Abstract
A simple, fast, and efficient method consisted of optimized dispersive liquid–liquid microextraction (DLLME) followed by UV–vis spectrophotometry was developed for determination of β-carotene in fruits and vegetables. Chloroform and methanol were chosen as extraction and disperser solvents, respectively. The extraction process was optimized using a central composite design (CCD) with the optimum points of 115 μL for volume of extraction solvent and 6.5 % (w/v) for salt concentration. Under the optimal conditions, the relative standard deviation (RSD, C = 500 μg L−1, n = 5), limit of detection (LOD), linear dynamic range (LDR), and coefficient of determination (R2) were 1.08 %, 2 μg L−1, 50–1,500 μg L−1, and 0.991, respectively. The present method consisted of a simple and fast sample preparation procedure without any antioxidant addition, saponification, and purification was used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
β-carotene (BC) is a natural compound belonging to the carotenoids (organic pigments) family that, in turn, are a part of the isoprenoids group. BC is the major carotenoid present in the human diet and organisms that shows pro-vitamin A activity. It is mainly present in plant sources of the human food and just partly present in animal-derived diet. Mainly green, yellow, orange, and red vegetables such as broccoli, brussels sprouts, peppers, tomatoes, spinach, carrots, sweet potato, pumpkin, and paprika as well as colored fruits like apricot, pink grapefruit, cherry, mango, papaya, and peach are rich in BC. β-carotene is also used as a coloring agent for foodstuffs like margarine, butter, and many soft drinks. In the human body, the concentration of BC in the serum is in the range of 0.34 to 0.89 μM, and in the liver is between 0 and 19.4 μM with an average level of 4.4 μM (Keijer et al. 2005, Rühl 2005).
β-carotene is the most important precursor of retinol and other retinoids. Moreover, it has certain other functional properties such as radical quenching, antioxidant, and anticarcinogenic activities, and regulation of cell proliferation (Kotake-Nara et al. 2001). The uptake of BC is necessary for the prevention and treatment of degenerative diseases related to oxidative stress, such as UV-mediated skin or eye diseases, neurodegenerative diseases, and cystic fibrosis. BC also reduces risk of lung and some types of cancer (Siems et al. 2005).
A literature review showed that a range of analytical techniques like UV–vis spectrophotometry (Craft and Soares 1992; Torrecilla et al. 2008; Tzouganaki et al. 2002; Zang et al. 1997), HPLC (Franko et al. 1998; Cámara et al. 2010), liquid chromatography-mass spectrometry (Frenich et al. 2005; Granado-Lorencio et al. 2010), ultra-performance liquid chromatography (Chauveau-Duriot et al. 2010), LC-MS-MS (Fang et al. 2003), Fourier transformation-Raman, attenuated total reflection infrared spectroscopy, and near-infrared spectroscopy (Baranska et al. 2006) have been successfully used for the analysis of carotenoids in different matrices.
Among these measurement techniques, the UV–vis absorption spectroscopy has mainly been used in quantitative (trace) analysis of metals, drugs, body fluids, and food due to its sensitivity, reproducibility, and ease of operation. Sensitivities in the 10−5 to 10−6 M range (with enrichment via solvent extraction), and precision of a few tenths of a percent are typical (Kellner et al. 2004). The majority of carotenoids exhibit absorption in the visible region of the spectrum between 400 and 500 nm that changes with the type of solvent (Zang et al. 1997). β-carotene demonstrates three different absorption peak at 435, 462, and 485 nm in chloroform with the λmax (wavelength of maximum absorbance) of 462 nm.
However, most of analytical systems are not capable to perform direct analysis of real samples without using a pretreatment technique. Depending on the type of working sample, removal of potential interferences, isolation, and/or preconcentration of analyte are necessary to enhance the selectivity and sensitivity of the proposed method. Therefore, several extraction methods such as liquid–liquid extraction, solid-phase extraction (SPE), and supercritical fluid extraction (Gomez-Prieto et al. 2003; Kozukue and Friedman 2003; Rozzi et al. 2002; Tzouganaki et al. 2002) have been applied for extraction of β-carotene from different matrices. In addition to the above mentioned pretreatment methods, a simple and efficient extraction/preconcentration technique was introduced by Assadi et al. in 2006 named dispersive liquid–liquid microextraction (DLLME). This method provides high recovery and enrichment factor within a very short time (a few second) (Rezaee et al. 2006). The DLLME method is based on the following two main steps: (1) first, a suitable mixture extraction and disperser solvents is injected rapidly into aqueous sample solution. Therewith, a cloudy solution, including tiny droplets of the extraction solvent dispersed entirely in the aqueous phase, is formed. Therefore, the large contact surface area between two phases leads to the establishment of a rapid equilibrium. (2) The cloudy solution is then centrifuged to separate the phases. Finally, the organic phase is removed and analyzed for determination of analyte(s) by an appropriate instrumental technique. The main parameters affecting the extraction efficiency are the type and volume of extraction and disperser solvents (Rezaee et al. 2010).
The present study was aimed to develop a simple, fast, and efficient method for determination of β-carotene in fruits and vegetables. Therefore, the DLLME method followed by UV–vis spectrophotometry was applied for this purpose. Response surface methodology with a central composite design (Almeida Bezerra et al. 2008) was used for optimization of the main parameters of the method.
Experimental
Instruments
UV–vis spectra were acquired on a PerkinElmer Lambda-850 spectrophotometer (PerkinElmer Life and Analytical Sciences, Waltham, Mass, USA) using a couple of 1-cm optical pathlength micro-cuvettes (Fischer Scientific, USA) with a sample volume of 0.1 mL. A 100-μL Hamilton syringe (Bonaduz, Switzerland) was used to transfer the preconcentrated sample solutions into the micro-cuvettes. Centrifuges were performed by a Hermel-Z200A (Hemel Labortechnik, Wehingen, Germany). A vortex mixer (Velp Scientifica, Milan, Italy) was used for homogenization of mixtures.
Chemicals and Reagents
β-carotene 95 % was purchased from Sigma-Aldrich (St. Louis, Mo, USA). Chloroform, methanol, and sodium chloride with the purity higher than 99 % were purchased from Merck Chemicals (Darmstadt, Germany). The standard stock solution (1,000 mg L−1) of β-carotene was prepared in chloroform. Working solutions were daily prepared by diluting the standard stock solution with methanol.
The Procedure
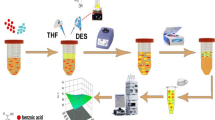
Five microliter of aqueous solution of NaCl 6.5 % (w/v) was placed in a 12-mL conical glass test tube. Then 1 mL of methanol (disperser solvent) containing 500 μg L−1 β-carotene and 115 μL chloroform (extraction solvent) was injected rapidly into the solution by using a 2-mL syringe. Thereby, a stable cloudy solution (containing droplets of chloroform dispersed into the aqueous phase) was formed. In this step, β-carotene was extracted into the chloroform droplets. After centrifugation at 4,000 rpm for 3 min, the chloroform phase was sedimented at the bottom of the test tube. The sedimented phase (consisted of the preconcentrated β-carotene) was removed by a 100-μL Hamilton syringe and placed into the micro-cuvette. UV–vis spectrophotometry analysis was carried out in the wavelength range from 400 to 500 nm with a data interval of 1 nm and scanning speed of 266.75 nm min−1. A spectral bandwidth of 2 nm and detector response of 0.2 s was selected for all measurements.
Data Analysis
The PerkinElmer UV Winlab software package was used for collecting and organizing of the data and recording the UV–vis spectra. Designing the experiments for the central composite design, analyzing and modeling the data, analysis of variance, and constructing the related plots were performed by using a trial version of “Design-Expert 7.1.3” (Stat-Ease Inc., Minneapolis, USA).
Result and Discussion
Effect of Extraction Solvent Type
The major requirements for selection of a solvent for UV–vis spectrometry are the transparency throughout the target wavelength region and the capability to dissolve sufficient quantity of the sample to obtain well-defined peaks (Skoog et al. 1996). Moreover, it must be immiscible with aqueous phase and has density higher than water. According to the literature review, the best solvents for carotenoids are chloroform (density 1.48 g mL−1), dichloromethane (density 1.33 g mL−1), and tetrahydrofuran (THF, density 0.889 g mL−1) in which the solubility may attain values of 1,000 to 10,000 mg L−1 (Craft and Soares 1992). However, since the solvents with higher density than water were preferred, THF was not considered for further experiments. Therefore, chloroform and dichloromethane were examined in accordance with the proposed procedure. Despite higher solubility and molar absorptivity of β-carotene in dichloromethane (6,000 mg L−1, λmax, 460 nm, molar absorptivity, 128,300 L mol−1 cm−1) in comparison to chloroform (solubility, 2,000 mg L−1, λmax, 462 nm, molar absorptivity, 125,000 L mol−1 cm−1), the maximum recovery (in percent) with higher repeatability was achieved by using chloroform (Fig. 1). This is due to lower solubility of chloroform in water (8 g L−1) that leads to higher sedimented phase. In the case of dichloromethane with 20 g L−1 solubility in water, by using 100 μL, the maximum volume of sedimented phase was only 30 μL. Thus, chloroform was chosen as extractor solvent for further experiments.
Influence of extractor and disperser solvent type on the extraction efficiency. Extraction conditions: Extraction solvent (chloroform and dichloromethane), 100 μL; salt concentration, 10 % w/v; concentration of β-carotene, 500 μg L−1; absorbance measured at 462 nm. The error bars represent the standard deviation of the measurements (n = 3)
Effect of Disperser Solvent Type
The most important characteristic of disperser solvent is good miscibility with both extracting solvent and aqueous solution. Therefore, methanol, ethanol, and acetone were tested for this purpose. In the test experiments, the 500 μg L−1 of β-carotene was extracted by 100 μL extraction solvent in a 10 % w/v solution of NaCl. The results in Fig. 1 showed that the maximum recovery (in percent) was obtained using methanol as the disperser solvent. Therefore, it was considered in further experiments.
Response Surface Methodology Optimization
In order to achieve the highest possible efficiency of the proposed method and obtaining the optimal conditions at which the best possible response is produced, a rotatable and orthogonal central composite design (CCD) was applied. Rotatability of the design provides constant variance of the estimated response corresponding to all new observation points that are at the same distance from the center point of the design. An experimental design is orthogonal if each factor can be evaluated independently of all the other factors. A CCD is a combination of factorial points (Nf = 2f), axial points (Na = 2f), and a set of center points (N0), where f is the number of factors (Morgan. 1991; Zeaiter et al. 2004). Table 1 represents the factors, their symbols, and levels.
The value of α and N0 needed to ensure orthogonality and rotatability was calculated from Eqs. (1) and (2), respectively.
Accordingly, the axial spacing and N0 were equal to ±1.414 and 8, respectively. The total number of experiments (N) needed to perform CCD was obtained equal to 16 by using Eq. (3) as follows:
The experiments were randomized in order to minimize the effect of uncontrolled factors. The design matrix for CCD including the experiments and the related responses is given in Table 2.
According to the experimental results of performing the central composite design, a quadratic polynomial model (Eq. (4)) with the most reasonable statistics was established. This model in terms of the actual values of the significant effects, consists of two main effects (S and E), one two-factor interaction effect (ES), and two quadratic effects (E2 and S2).
Where A is the absorbance of the solution, E is the volume of extraction solvent, and S is salt concentration. The sign and absolute value of the coefficients shows the direction and the weight of relationship between the terms and absorbance, respectively. An interaction occurs when the response is different depending on the settings of two factors. The interaction plots make it easy to interpret two factor interactions. They will appear with two non-parallel lines, indicating that the effect of one factor depends on the level of the other (Fig. 2). The “I beam” range symbols on the interaction plots are the result of least significant difference calculations. If the plotted points fall outside the range, the differences are unlikely to be caused by error alone and can be attributed to the factor effects. If the “I beams” overlap, there is not a significant difference (95 % confidence is default) between the two points.
To analyze the validity of the model and significance of the effects, analysis of variance (ANOVA) was performed on the entire dataset (Table 3). The F value which is the test for comparing the variance associated with a term with the residual variance is equal to 132.87 for the model and thus implies the model is significant. The lack of fit F value of 3.83 indicates that it is not significant relative to the pure error. In addition, there are three determination coefficients including R2, adjusted-R2, and adequate precision that demonstrate quality of the model. R2 shows the amount of variations around the mean and was equal to 0.991. The adjusted R2 is inversely related to the number of model terms and was equal to 0.9778. Adequate precision is a signal to noise ratio that compares the range of the predicted values at the design points to the average prediction error. The ratios greater than four indicate adequate model discrimination. In this case, it is equal to 38. Adequate precision is calculated using the following Eq. (5):
Where p is the number of model parameters (including intercept (b0) and any block coefficients), σ2 is residual MS from ANOVA table, and n is the number of experiments (Design-Expert 7.1.3" (Stat-Ease Inc., Minneapolis, USA).
For graphical interpretation of the significant interaction of the model (ES), three-dimensional response surface plot of the model was considered. Figure 3 shows the simultaneous effect of volume of extraction solvent (E) and salt concentration (S) on the response. In the present study, the volume of the extraction solvent was investigated in the range of 100 to 200 μL. With the volumes lower than 100 μL, the volume of the sedimented phase after centrifugation was not enough for UV–vis analysis. When the volume of the extraction solvent increases from 100 to 200 μL, the response (absorbance) was decreased. This decrease is related to the fact that with increasing the volume of chloroform, the dilution effect predominates over the extraction capacity and thus the extraction efficiency decreased. The influence of salt concentration on the performance of the method was studied in 5–15 % (w/v) range. Figure 3 illustrates that at higher volumes of the extraction solvent the salt concentration produced no considerable effect on the response. However, at lower volumes, the response decreases moderately with increasing the salt concentration. This phenomenon is attributed to the increased viscosity of the aqueous solution that overcomes the salting-out effect.
Finally, optimum value of the significant parameters was calculated using the numerical optimization option of the Design-Expert software package 7.1.3. In this method, the goal for the parameters (volume of extraction solvent and salt concentration) was set “within range.” And the response was set at “target” 0.5, where the indeterminate errors in the measurement of absorbance is minimum. The goals were combined into an overall desirability solution. Although the desirability is normally used for optimization of multi-responses designs, but any individual response may also be considered to show the optimum point. The program seeks to maximize this function. The goal seeking begins at a random starting point and proceeds up the steepest slope to a maximum. By starting from several points in the design space, chances improve for finding the “best” local maximum. Therefore, the optimal conditions were obtained as follows: volume of extraction solvent (chloroform), 115 μL; and salt concentration, 6.5 (w/v %). To evaluate the accuracy of the results obtained by the response surface model, the method was carried out at the optimum conditions. The experimental response with five replicates was 0.4987. The results showed a good agreement between optimum calculated response (0.5022) and experimental response.
Analytical Figures of Merit
Under the optimal conditions (volume of extraction solvent, 115 μL; and salt concentration, 6.5 % w/v), the analytical characteristics of the method for determination of β-carotene was obtained. The calibration graph was constructed with nine concentration levels of β-carotene in methanol in the range of 50–1,500 μg L−1 at 462 nm and was characterized with coefficient of determination (R2) of 0.991. Limit of detection based on 3Sd/m (where Sd and m are standard deviation of the blank and slope of calibration graph, respectively) was 2 μg L−1. The limit of quantification was calculated equal to 7 μg L−1 using 10Sd/m.
Repeatability and reproducibility of the proposed method were determined by intraday and interday measurements, respectively. The intraday relative standard deviation (C = 500 μg L−1, n = 5) was 1.08 % and interday RSD (C = 500 μg L−1, n = 5, in three successive days) was 4.23 %.
Real Sample Analysis
To investigate the applicability and efficiency of the proposed method, the fresh and unprocessed (raw) fruits and vegetables consisted of carrot, mango, pumpkin, persimmon, beetroot, green beans, chili pepper, cauliflower, lettuce, cabbage, broccoli, and turnip were tested. For this purpose, 100 mg of the sample was placed in a test tube and 5-mL distilled water was added to it. Then, the mixture was homogenized by vortexing at 2,500 rpm for 3 min. Afterward, the extraction was performed on 0.5 mL of the mixture according to the proposed procedure under the optimal conditions. The amount of β-carotene in each sample was obtained and given in Table 4. Moreover, for the evaluation of matrix effect, each sample was spiked with two standard solutions of β-carotene (5 and 10 μg L−1) and tested in accordance with the proposed procedure. Then, the relative recoveries were calculated using Eq. (6) and given in Table 4.
Where Cfound, Creal, and Cadded are the concentrations of analyte after addition of known amount of standard in the real sample, the concentration of analyte in real sample, and the concentration of known amount of standard which was spiked to the real sample, respectively. The obtained relative recoveries ranging from 94.70 to 99.80 % represent no significant matrix interference; therefore, the method did not require further clean-up during the procedure.
Conclusion
β-carotene is one of the main antioxidant nutrients in human diet that is partially supplied by fruits and vegetables. The β-carotene content of fruits reflects partly nutritional value of them. Therefore, the determination of vitamins, as essential factors in the diet, in plant sources of the human food is of practical importance since they concern with human health. In the present work, the DLLME method combined with UV–vis spectrometry was developed for determination of trace levels of β-carotene in the complex matrix of fruits and vegetables. The method was optimized and the results were modeled statistically with the aid of a CCD. In addition to successful quantification of β-carotene, the proposed method offered advantages such as simplicity, speed, sensitivity, low cost, and high efficiency with good reproducibility. Furthermore, the procedure is environmentally friendly because it consumes very low volumes of organic solvents (1 mL of methanol and 115 μL chloroform) without using even more toxic chemicals.
References
Almeida Bezerra M, Erthal Santelli R, Padua Oliveira E, Silveira Villar L, Ame′lia Escaleira L (2008) Talanta 76:965
Baranska M, Schütze W, Schulz H (2006) Anal Chem 78:8456
Cámara M, Torrecilla JS, Caceres JO, Sánchez Mata MC, Fernández-Ruiz V (2010) J Agric Food Chem 58:72
Chauveau-Duriot B, Doreau M, Nozière P, Graulet B (2010) Anal Bioanal Chem 397:777
Craft NE, Soares JH (1992) J Agric Food Chem 40:431
Fang L, Pajkovic N, Wang Y, Gu C, Van Breemen RB (2003) Anal Chem 75:812
Franko M, Van de Bovenkamp P, Bicanic D (1998) J Chromatogr B 718:47
Frenich AG, Torres MEH, Vega AB, Vidal JLM, Bolaños PP (2005) J Agric Food Chem 53:7371
Gomez-Prieto MS, Caja MM, Herraiz M, Santa Maria G (2003) J Agric Food Chem 51:3
Granado-Lorencio F, Herrero-Barbudo C, Blanco-Navarro I, Pérez-Sacristán B (2010) Anal Bioanal Chem 397:1389
Keijer J, Bunschoten A, Palou A, Franssen-van Hal NLW (2005) Biochim Biophys Acta 1740:139–146
Kellner R, Mermet J, Otto M, Valcárcel M, Widmer HM (2004) Analytical Chemistry, a modern approach to analytical science, 2nth edn. Wiley-VCH, Germany
Kotake-Nara E, Kushir M, Zhang H, Sugawara T, Miyashita K, Nagao A (2001) J Nutr 131:3303
Kozukue N, Friedman M (2003) J Sci Food Agric 83:195
Morgan E (1991) Chemometrics: Experimental Design Chapter 2. John Wiley, London
Rezaee M, Assadi Y, Milani-Hosseini MR, Aghaee E, Ahmadi F, Berijani S (2006) J Chromatogr A 1116:1
Rezaee M, Yamini Y, Faraji M (2010) J Chromatogr A 1217:2342
Rozzi NL, Singh RK, Vierling RA, Watkins BA (2002) J Agric Food Chem 50:2638
Rühl R (2005). Biochim Biophys Acta 1740:162
Siems W, Wiswedel I, Salerno C, Crifo ‵C, Augustin W, Schild L, Langhans CD, Sommerburg O (2005) J Nutr Biochem 16:385
Skoog DA, West DM, Holler FJ (1996) Fundamentals of Analytical Chemistry, 7th edition. Sanders College Publishing, Philadelphia, pp 567–568, PA 19106 USA
Torrecilla JS, Cámara M, Fernández-Ruiz V, Piera G, Caceres JO (2008) J Agric Food Chem 56:6261
Tzouganaki ZD, Alta-Politou J, Koupparis MA (2002) Anal Chim Acta 467:115
Zang LY, Sommerburg O, Van Kuijk FJGM (1997) Free Radic Biol Med 23:1086
Zeaiter M, Roger JM, Bellon-Maurel V, Rutledge DN (2004) TrAC Trends Anal Chem 23:157
Conflict of Interest
Hassan Sereshti declares that he has no conflict of interest. Mohammad Ahmadvand declares that he has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sereshti, H., Ahmadvand, M. & Asgari, S. A Rapid Quantification of β-Carotene in Fruits and Vegetables by Dispersive Liquid–Liquid Microextraction Coupled with UV–Vis Spectrophotometry: Optimized by Response Surface Methodology. Food Anal. Methods 7, 1481–1488 (2014). https://doi.org/10.1007/s12161-013-9777-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-013-9777-3