalic acid esters (PAEs), chemicals of concern since a few decades ago that are widely used as plasticizers in food processing and packaging, is presented taking into account the background of such compounds, the metabolism, human exposure to PAEs, the sources and occurrence in food as well as the toxicological aspects and human health effects. In addition, 45 novel research articles that were published between 2002 and 2017 were identified and their results were tabulated showing the PAEs analysed, food matrix of PAEs, methods of sample preparation/extraction, methods of instrumental analysis and quantitation, percentage recovery and limit of detection (LOD) of the instrument for ease of comparison and referencing. In general, it was found that in the last 15 years, the number of PAEs analysed has increased from the commonly analysed 8 PAEs, namely dimethyl phthalate (DMP), diethyl phthalate (DEP), diisobutyl phthalate (DIBP), di-n-butyl phthalate (DBP), butyl benzyl phthalate (BBP), dicyclohexyl phthalate (DCHP), di-n-octyl phthalate (DNOP) and di-(2-ethylhexyl) phthalate (DEHP) to as many as 23 PAEs. The methods of sample preparation have also progressed from the simple liquid-liquid extraction using organic solvents to solid-phase microextraction techniques to the more recent head-space or direct immersion solid-phase microextraction methods. Whereas for the analysis of PAEs, gas chromatography and liquid chromatography are still the preferred methods with improved LOD of analysis ranging from approximately 10 ppm for fatty foods to 1–60 ppt for water, juices and cooking oil samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food products are complex mixtures comprising of naturally occurring compounds such as lipids, carbohydrates, proteins, vitamins, organic acids and aromas and other different substances which generally originate from mechanical procedures, agrochemical treatments and packaging materials. They are produced and distributed around the world, hence prompting extremely stringent regulations to ensure the nourishment quality and safety in reference to food contaminants (Gallart-Ayala et al. 2013). Food contaminants may be referred to as the presence of an array of redundant chemical compounds other than accustomed ingredients or natural food constituents which can derive from field environmental pollutants. For instance, these contaminants may include but not limited to those derived from chemical industrial waste which may be waterborne or airborne, from leached pesticides or chemicals used in agricultural practices, those that are introduced through inattentiveness in transporting raw products or in the procedures during food transformation processes, as well as those that may arise from unsuitable packaging materials (Moret et al. 2012). Grob et al. (2006) reported that educated consumers had listed pesticides as the main source of food contamination, followed by environmental chemicals such as polychlorinated biphenyl (PCBs) and veterinary drugs and only a few would acknowledge food packaging materials in defiance of the fact that the measure of material migrating from food packaging into food may be 100 times more prominent than the contribution of pesticides and environmental pollutants (Moret et al. 2012).
Food packaging may contain chemical (organic or inorganic) food packaging contaminants that may be intentionally added for a technical purposes, the presence of impurities from starting materials or manufacturing by-products, or the presence of contaminants arising from packaging or material recycling. The migration of different types of chemicals particularly phthalates from the packaging into food is highly diverse depending on the type of packaging materials as summarized in Table 1. The physiochemical properties of the migrant such as the level of contamination of lipophilic substances in high-fat-content food, storage temperature and duration of storage, would also affect the extent to which the migration may occur. For non-inert materials such as plastics, elastomers, paper and board, chemical contaminants may migrate from the outside of the packaging or from the packaging material itself. Paper-based packaging materials tend to have large pore size that permits migration of small molecules from the outside into the food. For example, when beverage cartons or paper cups are stacked on top of each other, the outside layer comes into contact with its inside layer and thus transferring chemical contaminants, such as printing ink components, to the direct food contact side (Muncke 2014). Inert materials such as stainless steel, glazed ceramic or raw glass may contain heavy metals. These chemicals are often on the inside surface and hence are in direct contact with the food and can migrate into food by surface exchange. Furthermore, plasticizers like epoxidized soybean oil or phthalates can contaminate glass-packaged oily foods following migration of chemical contaminants from the closure’s gasket and hence, attentive manufacturing is required. The migration of chemical contaminants into food may also occur when small-sized monomers are released from the degradation of polymer which is often the case of reusable food contact materials like plastic kitchenware. A polymer will degrade and subsequently release monomers under highly acidic or alkaline conditions (Muncke 2014). Another factor that may affect the migration of the contaminants is temperature as performed by Jeddi et al. (2015) in which she concluded that drinking water from polyethylene terephthalate (PET)-bottles stored at high temperature (>25 °C) would cause significant phthalates migration compared to low temperature.
Phthalates
Background
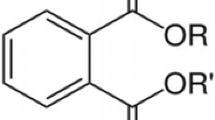
Phthalates, or commonly known as phthalic acid esters (PAEs), are derivatives of phthalic acid, the esters of 1,2-benzenedicarboxylic acid in which the acid groups are in the ortho-position (Gallart-Ayala et al. 2013; Muncke 2014; Van Holderbeke et al. 2014; Ventrice et al. 2013; Benson 2014; Cirillo et al. 2013). PAEs are synthetic organic chemicals that were introduced in the 1920s (Fasano et al. 2012). They were manufactured by reacting phthalic anhydride with various alcohols (Benson 2014) starting from methanol (MeOH) and ethanol (EtOH) for the smaller compounds, up to iso-decanol, straight chain or with some branching, producing a large variety of PAEs and thus providing a wide range of different properties for different possible uses (Moret et al. 2012). The physico-chemical characteristics of PAEs, and consequently their applications and uses, vary with the chemical structure of the side chains in which the PAEs can be classified into (1) low molecular weight PAEs with R and Rˈ side chains with up to six carbons and classified as very dangerous substances in Europe and in REACH (Registration, Evaluation, Authorization, and Restriction of Chemical substances) and (2) high molecular weight PAEs with side chains of more than six carbons but do not appear as substances that can cause problems to health (Moret et al. 2012; Ventrice et al. 2013). PAEs with shorter alkyl chain such as dimethyl phthalate, diethyl phthalate and dibutyl phthalate are commonly used in cosmetics and personal care products while longer branching alkyl chain such as butylbenzyl phthalate, dicyclohexyl phthalate, diethylhexyl phthalate and di-n-octyl phthalate are widely used as plasticizers (Cirillo et al. 2013). Figure 1 shows the general chemical structure of PAEs and the chemical structure, acronym, CAS number, molecular weight, boiling point, density and the uses of the most commonly used PAEs namely dimethyl phthalate, diethyl phthalate, diisobutyl phthalate, di-n-butyl phthalate, butyl benzyl phthalate, dicyclohexyl phthalate, di-n-octyl phthalate and di-(2-ethylhexyl) phthalate as listed in Table 2.
Metabolism of Phthalates
The metabolism and elimination of phthalates are rather complex, requiring three distinct steps, for example the metabolism of DEHP as illustrated in Fig. 2. The first step occurs at different parts of the body, for instance the mouth or skin, stomach, intestines or blood where the phthalate diester is cleaved into respective hydrolytic monoesters. In the second step, modification of the alkyl chain of the resulting hydrolytic monoester by various oxidation reaction takes place, in which the extent of the oxidative modification increases as the alkyl chain length of the phthalate monoester increases. This therefore decreases their water solubility as oxidative metabolites are more water soluble than the corresponding hydrolytic monoester. The low molecular weight phthalates are often metabolized to their hydrolytic monoesters (primary metabolites) whereas the high molecular weight phthalates of 8 or more carbons in the alkyl chain are metabolized to their hydrolytic monoesters, which are then transformed into oxidative products (secondary metabolites). Finally in the third step, conjugation of both the hydrolytic monoester and the oxidized secondary metabolites with glucuronic acid occur which are eventually excreted in urine (Yen et al. 2011; Koch and Calafat 2009).
Human Exposure to Phthalates
PAEs are primarily used as plasticizers and solvents as well as stabilizers for colour and fragrances (Duty et al. 2005a). They are also present in printing inks, lacquers, building materials (flooring, furniture and electric cables) (Ni et al. 2016; Butte and Heinzow 2002), paints, pesticides, baby toys, personal care and cosmetics (deodorants, perfumes and hair products), pharmaceutical products as well as medical devices (Fig. 3) (Du et al. 2016; Del Carlo et al. 2008; Shen 2005; Cinelli et al. 2013; Gómez-Hens and Aguilar-Caballos 2003; Sathyanarayana et al. 2008; Vera et al. 2011). Extensive industrial applications of PAEs in these many products have caused widespread exposure to human mainly through ingestion, inhalation, dermal contact (Cirillo et al. 2013; Adibi et al. 2003; Rudel et al. 2003) and medical devices (He et al. 2015; Zeman et al. 2013; Jeddi et al. 2015; Schecter et al. 2013; Swan 2008). It is possible to find PAEs in the environment (Rudel et al. 2003; Staples et al. 2008) as they are easily released into water, air and soil and are released slowly from worn down manufactures without PAEs being chemically bound in plastics or other products (Gómez-Hens and Aguilar-Caballos 2003). Although PAEs can be easily degraded in atmosphere by oxygen and UV radiation, they may persist for a long time in solution.
Ingestion
PAEs ingestion may occur through food, water and from the uses of their packaging particularly DEHP (Schettler 2006). In fact, the main source of PAEs exposure in the general population is from dietary intake (Cirillo et al. 2013; Fasano et al. 2012; He et al. 2015; Sioen et al. 2012; US Agency for Toxic Substances and Disease Registry 2012; Fromme et al. 2007; Wormuth et al. 2006). PAEs are mainly used as plasticizers due to their ability in increasing flexibility, workability and durability. However, Jeddi et al. (2015) and supported by Dewalque et al. (2014) have reported that children are more exposed than adults as they consume more food and water such as breast milk, infant formulas and plastic-packed food per unit body weight. In addition, they are also exposed by indoor dust and by sucking plastic teats, toys and mouthing contaminated hands and other objects (Sathyanarayana et al. 2008; Jeddi et al. 2015; Clark 2003; Calafat et al. 2004; Mortensen et al. 2005). Other than that, human are also exposed to PAEs by ingestion of dust from floor and carpet tile and products used in automotive interiors (Benson 2014).
Inhalation
Inhalation of PAEs can occur mainly from house dust and indoor air including inside automobiles where PAEs release from plasticized components can occur. However, PAEs exposure through dust and indoor air depends on the PAE sources such as building materials, PVC flooring and furnishing and PVC accessories. A study by Oie et al. (1997) reported a mean total PAE content of 960 μg/g of dust in 38 homes in Norway with DEHP as the main compound. Another study by Rudel et al. (2001) has reported a total PAEs concentration ranged from 0.3 to 524 μg/g dust and from 0.005 to 28 μg/m3 indoor air from 120 US homes with DEHP ranging from 20 to 114 ng/m3 and DBP ranging from 101 to 431 ng/m3. Hobbies such as clay modelling may also represent as a source of PAEs exposure by inhalation in which polymer clay is reported as a major source of air-dispersed PAEs. This material is softened by various PAEs and can be dispersed in air during the firing of the modelled clay. Another source for the PAEs inhalation is from the use of perfumes and hairsprays (Cirillo et al. 2013; Blount et al. 2000).
Dermal Contact
Direct contact with clothing, personal care products, synthetic modelling clay, cleaning products, insecticides and denture materials that contain PAEs may lead to absorption of PAEs through the skin. In terms of frequency of use, Blount et al. (2000) has considered personal care products such as cosmetics as the main exposure source for women. A study by Duty et al. (2005b) suggested that the main source of exposure for men was from using eau de cologne and aftershave. This is due to the uses of PAEs as lubricants in cosmetics and personal care products. In the case of infants, PAEs exposure is mostly from their mothers who use lotions, powders and shampoo as studied by Sathyanarayana et al. (2008).
Medical Devices
Medical devices have been shown to expose human to PAEs via ingestion mainly from the use of PVC bags which are softened by DEHP that are employed in enteral nutrition. The leaching of the plasticizer may be caused by the lipid content of the enteral/nutritional formulas (Cirillo et al. 2013). In addition, many drugs and medicines such as antibiotics, antihistamines, laxatives, herbal preparations and nutritional supplements are coated with synthetic polymers containing PAEs that can leach into the gastrointestinal tract during drug release and thus become an important source of PAEs ingestion (Hauser et al. 2004; Hernández-Díaz et al. 2009). Furthermore, exposure from medical devices such as intravenous exposure may also occur. DEHP which is often the main PAE is released from PVC devices that are normally employed for intravenous therapies such as transfusion of blood and blood products, extracorporeal membrane oxygenation and dialysis (Lee et al. 1999). As reported by Calafat et al. (2004) and Green et al. (2005), premature babies undergoing intensive medical care in neonatal intensive care units were found to be exposed to higher concentration of DEHP than adults.
Medical devices may also be a source of human exposure to PAEs by inhalation with regard to respiratory therapy. DEHP may transfer into respiratory gases passing through tubes that are made of PVC plasticized by PAEs (Cirillo et al. 2013).
Sources and Occurrence of PAEs in Food
Between 2009 and 2011, by the order of Belgian Federal Public Service of Health, Food Chain Safety and Environment, a Belgian research project PHTAL (acronym for phthalate) was conducted whose main objectives were to obtain data of phthalates in all kinds of food products and packaging materials sold in the Belgian market (Fierens et al. 2012), to understand possible contamination pathways of phthalates in the Belgian food market (Van Holderbeke et al. 2014) and to estimate dietary exposure to phthalates of the Belgian population (Sioen et al. 2012). Contamination of PAEs in food is found to be most likely due to their transfer from materials in contact with the food during processing, handling or transportation (Wormuth et al. 2006; Sakhi et al. 2014; Cao 2010). As the PAEs are not chemically bonded to polymers but remain present as a freely mobile and leachable phase, they can potentially leach and easily migrate (Ni et al. 2016; Moskovkin 2002) into food and beverages from the enclosing materials (Gómez-Hens and Aguilar-Caballos 2003). This has therefore made food as a major source of exposure of phthalates in humans (Fasano et al. 2012; Fromme et al. 2007; Wormuth et al. 2006; Clark et al. 2011; Rudel and Pevorich 2009). In addition to this, this project also found that PAEs were illegally substituted for food grade emulsifiers in formatting clouding agents that are meant to provide turbidity to selected food products, mainly beverages (Self and Wu 2012; Espachs-Barroso et al. 2005), and were also used to give a characteristic colour, flavour and mouth-feel in beverages (Jasentuliyana et al. 1998).
Toxicological Aspects and Human Health Effects
The toxicity of PAEs to human being has been reported over 20 years ago (Chronic Hazard Advisory Panel 1985; Ventrice et al. 2013; Moret et al. 2012; Ni et al. 2016; Martino-Andrade and Chahoud 2010; Okamoto et al. 2011) prompting concerns on the development of reproductive systems (He et al. 2015; Martino-Andrade and Chahoud 2010; Matsumoto et al. 2008; Kamrin 2009; Fisher 2004; Scholz 2004). PAEs have been categorized as a “chemical of concern” by the United States Environmental Protection Agency (EPA) (Cao et al. 2016; U.S. EPA 2012) and are classified by most countries as carcinogenic, mutagenic and toxic to reproductive health (Gallart-Ayala et al. 2013). PAEs are considered to be potential endocrine-disrupting chemicals (EDC) (European Union Risk Assessment Report 2003; Cariou et al. 2016; Chauvigné et al. 2009; Eveillard et al. 2009) which are compounds of known toxicity even at low concentrations that are able to mimic or block the action of natural hormones affecting the normal biology function in animals and humans and are able to interfere with androgen signalling and production (Cacho et al. 2012; Laws et al. 2000).
Exposure in male adults, mainly to DEHP, may cause alterations in pulmonary functions and sperm properties resulting in reduced sperm counts and mobility in such a way that it can cause seminiferous tubule atrophy, decreased testis weight, decreased sperm production and decreased testicular zinc level, in which testicular effects can lead to infertility (Ventrice et al. 2013; Foster et al. 1980; Li et al. 2012a). With regard to women, it was observed that the target of PAEs toxicity was in ovaries and in particular steroid hormone production (Ventrice et al. 2013).
Even though PAEs are rapidly hydrolysed into their corresponding monoesters and then metabolized and eventually excreted with urine and faeces (Itoh et al. 2005), they have been detected in serum, amniotic fluids and breast milk (Ghisari and Bonefeld-Jogersen 2009) and hence showing a negative relationship between high PAEs exposure and children’s intelligence and behaviour as reported by several epidemiological studies (Nelson 1991; Ventrice et al. 2013; Cho et al. 2010). Human exposure to PAEs (mainly DEHP) can begin in utero, resulting in a shorter pregnancy duration (Yen et al. 2011; Latini et al. 2003). In addition to this, from the recent epidemiological studies, PAEs exposure have also been associated with shorter gestational age (Whyatt et al. 2009; Adibi et al. 2009), shorter anogenital distance (Suzuki et al. 2011; Marsee et al. 2006), precocious puberty (Lomenick et al. 2010), pubertal gynecomastia (Durmax et al. 2010), premature thelarche (McKee 2004), low birth weight (Zhang et al. 2009), attention deficit hyperactivity disorder (Kim et al. 2009; Engel et al. 2010), low intelligence quotient (Cho et al. 2010), thyroid dysfunction and growth retardation (Boas et al. 2010) and hypospadias (Ormond et al. 2009) in infants and children.
As mentioned above, PAEs are considered to be carcinogenic in which they are responsible in causing cancer due to the peroxisome-proliferator-activated-receptor-α (PPARα) activated by the ability of PAE monoesters that increases as the chain length increases (Cirillo et al. 2013; Bility et al. 2004). Studies in rodents have shown that PAEs can cause hepatic cancer, liver tumours, testicular Leydig cell and pancreatic acinar cell tumours (Ventrice et al. 2013; Cirillo et al. 2013).
Other health effects caused by PAEs exposure also include airway remodelling causing asthma, allergies (Ventrice et al. 2013; Jaakkola et al. 1999; Jaakkola and Knight 2008) and respiratory symptom (Cirillo et al. 2013; Polakoff et al. 1975; Falk and Portnoy 1976; Brooks and Vandervort 1977; Eisen et al. 1985; Markowitz et al. 1989; Nielsen et al. 2007) obesity and diabetes due to low testosterone level (Ding et al. 2006; Selvin et al. 2007) and autism spectrum disorders (ASDs) (Weintraub 2011) as PAEs can also interfere with neurological development. Several animal studies have also revealed that their effect on the dopamine system in the central nervous system in which a low dose of PAEs can impair tyrosine hydroxylase immunoreactivity (Ishido et al. 2004) causing the loss of mid-brain dopaminergic neurons and thus decreasing tyrosine hydroxylase biosynthetic activity (Tanida et al. 2009).
Analysis Approach
It is particularly difficult to perform an effective measurement of PAEs content in food as food samples can be easily contaminated within laboratory environment and activities as glassware, solvents and reagents used may contain traces of PAEs. Hence, analysis of PAEs contamination in/from food packaging migrating into food products represents a challenging task that will necessitate suitable precautions to avoid any contamination. Due to the complexity of matrices and low concentration levels expected in samples, assessment of PAEs contamination would thus require efficient pre-concentration and clean-up procedures to ensure the quality of analytical determination results. Other typical steps required in the analysis procedures for food sample preparation are sampling, homogenization and extraction (Cirillo et al. 2013).
Sample preparation is a more or less complex procedure in accordance to the characteristics of the food matrix. In parallel to assessing the migration of chemicals from packaging into food, determining the migration of chemicals from packaging into food simulants (extractant used as food substitutes for analysis; solvents, oil or polymeric resin that can be used to mimic chemical properties of food to simplify the chemical analysis of migrants from food contact materials) may also be of interest as they represent a different group of food. Food simulants vary depending on their chemical properties as shown in Table 3 in which they may migrate variably depending on migration test conditions such as temperature and period of exposure. However, chemical migration into actual food is expected to be lower than the migration into food simulants; hence, food simulants are believed to overestimate the real migration. In contrast, all food simulants can be used for overall migration testing such as in assessing a mixture of chemicals that can migrate from the entire packaging into food. The use of distilled water as food simulant is also common for this purpose (unspecific analysis) (Muncke 2014).
Blank Problems
Due to the widespread use of products containing PAEs, PAEs have become ubiquitous environmental contaminants. They therefore have become the main cause of blank problems as well as increasing the risk of secondary contamination that may occur during sampling, sample preparation, extraction and/or instrumental analysis and therefore leading to overestimated contamination levels. As PAEs are commonly present in laboratory environment, Frankhauser-Noti and Grob (2007) reported the presence of DBP and DEHP in the laboratory air to be 3 and 2.4 μg/m3 respectively; in organic solvents and chemicals for instance 100 μg/L of DBP and DEHP were found in commercially available hexane (Grob et al. 2006), which are adsorbed on glassware and other devices used for the analysis. PAEs are also present in materials commonly used in laboratory activities such as tubing, caps, stoppers, glass wool, filter paper or fibres, cartridges and stir bar used in specific sample preparations. In addition, a 1.5-mL autosampler vial was also estimated to contain 10 and 4 ng of DBP and DEHP, respectively (Frankhauser-Noti and Grob 2007).
It is best to keep PAEs analysis to be as quick and as simple as possible by keeping the sample preparation to minimum, with minimal extraction steps and pre-concentration of the extracts, which can be done by minimizing the use of solvents and chemicals, glassware and the exposure of sample in air. In order to reduce the primitive contamination in solvents, redistilling the solvents can be done even though Frankhauser-Noti and Grob reported redistilling solvent is not efficient enough and is not always possible in routine analysis laboratories as contamination during and after distillation is still possible. However, they reported that the best solution was to perform a dispersive solid-phase extraction (SPE) in distilled solvent such as adding active aluminium oxide to reservoir which is able to absorb the presence of all polar materials in the solvent, taking into account the amount of aluminium oxide added and the time of shaking to allow aluminium oxide to absorb PAEs present in the solvent. However, this alternative is only applicable for organic solvents and not in more polar solvents as PAEs would be extracted from aluminium oxide instead of being purified. The solvent bottles then should be closed after use to avoid contact with air.
With regard to the glassware used, removal of more than 90% of DBP and DEHP (Frankhauser-Noti and Grob 2007) can be achieved by solvent rinsing followed by heating at 400 °C for 1–2 h (David et al. 2003) or heated in the oven at 400 °C for several hours or overnight and then kept in a desiccator containing aluminium oxide or covered with aluminium foil to avoid adsorption of PAEs from the air. For materials that cannot be cleaned by heating, they should be rinsed with purified solvent drawn from a bottle containing aluminium oxide. In conclusion, it is best not to expose any solvents and materials used to the air during the preparation, extraction steps until the end of the analysis determination (Moret et al. 2012).
Water and Beverages
In general, PAEs from non-fatty liquid samples such as water, beverages and alcoholic solution can be extracted commonly by liquid-liquid extraction (LLE) in which the sample is mixed with organic solvents with higher affinity for PAEs in order to change the equilibrium in favour of the organic solvent with no additional clean-up required. Different extraction solvents can be used as proposed by several researches for instance dichloromethane (CH2Cl2) (Shelton et al. 1984; Bošnir et al. 2007; Fierens et al. 2012; He et al. 2015; Paz Otero et al. 2015), n-hexane (Holadová and Hajŝlova 1995), cyclohexane (Tienpoint et al. 2005), diethyl ether (Ejlertsson and Svensson 1995) and ethyl acetate (Jonsson and Borén 2002). However, in comparison with conventional LLE (Ostrovský et al. 2011), Rezaee et al. (2006) has proposed using dispersive liquid-liquid microextraction (DLLME) of better efficiency, simplicity and rapidity in 2006. Later on in 2011, ultrasound DLLME using carbon tetrachloride (CCl4) as extractant was applied to extract six PAEs in bottled milks. In 2013, ultrasound-vortex-assisted extraction was established by Cinelli et al. (2013) to extract PAEs in wine. Despite the advantages of DLLME, its main drawback is the use of chloro-containing organic extractants that could lead to environmental pollution. Therefore, recyclable ionic liquids (salt in liquid state for example sodium chloride), which are non-volatile and non-toxic were used as green extractants and were combined with DLLME to extract PAEs from alcoholic beverages (Fan et al. 2014). However ionic liquids are unstable and tend to decompose when in touch with metallic catalysts. In addition a few toxic solvents and extremely complex purification process are required to synthesize an ionic liquid and thus leading to high cost limiting their wide application (Yang et al. 2015). In 2016, Pérez-Outerial et al. determined PAEs in liquid samples by UA-DLLME (ultrasound-assisted-dispersive liquid-liquid microextraction) followed by solidification of floating organic drop by using n-hexadecane as extracting solvent.
Other than LLE, solid-phase extraction (SPE) is also a commonly used method where SPE columns use polar stationary phase such as C18 (Khedr 2013), C8, polystyrene, XAD-2 adsorbents (Cinelli et al. 2014) and multiwall carbon nanotubes (MWCNTs) (Casajuana and Lacorte 2003; Cai et al. 2003; Mohamed and Ammar 2008; Del Carlo et al. 2008) to selectively adsorb PAEs while polar compounds that are not of interest are eluted and separated from analytes of interest. Even though this technique allows the use of solvents to be reduced and hence improving extraction efficiency and yielding more purified extracts, it is time consuming and it often requires extensive sample handling and treatment of sample prior to analysis, which then lead to high blank values. In addition to the conventional SPE, magnetic solid-phase extraction (MSPE) using magnetic nanosorbents incorporating multiwall carbon nanotubes (MWCNTs) (Guan et al. 2010), single-walled carbon nanotubes (SWCNTs) (Rastkari et al. 2010) and graphene (Wu et al. 2011) have been employed. In 2011, analysis of PAEs in water was performed using a newly synthesized polypyrrole-coated Fe3O4 magnetic microsphere (Meng et al. 2011) and later on in 2013, it was applied to analysis of PAEs in soybean milk (Wang et al. 2013). In the same year, Tahmasebi et al. (2013) has successfully synthesized a novel type of polythiophene-coated Fe3O4 supermagnetic nanosorbent as a new sorbent for SPE in analysis of PAEs in water.
In contrast to SPE, solid-phase microextraction (SPME) is more efficient, simple and solvent-free and does not require any prior sample preparation. The conventional SPME device looks like a syringe composed of fused silica fibre coated with a thin layer of sorbents which plays an important role in its high selectivity and fixed with a needle that usually employs a miniature automatic device to integrate sampling, extraction, purification, concentration and injection in one procedure (Moret et al. 2012). This technique permits simpler sample preparation and reduce the risk of secondary contamination. The extraction of target analytes from liquid samples can be performed either by direct immersion SPME (DI-SPME) or headspace SPME (HS-SPME) in which both techniques were used in several studies for determination of water since late 1990s (Peñalver et al. 2000, 2001; Luks-Betlej et al. 2001; Polo et al. 2005; Montuori et al. 2008; Cao 2008). In recent years, Carillo et al. (2007, 2008) have developed a method based on HS-SPME to extract PAEs from wine samples using PDMS/DVB (polydimethylsiloxane/divinylbenzene) fibre. However, the fibres showed tendency to break and are relatively expensive.
Another approach of analysing liquid samples is stir bar sorptive extraction (SBSE) in which PDMS is also used as a coating for the stir bar, which is usually immersed in sample solution to extract the target analytes and then thermally desorbed for separation and detection. This method allows a higher performance resulting in higher sample capacity and recovery, thus giving better sensitivity than LLE and SPE. In the case of liquid samples, no clean-up procedure is necessary when using this approach.
Oils and Fatty Extracts
When extracting PAEs from fatty matrices, it is crucial to apply clean-up procedures due to the co-extraction of the lipid components mostly represented by triacylglycerols. The two commonly clean-up procedures applied are liquid-liquid partition with acetonitrile (ACN) (Sørensen 2006) and gel permeation chromatography (GPC) both of which involve liquid-liquid partition to remove fats and oils in fatty extracts that is often performed by size exclusion chromatography (SEC), where extracts are injected onto a column packed with Biobeads SX3 or PLgel and eluted with CH2Cl2/cyclohexane (Tsumura et al. 2001; Castle et al. 1988, 1990) or ethyl acetate/cyclohexane (Blüthgen and Heeschen 1998; Petersen and Breindahl 2000) or pentane/methyl tert-butyl ether (MTBE) (Hogberg et al. 2008). In addition to this, the column may be packed with Florisil, silica gel or other phases to perform the clean-up.
With regard to liquid fatty matrices, extraction methods will be briefly described in the solid foods section below. In the case of vegetable oils, PAEs determination can be analysed by conventional LLE technique usually performed by using ACN followed by clean-up using different SPE phase such as silica or Florisil. Treatment with aluminium oxide prior to clean-up step was proposed by Mariani et al. (2006) to remove co-extracted free fatty acids that may cause interference in the chromatographic analysis.
New approach for the extraction of PAEs from virgin oil samples has been developed in López-Feria et al. 2009 by López-Feria et al. using surfactant-coated carbon nanotubes as extractant where the phase containing the extract analytes is transferred into a headspace vial and added with sodium chloride to facilitate the release of the target analytes to the headspace. Other more recent approaches to further simplify sample preparation are SPME and the analysis with PTV injection system (programmed temperature vaporizer). Frankhauser-Noti and Grob (2006) have described PTV injection technique as a very useful method as it allows direct injection of a diluted oily solution without prior extraction and clean-up steps in which the injector is kept below the solvent evaporation temperature during the injection of sample and is then rapidly heated. The analytes present in the sample are subsequently evaporated as characterized by different volatilities and then compounds of interest are transferred into the separation column leaving high-boiling components in the inlet to avoid their entrance in the analytical column. A system known as backflush system will then allow the cleaning of the pre-column and the inlet. Although PTV injector and the convention split/splitless injector are rather similar in injecting the sample into a liner place inside the volatilizing chamber where it is evaporated, the difference between the two is that the PTV injector can be rapidly heated and cooled during the injection and analysis, whereas the conventional split/splitless injector only works in isothermal conditions. Therefore, the PTV injection technique is able to simplify sample preparation and eliminate problems of secondary contamination.
Solid Foods
The commonly used method of extraction of PAEs from non-fatty solid foods after homogenization such as fruits and vegetables is LLE by direct extraction with ACN or mixtures of ACN and water in which some cases need to be followed up by a further extraction with 1:1 mixtures of n-hexane/CH2Cl2 or cyclohexane/CH2Cl2 (Page and Lacroix 1995; Lau and Wong 1996). A less common technique of Soxhlet liquid extraction (SLE) is also used which has been proposed by Sablayrolles et al. (2005) where frozen, lyophilized and ground samples are extracted with n-hexane. The extract can then be purified using a Florisil SPE cartridge after concentration and finally target compounds are eluted by a 9:1 mixture of n-hexane/acetone.
In the case of fatty solid foods such as dairy products, meat products, chocolates and retail products, it is necessary to extract the lipid fraction first as PAEs may be co-extracted with it. This is then followed by LLE with a mixture of solvents such as of acetone/n-hexane as proposed by Page and Lacroix (1995), Castle et al. (1988) and MAFF (1996a, b, 1998), MeOH/n-hexane by Sharman et al. (1994) and Castle et al. (1990), n-hexane/CH2Cl2 by Yano et al. (2002), MeOH/n-hexane/MTBE by Sørensen (2006), pentane/acetone/n-hexane/MTBE by Hogberg et al. (2008), ACN/n-hexane by Page and Lacroix (1995), Tsumura et al. (2001, 2002, 2003) and Yano et al. (2005) or with singly solvent such as n-hexane as proposed by Guo et al. (2010) and Jarošová (2006), CH2Cl2 by Page and Lacroix (1995), pentane by Petersen and Breindahl (2000) and ACN by Cariou et al. (2016).
In addition to the LLE step, Guo et al. (2010) proposed the addition of aluminium oxide and sodium chloride solution to decrease interference from proteins, fats and other components whereas Tsumura et al. (2001, 2002, 2003) has proposed the addition of sodium chloride to eliminate water for PAEs analysis in fresh foods. On the other hand, Page and Lacroix (1995), Yano et al. (2002) and Petersen and Breindahl (2000) proposed treatment with potassium hydroxide, potassium oxalate or other destabilizing agents to damage the phospholipid-protein membrane of the fat globules in PAEs analysis of cheese, milk, infant foods and other dairy products. Likewise, sodium sulphate is also used to remove water from the extract followed by evaporation to concentrate the sample extract under nitrogen flow and finally redissolving the residue with various solvents. PAEs analysis in fatty extracts should undergo clean-up procedures such as gel permeation chromatography (GPC) (Fierens et al. 2012) and gas-purge microsyringe extraction (GP-MSE) (He et al. 2015). Other extraction methods include the use stir bar sorptive extractive (SBSE) (Cacho et al. 2012), direct analysis in real time–standardized voltage and pressure (DART-SVP) (Self and Wu 2012) and ultrasonic extraction (UE) (He et al. 2015; Cacho et al. 2012).
Packaging Materials
In the analysis of PAEs determination in food packaging materials, it is necessary to apply migration tests using food simulants and standardized migration test conditions depending on the type of food. Fasano et al. (2012) has performed two extraction methods to analyse PAEs which are incubation for 10 days at 40 °C and ultrasonic extraction in which the food simulants are extracted by SPE. In the same year, Cacho et al. (2012) has proposed a method in determining PAEs in vegetables and migration studies from their packages by SBSE with prior extraction with ethanol.
Another method for PAEs analysis from packaging is ultrasonic method with n-hexane as the extracting solvent as performed by Fierens et al. (2012) and later on by Van Holderbeke et al. (2014). However, Van Holderbeke has employed modifications from the original method by Fierens which include adding a step where exchanging the sample extracts was done with CH2Cl2 followed by purification with GPC.
Nevertheless, a direct analysis of the sample extracts from paper and board packaging was performed by Nerin et al. (2002) with supercritical fluid extraction (SFE) using ethanol that did not require pre-treatment of the samples. In addition, Soxhlet extraction using hexane is also applied to analyse PAEs in polymer-coated sample cup. A more recent approach of PAE determination is the development of magnetic dummy molecularly imprinted dispersive solid-phase extraction (MAG-MIM-dSPE) (Qiao et al. 2014) for selective determination of PAEs in plastic bottled beverages using DINP (diisononyl phthalate) as a template mimic resulting in a successful analysis of 5 PAEs.
Other Extraction Methods
In 2012, dispersive SPE (d-SPE) approach was performed for clean-up of 17 PAEs in fatty food after being extracted with organic solvents (Li et al. 2012b). In 2013, 15 PAEs were analysed in vegetable juices by using hollow fibre-liquid phase microextraction (HF-LPME) (Zhu et al. 2013). Later on, a new extraction method based on membrane filtration-enrichment using nylon membrane as solid-phase support was proposed by Chen et al. (2014).
As proposed by Anastassiades et al. (2003), a method known as QuEChERs (quick, easy, cheap, effective, rugged and safe) was first used to extract pesticides from foods in which the procedures include homogenization of sample, extraction with ACN, dehydration with magnesium sulphate followed by removal of impurity with primary secondary amine (PSA) and finally analysis using GC-MS or LC-MS. Later on in 2014, QuEChERs was successfully applied in extracting 23 PAEs from grape jelly, seasoning powder, egg noodles and grapefruit sauce (Xu et al. 2014, Yang et al. 2015).
Instrumental Determination
The most common quantitative methods used for PAEs determination are mainly gas chromatography (GC) and liquid chromatography (LC). PAEs have low molecular weight, relatively low polarity, thermally stable and sufficiently volatile to be analysed by GC methods. GC-MS equipped with a DB-5MS column coated with 5% phenyl-95% dimethylpolysiloxane carried out in selected ion monitoring (SIM) mode is one of the most widely used techniques for the analysis (Casajuana and Lacorte 2003; Blüthgen and Heeschen 1998; Petersen and Breindahl 2000; Frankhauser-Noti and Grob 2006; Yang et al. 2015; Gärtner et al. 2009). Even though low detection limit is strongly influenced by the secondary contamination problems, GC techniques however can allow low detection limits to be achieved especially by splitless injection. Other than GC-MS, electron ionization (EI)-MS, chemical ionization (CI)-MS using methane as the reagent gas in either positive or negative mode, GC-MS/MS under positive chemical ionization using isobutene as reagent gas and gas chromatography-flame ionization detector (GC-FID) have also been used for identification and quantification of PAEs in food samples (Moret et al. 2012).
LC such as HPLC using C18-columns running either in isocratic or gradient elution have also been widely used for the determination of PAEs in food samples due to its ability in analysing thermally-unstable and non-volatile organic chemicals (Moret et al. 2012; Yang et al. 2015). The recoveries (R), relative standard deviations (RSD), limit of detections (LOD) and limit of quantifications (LOQ) may vary when using different extraction and instrumental analysis which are summarized in Table 4 with the analytes stated only focusing on the most common PAEs. However, even though the analysis depends on the pre-treatment step, instrumental conditions and the sample matrix in which they are obtained, several studies had concluded that GC methods are able to obtain better LODs than HPLC methods (Moret et al. 2012; Bošnir et al. 2007; Ostrovský et al. 2011; Kozyrod and Ziaziaris 1989; Petersen 1991; Prokůpková et al. 2002).
Comparison of Sample Preparation Methods
Based on the literature review, the advantages and drawbacks of various sample preparation/extraction methods of PAEs analysis in food/beverages and food packaging materials are presented in Table 5. In the authors’ opinion, QuEChERS is an interesting method as its simplicity in sample preparation and extraction with the use of low quantities of organic solvent, low cost, as well as requiring only a short amount of time has gained particular interest from researchers. This method is highly efficient in detecting target compound where it has successfully extracted 23 PAEs from food samples when paired with HPLC-MS/MS. A recent study performed by Dong et al. (2017) using the QuEChERS-GC/MS method was able to analyse 14 PAEs from wheat samples; with satisfactory recoveries between 84.8–120.3% and RSD of 0.6–9.0% for intra-day and inter-day precision, respectively, whereas the LOD ranged from 0.1–2.5 μg/kg.
On the other hand, 3 different extraction methods as performed by Fierens et al. in 2012 has analysed as many as 400 food samples of various matrices. High-fat and low-fat food were extracted with acetone/n-hexane mixture followed by centrifugation and a clean-up by gel permeation chromatography, while a liquid-liquid extraction with dichloromethane was used for aqueous-based beverages and in the case of food packaging materials, ultrasonic extraction with n-hexane was carried out. This study has successfully analysed 8 PAEs with LOD levels in the range of 0.003–0.3 ppb, recoveries between 82 and 104% and RSD of below 14%.
In 2012, Guo et al. also did a study on analysing 9 PAEs from 78 various food samples which were sorted into 3 different matrices for extraction such as liquid samples, solid food sample and cooking oil. Although this study has only performed extraction simply by liquid extraction and a liquid-liquid partition clean-up in the case of solid food samples and cooking oil, it was able to achieve detection limit down to 1 ppt level.
Conclusion
The widespread use of products containing PAEs has caused growing concerns on their effects on human health; this has prompted researches in the development of sample preparation and analytical methods for the determination of PAEs in the last two decades. This review shows that there has been an increase in the number of PAEs being analysed in increasing types of food matrices especially in the last 5 years. Methods of sample preparation have progressed towards the use of green extractants as well as minimizing the use of organic solvent that could lead to environmental pollution. GC and HPLC with various detectors such as MS, FID and ECD are still widely used as the preferred instruments for the PAEs analysis with the levels of LOD improved to as low as 1 ppt level.
References
Adibi JJ, Perera FP, Jedrychowski W, Camann DE, Barr D, Jacek R, Whyatt RM (2003) Prenatal exposures to phthalates among women in New York City and Krakow, Poland. Environ Health Perspect 111(14):1719–1722
Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H et al (2009) Maternal urinary metabolites of di-(2-ethylhexyl) phthalate in relation to the timing of labor in a US multicentre pregnancy cohort study. Am J Epidemiol 169(8):1015–1024
Amanzadeh H, Yamini Y, Moradi M, Asl YA (2016) Determination of phthalate esters in drinking water and edible vegetable oil samples by headspace solid phase microextraction using graphene/polyvinylchloride nanocomposite coated fiber coupled to gas chromatography-flame ionization detector. J Chromatogr A 1465:38–46
Amiridou D, Voutsa D (2011) Alkylphenols and phthalates in bottled waters. J Hazard Mater 185(1):281–286
Anastassiades M, Lehotay SJ, Štajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/portioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431
Api AM (2001) Toxicological profile of diethyl phthalate: a vehicle for fragrance and cosmetic ingredients. Food Chemistry Toxicology 39:97–108
Benson RW (2014) Phthalates. Encyclopedia of Food Safety 2:438–443
Bility MT, Thompson JT, McKee RH, David RM, Butala JH, Heuvel JPV, Peters JM (2004) Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol Sci 82(1):170–182
Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ et al (2000) Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect 108(10):979–982
Blüthgen A, Heeschen WH (1998) Secretory and migratory contamination of cow milk with plasticizers, especially phthalic acid esters. Bulletin of the International Daily Federation 330:6–11
Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebӕk NE, Hegedüs L, Hilsted L et al (2010) Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect 118(10):1458–1464
Bonini M, Errani E, Zerbinati G, Ferri E, Girotti S (2008) Extraction and gas chromatographic evaluation of plasticizers content in food packaging films. Microchem J 90(1):31–36
Bošnir J, Puntarić D, Galić A, Škes I, Dijanić T, Klarić M et al (2007) Migration of phthalates from plastic containers into soft drinks and mineral water. Food Technol Biotechnol 45(1):91–95
Brooks SM, Vandervort R (1977) Polyvinyl chloride film thermal decomposition products as an occupational illness. 2. Clinical studies. J Occup Environ Med 19(3):192–196
Bueno-Ferrer C, Garrigós MC, Jiménez A (2010) Characterization and thermal stability of poly (vinyl chloride) plasticized with epoxidized soybean oil for food packaging. Polym Degrad Stab 95(11):2207–2212
Butte W, Heinzow B (2002) Pollutants in house dust as indicators of indoor contamination. Reviews in Environmental Contamination and Toxicology 175:1–46
Cacho JI, Campillo N, Viñas P, Hernández-Córdoba M (2012) Determination of alkylphenols and phthalate esters in vegetables and migration studies from their packages by means of stir bar sorptive extraction coupled to gas-chromatography-mass spectrometry. J Chromatogr A 1241:21–27
Cai YQ, Jiang GB, Liu JF, Zhou QX (2003) Multi-walled carbon nanotubes packed cartridge for the solid-phase extraction of several phthalate esters from water samples and their determination by high performance liquid chromatography. Analytica Chemica Acta 494(1):149–156
Calafat AM, Needhalm L, Silva MJ, Lambert G (2004) Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics 113(5):429–434
Cao XL (2008) Determination of phthalates and adipate in bottled water by headspace solid-phase microextraction and gas chromatography/mass spectrometry. J Chromatogr A 1178(1–2):231–238
Cao XL (2010) Phthalate esters in foods: sources occurrence and analytical methods. Compr Rev Food Sci Food Saf 9(1):21–43
Cao Y, Liu J, Liu Y, Wang J, Hao X (2016) An integrated exposure assessment of phthalates for the general population in China based on both exposure scenario and biomonitoring estimation approaches. Regul Toxicol Pharmacol 74:34–41
Carillo JD, Salazar C, Moreta C, Tena MT (2007) Determination of phthalates in wine by headspace solid-phase microextraction followed by gas chromatography-mass spectrometry: fibre comparison and selection. J Chromatogr A 1164(1):248–261
Carillo JD, Martinez MP, Tena MT (2008) Determination of phthalates in wine by headspace solid-phase microextraction followed by gas chromatography-mass spectrometry use of deuterated phthalates as internal standards. J Chromatogr A 1181:125–130
Cariou R, Larvor F, Monteau F, Marchand P, Bichon E, Dervilly-Pinel G et al (2016) Measurement of phthalate diesters in food using gas-chromatography-tandem mass spectrometry. Food Chem 196:211–219
Casajuana N, Lacorte S (2003) Presence and release of phthalic esters and other endocrine disrupting compounds in drinking water. Chromatographia 57(9–10):649–655
Casajuana N, Lacorte S (2004) New methodology for the determination of phthalate esters, bisphenol A, bisphenol A diglycidyl ether, and nonylphenol in commercial whole milk samples. J Agr Food Chem 52(12):3702–3707
Castle L, Mercer AJ, Startin JR, Gilbert J (1988) Migration from plasticized films into foods. III. Migration of phthalate, sebacate, citrate and phosphate esters from films used for retail food packaging. Food Additives & Contaminants 5(1):9–20
Castle L, Mayo A, Gilbert J (1989) Migration of plasticizers from printing inks into foods. Food Additives & Contaminants 6(4):437–443
Castle L, Gilbert J, Eklund T (1990) Migration of plasticizer from poly (vinylchloride) milk tubing. Food Additives & Contaminants 7(5):591–596
Chauvigné F, Menuet A, Lesne L, Chagnon MC, Chevrier C, Regnier J et al (2009) Time- and dose-related effects of di-(2-ethylhexyl) phthalate and its main metabolites on the function of the rat fetal testis in vitro. Environ Health Perspect 117(4):515–521
Chen G, Hu H, Wu T, Tong P, Liu B, Zhu B, Du Y (2014) Rapid and sensitive determination of plasticizer diethylhexyl phthalate in drink by diffuse reflectance UV spectroscopy coupled with membrane filtration. Food Control 35(1):218–222
Cho SC, Bhang SY, Hong YC, Shin MS, Kim BN, Kim JW et al (2010) Relationship between environmental phthalate exposure and the intelligence of school-age children. Environ Health Perspect 118:1027–1032
Cinelli G, Avino P, Notardonato I, Centola A, Russo MV (2013) Rapid analysis of six phthalate esters in wine by ultrasound-vortex-assisted dispersive liquid–liquid micro-extraction coupled with gas chromatography-flame ionization detector or gas chromatography–ion trap mass spectrometry. Anal Chim Acta 769:72–78
Cinelli G, Avino P, Notardonato I, Centola A, Russo MV (2014) Study of XAD-2 adsorbent for the enrichment of trace levels of phthalate esters in hydroalcoholic food beverages and analysis by gas chromatography coupled with flame ionization and ion-trap mass spectrometry detectors. Food Chem 146:181–187
Cirillo T and Cocchieri RA (2013) Phthalates in foods. In: Rose M and Fernandes A (ed) Persistent Organic Pollutants and Toxic Metals in Foods. Woodhead Publishing Limited, pp 334–366
Clark K (2003) Assessment of critical exposure pathways. In Series anthropogenic compounds. Springer Berlin, Heidelberg, pp 227–262
Clark KE, David RM, Guinn R, Kramarz KW, Lampi MA, Staples CA (2011) Modelling human exposure to phthalate esters: a comparison of indirect and biomonitoring estimation methods. Human and Ecological Risk Assessment: An International Journal 17(4):923–965
David F, Sandra P, Tienpoint B, Vanwalleghem F, Ikonomou M (2003) In Phthalate esters: the handbook of environmental chemistry. Springer editor, Berlin. 3(Q), pp 26–27
Del Carlo M, Pepe A, Sacchetti G, Compagnone D, Mastrocola D, Cichelli A (2008) Determination of phthalate esters in wine using solid-phase extraction and gas chromatography-mass spectrometry. Food Chem 111(3):771–777
Dewalque L, Charlier C, Pirard C (2014) Estimated daily intake and cumulative risk assessment of phthalate diesters in Belgian general population. Toxicol Lett 231(2):161–168
Ding EL, Song Y, Malik VS, Liu S (2006) Sex differences of endogenous sex hormones and risk of 2 types diabetes: a systematic review and meta-analysis. Journal of American Medical Association 295(11):1288–1299
Dong W, Sun B, Sun J, Zheng F, Sun X, Huang M, Li H (2017) Matrix effects in detection of phthalate esters from wheat by a modified QuEChERS method with GC/MS. Food Anal Methods 1–15. doi:10.1007/s12161-017-0892-4
Du L, Ma L, Qiao Y, Lu Y, Xiao D (2016) Determination of phthalate esters in teas and tea infusions by gas chromatography-mass spectrometry. Food Chem 197:1200–1206
Durmax E, Ozmert EN, Erkekoglu P, Giray B, Derman O, Hincal F, Yurdakok K (2010) Plasma phthalate levels in pubertal gynecomastia. Pediatrics 125(1):122–129
Duty SM, Ackerman RM, Calafat AM, Hauser R (2005a) Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect 113:1530–1535
Duty SM, Calafat AM, Silva MJ, Ryan L, Hauser R (2005b) Phthalate exposure and reproductive hormones in adult men. Hum Reprod 20(3):604–610
Eisen EA, Wegman DH, Smith TJ (1985) Across-shift changes in the pulmonary function of meat-wrappers and other workers in the retail food industry. Scand J Work Environ Health 11(1):21–26
Ejlertsson J, Svensson BH (1995) A review of the possible degradation of polyvinyl chloride (PVC) plastics and its components phthalic acid esters and vinyl chloride under anaerobic conditions prevailing in landfills. Department of Water and Environmental Studies, Linkoping University, Sweden 20pp
Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, Wolff MS (2010) Prenatal phthalates exposure is associate with childhood behaviour, and executive functioning. Environ Health Perspect 118(4):565–571
Espachs-Barroso A, Soliva-Fortuny RC, Martin-Belloso O (2005) A natural clouding agent from orange peels obtained using polygalacturonase and cellulose. Food Chem 92(1):55–61
European Union Risk Assessment Report. Dibutyl phthalates. European Communities. Luxemborg. 2003 (http:/www.dbp-facts.com/upload/documents/document30.pdf)
Eveillard A, Mselli-Lakhal L, Mogha A, Laserre F, Polizzi A, Pascussi J et al (2009) Di-(2-ethylhexyl)-phthalate (DEHP) activates the constitutive androstane receptor (CAR): a novel signalling pathway sensitive to phthalates. Biochem Pharmacol 77(11):1735–1746
Falk H, Portnoy B (1976) Respiratory tract illness in meat packers. Journal of American Medical Association 235(9):915–917
Fan Y, Liu SH, Xie Q (2014) Rapid determination of phthalate esters in alcoholic beverages by conventional ionic liquid dispersive liquid-liquid microextraction couple with high performance liquid chromatography. Talanta 119:291–298
Farajzadeh MA, Mogaddam MRA (2012) Air-assisted liquid–liquid microextraction method as a novel microextraction technique; application in extraction and preconcentration of phthalate esters in aqueous sample followed by gas chromatography–flame ionization detection. Anal Chim Acta 728:31–38
Fasano E, Bono-Blay F, Cirillo T, Montuori P, Lacorte S (2012) Migration of phthalates, alkylphenols, bisphenol A and di (2-ethylhexyl) adipate from food packaging. Food Control 27:132–138
Fierens T, Servaes M, Van Holderbeke M, Geerts L, De Henauw S, Sioen I, Vanermen G (2012) Analysis of phthalates in food products and packaging materials sold in Belgian market. Food Chem Toxicol 50(7):2575–2583
Fisher JS (2004) Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction 127(3):305–315
Food Safety Directorate, Ministry of Agriculture, Fisheries and Food (MAFF). Phthalates in food. Food Surveillance Information Sheet no. 82, 1996a
Food Safety Directorate, Ministry of Agriculture, Fisheries and Food (MAFF). Phthalates in infant formulas. Food Surveillance Information Sheet no. 83, 1996b
Food Safety Directorate, Ministry of Agriculture, Fisheries and Food (MAFF). Phthalates in infant formulae-follow-up survey. Food Surveillance Information Sheet no. 168, 1998
Foster PM, Thomas LV, Cook MW, Gangolli SD (1980) Study of the testicular effects and changes in zinc excretion produced by some n-alkyl phthalates in the rat. Toxicol Appl Pharmacol 54:392–398
Frankhauser-Noti A, Grob K (2006) Injector-internal thermal desorption from edible oils performed by programmed temperature vaporizing (PTV) injection. J Sep Sci 29(15):2365–2374
Frankhauser-Noti A, Grob K (2007) Blank problems in trace analysis of diethylhexyl and dibutyl phthalate: investigation of the sources, tips and tricks. Analytica Chemica Acta 582(2):353–360
Fromme H, Gruber L, Schlummer M, Wolz G, Boehmer S, Angerer J et al (2007) Intake of phthalates and di (2-ethylhexyl) adipate: result of the integrated exposure assessment survey based on duplicate diet samples and biomonitoring data. Environ Int 33(8):1012–1020
Gallart-Ayala H, Núñez O, Lucci P (2013) Recent advances in LC-MS analysis of food-packaging contaminants. Trends Anal Chem 42:99–124
Gärtner S, Balski M, Koch M, Nehls I (2009) Analysis and migration of phthalates in infant food packed in recycled paperboard. Journal of Agricultural Food Chemistry 57(22):10675–10681
Ghisari M, Bonefeld-Jogersen EC (2009) Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett 189(1):67–77
Gómez-Hens A, Aguilar-Caballos MP (2003) Social and economic interest in the control of phthalic acid esters. Trends Anal Chem 22(11):847–857
Green R, Hauser R, Calafat AM, Weuve J, Schettler T, Ringer S, Hu H (2005) Use of di (2-ethylhexyl) phthalate-containing medical products and urinary levels of mono (2-ethylhexyl) phthalate in neonatal intensive care unit infants. Environ Health Perspect 113(9):1222–1225
Grob K, Biedermann M, Scherbaum E, Roth M, Rieger K (2006) Food contamination with organic materials in perspective: packaging materials as the largest and least controlled source? A view focusing on the European situation. Critical Review in Food Science and Nutrition 46(7):529–535
Guan Y, Jiang C, Hu C, Jia L (2010) Preparation of multi-walled carbon nanotubes functionalized magnetic particles by sol–gel technology and its application in extraction of estrogens. Talanta 83(2):337–343
Guo Z, Wang S, Wei D, Wang M, Zhang H, Gai P, Duan J (2010) Development and application of a method for analysis of phthalates in ham sausages by solid-phase extraction and gas chromatography-mass spectrometry. Journal of Meat Science 84(3):484–490
Guo Y, Zhang Z, Liu L, Li Y, Ren N, Kannan K (2012) Occurrence and profiles of phthalates in foodstuffs from China and their implications for human exposure. J Agric Food Chem 60(27):6913–6919
Hauser R, Duty S, Godfrey-Bailey L, Calafat AM (2004) Medications as a source of human exposure to phthalates. Environ Health Perspect 112(6):751–753
Hayasaka Y (2014) Analysis of phthalates in wine using liquid chromatography tandem mass spectrometry combined with a hold-back column: chromatographic strategy to avoid the influence of pre-existing phthalate contamination in a liquid chromatography system. J Chromatogr A 1372:120–127
He J, Lv R, Zhu J, Lu K (2010) Selective solid-phase extraction of dibutyl phthalate from soybean milk using molecular imprinted polymers. Anal Chim Acta 661(2):215–221
He M, Yang C, Geng R, Zhao X, Hong L, Piao X et al (2015) Monitoring of phthalates in foodstuffs using gas-purge microsyringe extraction couled with GC-MS. Anal Chim Acta 879:63–68
Hernández-Díaz S, Mitchell AA, Kelley KE, Calafat AM, Hauser R (2009) Medications as a potential source of exposure to phthalates in the U.S. population. Environ Health Perspect 117(2):185–189
Heudorf U, Mersch-Sundermann V, Angerer J (2007) Phthalates: toxicology and exposure. Int J Hyg Environ Health 210(5):623–634
Hogberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM et al (2008) Phthalate diesters and their metabolites in human breast milk, blood or serum and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect 116(3):334–339
Holadová K, Hajŝlova J (1995) A comparison of different ways of sample preparation for the determination of phthalic acid esters in water and plant matrices. Int J Environ Anal Chem 59(1):43–57
Hosaka A, Watanabe A, Watanabe C, Teramae N, Ohtani H (2015) Polymer-coated sample cup for quantitative analysis of semi-volatile phthalates in polymeric materials by thermal desorption-gas chromatography–mass spectrometry. J Chromatogr A 1391:88–92
Houlihan J, Brody C, Schwan B (2002) Not too pretty, phthalates, beauty products and the FDA. Environmental Working Group. http://www.safecosmetics.org/downloads/NotTooPretty_report.pdf. Accessed Dec 2016
Ishido M, Masuo Y, Sayato-Suzuki J, Oka S, Niki E, Morita M (2004) Dicyclohexylphthalate causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J Neurochem 91(1):69–76
Itoh H, Yoshida K, Masunaga S (2005) Evaluation of the effect of governmental control of human exposure to two phthalates in Japan using a urinary biomarker approach. Int J Hyg Environ Health 208(4):237–245
Jaakkola JJ, Knight TL (2008) The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: a systematic review and meta-analysis. Environ Health Perspect 116(7):845–853
Jaakkola JJ, Oie L, Nafstad P, Botten G, Samuelsen SO, Magnus P (1999) Interior surface materials in the home and the development of bronchial obstruction in young children in Oslo, Norway. Am J Public Health 89:188–192
Jarošová ALŽBETA (2006) Phthalic acid esters (PAEs) in the food chain. Czech Journal of Food Science 24:223–231
Jasentuliyana N, Toma RB, Klavons JA, Medora N (1998) Beverage cloud stability with isolated soy protein. J Sci Food Agric 78(3):389–394
Jeddi MZ, Rastkari N, Ahmadkhaniha R, Yunesian M (2015) Concentrations of phthalates in bottled water under common storage conditions: do they pose a health risk to children? Food Res Int 69:256–265
Jonsson S, Borén H (2002) Analysis of mono-and diesters of o-phthalic acid by solid-phase extractions with polystyrene-divinylbenzene-based polymers. J Chromatogr A 963(1):393–400
Kamrin MA (2009) Phthalate risks, phthalate regulation, and public health: a review. Journal of Toxicology and Environmental Health, Part B Critical Review 12(2):157–174
Khedr A (2013) Optimized extraction method for LC-MS determination of bisphenol A, melamine, and di (2-ethylhexyl) phthalate in selected soft drinks, syringes and milk powder. J Chromatogr B 930:98–103
Kim BN, Cho SC, Kim Y, Shin MS, Yoo HJ, Kim JW et al (2009) Phthalates exposure and attention-deficit/hyperactivity disorder in school-age children. Biol Psychiatry 66(10):958–963
Koch HM, Calafat AM (2009) Human body burdens of chemicals used in plastic manufacture. Philosophical Transactions of the Royal Society B: Biological Sciences 364(1526):2063–2078
Koch HM, Christensen KLY, Harth V, Lorber M, Brüning TH (2012) Di-n-butyl phthalate (DnBP) and diisobutyl phthalate (DiBP) metabolism in a human volunteer after single oral doses. Arch Toxicol 86(12):1829–1839
Koo HJ, Lee BM (2004) Estimated exposure to phthalates in cosmetics and risk assessment. J Toxicol Environ Health A 67:1901–1914
Kozyrod RP, Ziaziaris J (1989) A survey of plasticizer migration into foods. J Food Prot 52(8):578–580
Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P (2003) In utero exposure to di-(2-ethyl hexyl) phthalate and duration of human pregnancy. Environ Health Perspect 111(14):1783–1785
Lau OW, Wong SK (1996) Determination of plasticizers in food by gas chromatography-mass spectrometry with ion-trap mass detection. J Chromatogr A 737(2):338–342
Laws SC, Carey SA, Ferrell JM, Bodman GJ, Cooper RL (2000) Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol Sci 54(1):154–167
Lee JH, Kim KO, Ju YM (1999) Polyethylene oxide additive entrapped polyvinyl chloride as a new blood bag material. J Biomed Mater Res 48(3):328–334
Leivadara SV, Nikolaou AD, Lekkas TD (2008) Determination of organic compounds in bottled waters. Food Chem 108(1):277–286
Li N, Liu T, Zhou L, He J, Ye L (2012a) Di-(2-ethylhexyl) phthalate reduces progesterone levels and induces apoptosis of ovarian granulosa cell in adult female ICR mice. Environ Toxicol Pharmacol 34(3):869–875
Li T, Tang Z, Hong WX (2012b) Determination of 17 phthalic acid esters in fatty food by dispersive solid phase extraction-gas chromatography-mass spectrometry. Chin J Anal Chem 40:391–396
Li C, Xu J, Chen D, Xiao Y (2016) Detection of phthalates migration from disposable tablewares to drinking water using hexafluoroisopropanol-induced catanionic surfactant coacervate extraction. J Pharm Anal 6(5):292–299
Lin J, Chen W, Zhu H, Wang C (2015) Determination of free and total phthalates in commercial whole milk products in different packaging materials by gas chromatography-mass spectrometry. J Dairy Sci 98(12):8278–8284
Lomenick JP, Calafat AM, Castro MSM, Mier R, Stenger P, Foster MB, Wintergerst KA (2010) Phthalate exposure and precocious puberty in females. J Pediatr 156(2):221–225
López-Feria S, Lucena R, Cárdenas S, Valcárcel M (2009) Surfactant coated nanotubes for the liquid-liquid extraction of phthalates and other migrants in virgin olive oils. Anal Bioanal Chem 395(3):737–746
Luks-Betlej K, Popp P, Janoszka B, Paschke H (2001) Solid-phase microextraction of phthalates from water. J Chromatogr A 938(1):93–101
Luo YB, Yu QW, Yuan BF, Feng YQ (2012) Fast microextraction of phthalate acid esters from beverage, environmental water and perfume samples by magnetic multi-walled carbon nanotubes. Talanta 90:123–131
Makkliang F, Kanatharana P, Thavarungkul P, Thammakhet C (2015) Development of magnetic micro-solid phase extraction for analysis of phthalate esters in packaged food. Food Chem 166:275–282
Mariani C, Venturini S, Grob K (2006) Presence of phthalates in vegetable oils. Rivista Italiana Delle Sostanze Grasse 83(6):251–256
Markowitz JS, Gutterman EM, Schwartz S, Link B, Gorman SM (1989) Acute health effects among firefighters exposed to a polyvinyl chloride (PVC) fire. Am J Epidemiol 129(5):1023–2031
Marsee K, Woodruff TJ, Axelrad DA, Calafat AM, Swan SH (2006) Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect 114:805–809
Martino-Andrade AJ, Chahoud I (2010) Reproductive toxicity of phthalate esters. Molecular Nutrition and Food Research 54(1):148–157
Matsumoto M, Hirata-Koizumi M, Ema M (2008) Potential adverse effects of phthalic acid esters on human health: a review of recent studies on reproduction. Regul Toxicol Pharmacol 50(1):37–49
McKee RH (2004) Phthalate exposure and early thelarce. Environ Health Perspect 112:A541-A543
Meng J, Bu J, Deng C, Zhang X (2011) Preparation of polypyrrole-coated magnetic particles for micro solid-phase extraction of phthalates in water by gas chromatography–mass spectrometry analysis. J Chromatogr A 1218(12):1585–1591
Mohamed MA, Ammar AS (2008) Quantitative analysis of phthalates plasticizers in traditional Egyptian foods (Kouschary and Foul Medams), black tea, instant coffee and bottled waters by solid phase extraction-capillary chromatography–mass spectrometry. Am J Food Technol 3(5):341–346
Montuori P, Jover E, Morgantini M, Nayona JM, Triassi M (2008) Assessing human exposure to phthalic acid and phthalate esters from mineral water stored in polyethylene terephthalate and glass bottles. Food Addit Contam 25(4):511–518
Moret S, Marega M, Conte LS, Purcaro G (2012) Sample preparation techniques for the determination of some food contaminants (polycylic aromatic hydrocarbons, mineral oils and phthalates). Comprehensive Sampling and Sample Preparation 4:340–351
Mortensen GK, Main KM, Andersson AM, Leffers H, Skakkebӕk NE (2005) Determination of phthalates monoesters in human milk, consumer milk, and infant formula by tandem mass spectrometry (LC-MS-MS). Anal Bioanal Chem 382(4):1084–1092
Moskovkin AS (2002) Chromatographic-mass-spectrometric determination of toxic substances liberated from polymeric materials. J Anal Chem 57(6):507–512
Muncke J (2014) Food packaging contaminants. Encyclopedia of Food Safety 2:430–437
Nelson KB (1991) Prenatal and perinatal factors in the etiology of autism. Pediatrics 87(5):761–766
Nerin C, Asensio E, Jiménez C (2002) Supercritical fluid extraction of potential migrants from paper and board intended for use as food packaging materials. Anal Chem 74(22):5831–5836
Ni X, Xing X, Cao Y, Cao G (2016) Determination of phthalates in food packing materials by electrokinetic chromatography with polymeric pseudostationary phase. Food Chem 190:386–391
Nielsen GD, Larsen ST, Olsen O, Lovik M, Poulsen LK, Glue C, Wolkoff P (2007) Do indoor chemicals promote development of airway allergy? Indoor Air 17(3):236–255
Oie L, Hersoug LG, Madsen JO (1997) Residential exposure to plasticizers and its possible role in the pathogenesis of asthma. Environ Health Perspect 105(9):972–978
Okamoto Y, Ueda K, Kojima N (2011) Potential risks of phthalate esters: acquisition of endocrine-disrupting activity during environmental and metabolic processing. J Health Sci 57(6):497–503
Ormond G, Nieuwenhuijsen MJ, Nelson P, Toledano MB, Iszatt N, Geneletti S, Elliott P (2009) Endocrine disruptors in the workplace, hair spray, folate supplementation, and risk of hypospadias: case-control study. Environ Health Perspect 117(2):303–307
Ostrovský I, Čabala R, Kubinec R, Górová R, Blaško J, Kubincová J et al (2011) Determination of phthalate sum in fatty food by gas chromatography. Food Chem 124(1):392–395
Otero P, Saha SK, Moane S, Barron J, Clancy G, Murray P (2015) Improved method for rapid detection of phthalates in bottled water by gas chromatography-mass spectrometry. J Chromatogr B 997:229–235
Page BD, Lacroix GM (1995) Food Additives & Contaminants 12(1):125–151
Chronic Hazard Advisory Panel (1985) Report to the U.S. Consumer Product Safety Commission on Di(2-Ethylhexyl)Phtalate
Peñalver A, Pocurull E, Borrull F, Marcé RM (2000) Determination of phthalate esters in water samples by solid-phase microextraction and gas chromatography with mass spectrometric detection. J Chromatogr A 872(1):191–201
Peñalver A, Pocurull E, Borrull F, Marcé RM (2001) Comparison of different fibers for the solid-phase microextraction of phthalate esters from water. J Chromatogr A 922(1):377–384
Pérez-Outerial J, Millán E, Garcia-Arrona R (2016) Determination of phthalates in food simulants and liquid samples using ultrasound-assisted dispersive liquid-liquid microextraction followed by solidification of floating organic drop. Food Control 62:171–177
Petersen JHJ (1991) Survey of di-(2-ethylhexyl) phthalate plasticizer contamination of retail Danish milks. Journal of Food Additives & Contaminants 8(6):701–106
Petersen JH, Breindahl T (2000) Plasticizers in total diet samples, baby food and infant formulae. Food Additives & Contaminants 17(2):133–141
Polakoff PL, Lapp NL, Roger R (1975) Polyvinyl chloride pyrolysis products: a potential cause for respiration impairment. Arch Environ Health 30(6):269–271
Polo M, Llompart M, Garcia-Jares C, Cela R (2005) Multivariate optimisation of solid-phase microextraction method for the analysis of phthalate esters in environmental waters. J Chromatogr A 1072(1):63–72
Prokůpková G, Holadová K, Poustka J, Hajšlová J (2002) Development of a solid-phase microextraction method for the determination of phthalic acid esters in water. Analytica Chemica Acta 457(2):211–223
Qiao J, Wang M, Yan H, Yang G (2014) Dispersive solid-phase extraction based on magnetic dummy molecularly imprinted microspheres for selective screening of phthalates in plastic bottle beverages. J Agric Food Chem 62(13):2782–2789
Ranjbari E, Hadjmohammadi MR (2012) Magnetic stirring-assisted dispersive liquid–liquid microextraction followed by high performance liquid chromatography for determination of phthalate esters in drinking and environmental water samples. Talanta 100:447–453
Rastkari N, Ahmadkhaniha R, Samadi N, Shafiee A, Yunesian M (2010) Single-walled carbon nanotubes as solid-phase microextraction adsorbent for the determination of low-level concentrations of butyltin compounds in seawater. Anal Chim Acta 662(1):90–96
Rezaee M, Assadi Y, Hosseini MRM, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid-liquid microextraction. J Chromatogr A 1116(1):1–9
Rios JJ, Morales A, Márquez-Ruiz G (2010) Headspace solid-phase microextraction of oil matrices heated at high temperature and phthalate esters determination by gas chromatography multistage mass spectrometry. Talanta 80(5):2076–2082
Romero-Franco M, Hernández-Ramírez RU, Calafat AM, Cebrián ME, Needham LL, Teitelbaum S et al (2011) Personal care product use and urinary levels of phthalate metabolites in Mexican women. Environ Int 37(5):867–871
Rudel RA, Pevorich LJ (2009) Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ 43:170–181
Rudel RA, Brody JG, Spengler JD, Korn LR, Vallarino J, Geno PW et al (2001) Identification of selected hormonally active agents and animal mammary carcinogens in commercial and residential air and dust samples. Journal of the Air, Waste Management Association 51(4):499–513
Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG (2003) Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol 37(20):4543–4553
Sablayrolles C, Montréjaud-Vignoles M, Benanou D, Patria L, Treilhou M (2005) Development and validation of methods for the trace determination of phtahalates in sludge and vegetables. J Chromatogr A 1072(2):233–242
Sakhi AK, Lillegaard ITL, Voorspoels S, Carlsen MH, Løken EB, Brantsӕter AL et al (2014) Concentrations of phthalates and bisphenol a in Norwegian foods and beverages and estimated dietary exposure in adults. Environ Int 73:259–269
Sathyanarayana S, Karr CJ, Lozano P, Brown E, Calafat AM, Liu F, Swan SH (2008) Baby care products: possible sources of infant phthalate exposure. Pediatrics 121(2):260–268
Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K et al (2013) Phthalate concentrations and dietary exposure from food purchased in New York State. Environ Health Perspect 121(4):473
Schettler T (2006) Human exposure to phthalates via consumer products. Int J Androl 29:134–139
Scholz N (2004) Ecotoxicity and biodegradation of phthalate monoesters. Chemosphere 53:921–926
Self RL, Wu WH (2012) Rapid qualitative analysis of phthalates added to food and nutraceutical products by direct analysis in real-time/orbitrap mass spectrometry. Food Control 25(1):13–16
Selvin E, Feinleb M, Zhang L, Rohrmann S, Rifai N, Nelson WG et al (2007) Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care 30(2):234–238
Serôdio P, Nogueira JMF (2006) Considerations on ultra-trace analysis of phthalates in drinking water. Water Res 40(13):2572–2582
Sharman M, Read WA, Castle L, Gilbert J (1994) Levels of di-(2-ethylhexyl) phthalate and total phthalate esters in milk, cream, butter and cheese. Food Additives & Contaminants 11(3):375–385
Shelton DR, Boyd SA, Tiedje JM (1984) Anaerobic biodegradation of phthalic acid esters in sludge. Environmental Science Technology 18(2):93–97
Shen HY (2005) Simultaneous screening and determination eight phthalates in plastic products for food use by sonication-assisted extraction/GC-MS methods. Talanta 66(3):734–739
Sioen I, Fierens T, Van Holderbeke M, Geerts L, Bellemans M, De Maeyer M et al (2012) Phthalates dietary exposure and food sources for Belgian preschool children and adults. Environ Int 48:102–108
Sørensen LK (2006) Determination of milk and milk products by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 20(7):1135–1143
Staples CA, Peterson DR, Parkerton TF, Adams WJ (2008) The environmental fate of phthalate esters: a literature review. Chemosphere 35(4):667–749
Suzuki Y, Yoshinaga J, Mizumoto Y, Serizawa H (2011) Foetal exposure to phthalate esters and anogenital distance. Environmental health perspectives. Int J Androl 35(3):236–244
Swan SH (2008) Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res 108(2):177–184
Tahmasebi E, Yamini Y, Moradi M, Esrafili A (2013) Polythiophene-coated Fe3O4 superparamagnetic nanocomposite: synthesis and application as a new sorbent for solid-phase extraction. Anal Chim Acta 770:68–74
Tanida T, Warita K, Ishihara K, Fukui S, Mitsuhashi T, Sugarawa T et al (2009) Fetal and neonatal exposure to three typical environment chemicals with different mechanisms of action: mixed exposure to phenol, phthalate, and dioxin cancels the effect of sole exposure on mouse midbrain dopaminergic nuclei. Toxicol Lett 189(1):40–47
Tienpoint B, David F, Dewulf E, Sandra P (2005) Pitfalls and solutions for the trace determination of phthalates in water samples. Chromatographia 61(7–8):365–370
Ting L, Zhi T, Wu-Xing H (2012) Determination of 17 phthalic acid esters in fatty food by dispersive solid phase extraction-gas chromatography-mass spectrometry. Chin J Anal Chem 40(3):391–396
Tsumura Y, Ishimitsu S, Kaihara A, Yoshii K, Nakamura Y, Tonogai Y (2001) Di (2-ethylhexyl) phthalate contamination of retail packed lunches caused by PVC gloves used in the preparation of foods. Food Additives & Contaminants 18(6):569–579
Tsumura Y, Ishimitsu S, Kaihara A, Yoshii K, Tonogai Y (2002) Phthalates, adipates, citrate and some of the other plasticizers detected in Japanese retail foods: a survey. J Health Sci 48(6):493–502
Tsumura Y, Ishimitsu S, Saito I, Sakai H, Tsuchida Y, Tonogai Y (2003) Estimated daily intake of plasticizers in 1-week duplicate diet samples following regulation of DEHP-containing PVC gloves in Japan. Food Additives & Contaminants 20(4):317–324
U.S. EPA (2012) Phthalates action plan http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/phthalates_actionplan_revised_2012-03-14.pdf
US Agency for Toxic Substances and Disease Registry 2012 Toxicological profile for di-2-ethylhexylphthalate. http://www.atsdr.cdc.gov
Van Holderbeke M, Geerts L, Vanermen G, Servaes K, Sioen I, De Henauw S, Fierens T (2014) Determination of contamination pathways of phthalates in food products sold on the Belgian market. Environ Res 134:345–352
Ventrice P, Ventrice D, Russo E, De Sarro G (2013) Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environ Toxicol Pharmacol 36(1):88–96
Vera P, Aznar M, Mercea P, Nerin C (2011) Study of hotmelt adhesives used in food packaging multilayer laminates. Evaluation of the main factors affecting migration into food. J Mater Chem 21(2):420–431
Wang Y, Fan Y, Gu JD (2004) Dimethyl phthalate ester degradation by two planktonic and immobilized bacterial consortia. International Biodeterioration & Biodegradation 53(2):93–101
Wang W, Ma R, Wu Q, Wang C, Wang Z (2013) Fabrication of magnetic microsphere-confined graphene for the preconcentration of some phthalate esters from environmental water and soybean milk samples followed by their determination by HPLC. Talanta 109:133–140
Weintraub K (2011) The prevelance puzzle: autism counts. Nature 479(7371):22–24
Whyatt RM, Adibi JJ, Calafat AM, Camann DE, Rauh V, Bhat HK et al (2009) Prenatal di (2-ethylhextl) phthalate exposure and length of gestation among in inner-city cohort. Pediatrics 124(6):1213–1220
Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K (2006) What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal 26(3):803–824
Wu Q, Zhao G, Feng C, Wang C, Wang Z (2011) Preparation of a graphene-based magnetic nanocomposite for the extraction of carbamate pesticides from environmental water samples. J Chromatogr A 1218(44):7936–7942
Xu Q, Yin X, Wang M, Wang H, Zhang N, Shen Y et al (2010) Analysis of phthalate migration from plastic containers to packaged cooking oil and mineral water. J Agric Food Chem 58(21):11311–11317
Xu D, Deng X, Fang E, Zheng X, Zhou Y, Lin L et al (2014) Determination of 23 phthalic acid esters in food by liquid chromatography tandem mass spectrometry. J Chromatogr A 1324:49–56
Yan H, Cheng X, Liu B (2011) Simultaneous determination of six phthalate esters in bottled milks using ultrasound-assisted dispersive liquid–liquid microextraction coupled with gas chromatography. J Chromatogr B 879(25):2507–2512
Yang C, Xue-Yan Y, Fang-Qing Z, Fei-Fei Q, Qian X (2013) Determination of phthalates esters in edible oil by high performance liquid chromatography with solid-membrane extraction based on electrospun nylon 6 nanofibrous membrane. Chin J Anal Chem 41(12):1837–1843
Yang J, Li Y, Wang Y, Ruan J, Zhang J, Sun C (2015) Recent advances in analysis of phthalate esters in foods. Trends Anal Chem 72:10–26
Yano K, Hirosawa N, Sakamoto Y, Katayama H, Moriguchi T, Joung KE et al (2002) Phthalate levels in beverages in Japan and Korea. Bull Environ Contam Toxicol 68(4):463–469
Yano K, Hirosawa N, Sakamoto Y, Katayama H, Moriguchi T, Asaoka K (2005) Phthalate levels in baby milk powders sold in several countries. Bull Environ Contam Toxicol 74(2):373–379
Yen TH, Lin-Tan DT, Lin JL (2011) Food safety involving ingestion of foods and beverages with phthalate-plasticizer-containing clouding agents. J Formos Med Assoc 110(11):671–682
Zeman FA, Boudet C, Tack K, Barneaud AF, Brochot C, Pery ARR et al (2013) Exposure assessment of phthalates in French pregnant women: results of the ELFE pilot study. Int J Hyg Environ Health 216(3):271–279
Zhang Y, Lee HK (2013) Low-density solvent-based vortex-assisted surfactant-enhanced-emulsification liquid–liquid microextraction combined with gas chromatography–mass spectrometry for the fast determination of phthalate esters in bottled water. J Chromatogr A 1274:28–35
Zhang Y, Lin L, Cao Y, Chen B, Zheng L, Ge RS (2009) Phthalate levels and low birth weight: a nested-case-control study of Chinese newborns. J Pediatr 155(4):500–504
Zhang H, Chen X, Jiang X (2011) Determination of phthalate esters in water samples by ionic liquid cold-induced aggregation dispersive liquid–liquid microextraction coupled with high-performance liquid chromatography. Anal Chim Acta 689(1):137–142
Zhu LP, Zhu T, Ma YP, Ni YF, Wang Y, Yan QC (2013) Rapid determination of 15 phthalates esters in vegetable juices by hollow fiber-liquid phase microextraction coupled with gas chromatography-mass spectrometry. Chin J Anal Chem 41:1019–1024
Acknowledgements
The authors would like to thank the Ministry of Education, Brunei Darussalam, and Universiti Brunei Darussalam for the provision of scholarship and research facilities to the graduate student.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
Nur Zatil Izzah Haji Harunarashid declares that she has no conflict of interest. Lee Hoon Lim declares that she has no conflict of interest. Mohammad Hilni Harunsani declares that he has no conflict of interest.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Haji Harunarashid, N.Z.I., Lim, L.H. & Harunsani, M.H. Phthalate Sample Preparation Methods and Analysis in Food and Food Packaging: a Review. Food Anal. Methods 10, 3790–3814 (2017). https://doi.org/10.1007/s12161-017-0938-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0938-7