Abstract
Graphene, as a kind of novel and interesting carbon material, has gained much attention in recent years. In this paper, a new sample preparation technique, graphene reinforced hollow fiber liquid phase microextraction (G-HF-LPME), was developed and used to pre-concentrate some phenylurea herbicides (chlortoluron, isoproturon, diuron, monolinuron, and buturon) in milk sample prior to high-performance liquid chromatography–ultraviolet detection. Different parameters influencing the extraction efficiency of the G-HF-LPME were investigated and optimized. Under the optimized conditions, a good linearity was observed in the range between 10.0 and 400.0 μg L−1 with the correlation coefficients ranging from 0.9911 to 0.9987. The limit of detection (S/N = 3) of the method was lower than 2.0 μg L−1. The developed method is simple, efficient, and has been successfully applied to the determination of the phenylureas in milk samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenylurea herbicides (PUHs) are a kind of pre- and postemergence herbicides and are widely used in agriculture. The herbicides are well-known for their effective control of weeds in various cereals, vegetables, aquatic plant, and small fruit crops (Charrêteur et al. 1998; Bautista et al. 1999; Elcombe et al. 2002). However, PUHs can enter the external environment through diverse pathways and pass to consumers through the food chain (Chou et al. 2009; Mou et al. 2008), which may cause environmental pollution and pose harm to human health. Thus, PUHs and their degradation products have caused great concern (Lundén and Noren 1998). The European Union regulates the use of PUHs and allows a permissible limit of 0.1 μg L−1 of any single herbicides in drinking water (Ruberu et al. 2000). Therefore, it is important to develop a sensitive analytical method with rapidness and reliability to satisfy the need for the determination of trace PUHs in different samples.
PUHs have been mostly determined by gas chromatography (GC) (Escuderos-Morenas et al. 2003; Gerecke et al. 2001) and high-performance liquid chromatography (HPLC) (Tamayo and Martin-Esteban 2005; Sagratini et al. 2007). However, most PUHs are not conducive to GC analysis without prior derivatization (Karg 1993; Scott 1993). For this reason, HPLC with different detectors has become the most commonly used techniques for the determination of the PUH residues.
As we all know, a sample preparation step is usually needed prior to an instrumental analysis. So far, different sample pretreatment techniques have been applied to extract PUHs from different samples. For example, solid phase extraction has been employed for the enrichment of some PUHs from water (Ruberu et al. 2000; Delapena et al. 2003), vegetables (Tamayo and Martin-Esteban 2005; Melo et al. 2005), and soil samples (Ferrer et al. 1999). Solid phase microextraction has been developed for the pretreatment of PUHs in aqueous samples (Sagratini et al. 2007; Mughari et al. 2007; Lin et al. 2003). Dispersive liquid–liquid microextraction and microwave assisted ionic liquid microextraction were performed to extract PUHs from water (Chou et al. 2009) and milk samples (Gao et al. 2010).
Recently, extraction techniques based on solvent microextraction approaches, such as hollow fiber liquid phase microextraction (HF-LPME) (Pedersen-Bjergaard and Rasmussen 1999), have been developed. HF-LPME is a relatively new miniaturized technique and has attracted considerable attention in the analytical science (Zhang et al. 2013; Song et al. 2012). It has the advantages such as high enrichments, low consumption of organic solvents, and excellent sample cleanup function. Also, the HF-LPME equipment is very simple and inexpensive (Ghambarian et al. 2012). HF-LPME can prevent large molecules from entering the lumen of the fiber and stabilize the extractant phase even when samples are stirred or vibrated vigorously (Xiao and Hu 2010).
In order to further improve the extraction efficiency of HF-LPME, carbon nanotubes (CNTs) reinforced hollow fiber liquid phase microextraction has been reported. Es’haghi et al. (2010) have developed a novel method of CNT reinforced hollow fiber solid/liquid phase microextraction to determine caffeic acid in medicinal plants. Zhao et al. (2011) have reported a CNT reinforced hollow fiber liquid phase microextraction method to extract some carbamate pesticides from water and fruit samples.
Graphene (G), which has attracted widespread attention lately, is a novel and two-dimensional carbon nanomaterial that possesses exceptional electronic, thermal, optical, adsorptive, and mechanical properties as well as extremely high specific surface area (Novoselov et al. 2004). These properties hold enormous promise for its applications in chemical analysis (Yang et al. 2010). Since the large delocalized π-electron system of graphene can form a strong π-stacking interaction with the benzene ring, it has been demonstrated to be an effective and applicable adsorbent for some extractions (Wang et al. 2012; Wu et al. 2011).
The outstanding adsorption performance of graphene could make it a promising alternative material to CNTs. An introduction of graphene into the acceptor phase in the lumen of the polypropylene hollow fiber would possibly has a higher adsorption capacity for the analytes while maintaining the cleanup effect of the HF-LPME. In the present work, graphene reinforced hollow fiber liquid phase microextraction (G-HF-LPME) was developed for the first time for the extraction of phenylurea residues in milk samples prior to their determination by HPLC–ultraviolet detection (UV). Some important experimental parameters affecting the extraction efficiency were optimized. The results showed that the method can be applied to the determination of herbicide residues in complex samples.
Experimental
Chemicals and Materials
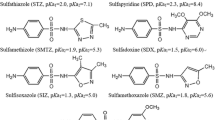
Graphite powder (50 meshes) was purchased from the Boaixin Chemical Reagent Company (Baoding, China). Chlortoluron, isoproturon, diuron, monolinuron, and buturon were purchased from Aladdin-reagent (Shanghai, China). The chemical structures of the herbicides are shown in Fig. 1. Chromatography-grade acetonitrile, methanol, and other chemicals (acetone, hydrochloric acid, sodium hydroxide, 1-octanol, ethyl acetate, tetrahydrofuran, and n-hexane) were purchased from Huaxin Chemical Reagent Company (Baoding, China). Sodium chloride (NaCl) was from Tianjin Fuchen Chemical Reagent Factory (Tianjin, China). The water used throughout the work was purified by a SZ-93 automatic double-distiller purchased from Yarong Biochemistry Instrumental Factory (Shanghai, China). An 85-2B temperature-controlled magnetic stirrer was obtained from Jintan (Jiangsu, China). Accurel Q 3/2 polypropylene hollow fiber membrane (200 μm thick wall, 600 μm inner diameter, and 0.2 μm average pore size) was bought from Membrana GmbH (Wuppertal, Germany).
HPLC Conditions
HPLC was carried out on a LC-20AT liquid chromatography (Shimadzu, Japan) with two LC-20AT VP pumps and a SPD-20A UV/vis detector. Chromatographic separations were performed on a Promosil C18 column (150 mm × 4.6 mm I.D., 5.0 μm) from Bonna-Agela technologies (Tianjin, China). The mobile phase was a mixture of acetonitrile–water (45:55 v/v) at a flow rate of 1.0 mL min−1. The UV monitoring wavelength was chosen at 244 nm.
Sample Pretreatment
Preparation of Standard Solutions and Milk Samples
A mixture stock solution containing each of chlortoluron, isoproturon, diuron, monolinuron, and buturon at 20.0 μg mL−1 was prepared in methanol. A series of standard solutions were prepared by mixing an appropriate amount of the stock solution with methanol in a 10-mL volumetric flask. All the standard solutions were stored at 4 °C in dark. Fresh milk was bought in the local market and stored at 4 °C, which was directly used for the following G-HF-LPME.
Preparation of Graphene Reinforced Hollow Fiber
Graphene was synthesized according to our previously reported method (Wu et al. 2012). For the preparation of the acceptor phase (AP), a certain amount of graphene was thoroughly dispersed in 1-octanol by ultrasonication at room temperature for 1.0 h.
The hollow fiber was cut into the segments of 7 cm long, which were washed with acetone in an ultrasonic bath for 5 min to remove the possible contaminants in the fiber and dried. Then, the fiber was soaked with 1-octanol for 30 s to impregnate the fiber pores and cleaned with water under ultrasonication for 1 min to remove the organic solvent on the surface and the inner wall. Then, 20 μL AP was injected into the lumen of the fiber. Then, both ends of the fiber were sealed together with a heated tweezer.
G-HF-LPME Procedure
Extraction was performed as follows: the prepared hollow fiber was immersed in 15 mL of sample solution, which contained 2.25 g NaCl and 10 μL 1-octanol in a 25-mL glass vial and stirred at 800 rpm for 30 min. After extraction, the fiber was taken out from the sample solution and the outside of the fiber was dried with filter paper. It was then transferred into a 0.5-mL centrifuge tube and 75 μL acetonitrile was added for desorption by vortexing for 1 min. Finally, 15 μL desorption solution was injected into HPLC for analysis.
Results and Discussion
Optimization of the G-HF-LPME Procedure
In this study, for the purpose of obtaining a high extraction efficiency, 15.0 mL sample solution spiked with 50.0 ng mL−1 each of the five phenylurea pesticides was used to optimize the experimental conditions. All the optimization experiments were conducted in triplicate, and the means of the results were used for evaluation.
Optimization of Organic Extraction Solvent
The choice of organic extraction solvents in HF-LPME plays a key role in achieving a good extraction performance for the analytes. There are several factors that should be considered. Firstly, the organic solvent should be compatible well with both the hollow fiber and the graphene. Secondly, it should have a low solubility in aqueous solution and a low volatility. Additionally, it should have a high partition coefficient for the analytes. Considering the above factors, several solvents including 1-octanol, ethyl acetate, tetrahydrofuran, and n-hexane were tested. As a result, 1-octanol gave the best extraction efficiency for all the analytes and therefore was selected.
The literature work (Ren et al. 2008) showed that adding small amount of the extraction solvent into the sample solution could increase the contact area between the extractant and the sample solution. In this work, the effect of the added volume of 1-octanol to the sample solution was evaluated in the range from 0 to 20 μL. It was found that the extraction efficiency reached the plateau when the volume of 1-octanol was increased to 10 μL. Therefore, 10 μL of 1-octanol was added to the sample solution.
Effect of the Concentration of Graphene
The effect of the concentration of graphene on the extraction capability was investigated in the range between 0 and 3 mg mL−1. As shown in Fig. 2a, when the concentration of graphene was increased from 0 to 2 mg mL−1, the peak areas of the analytes were increased and after that remained almost unchanged. Meanwhile, the amount of graphene exceeded 3 mg in 1 mL 1-octanol; the graphene would not be dispersed well.
Effect of the Sample Solution pH
The pH value of the sample solution is a crucial factor in the extraction of organic compounds. To assess the influence of sample solution pH on the extraction efficiency of the analytes, the pH of the sample solution was adjusted to a range of 2–10 by adding 1.0 mol L−1 sodium hydroxide solution or 1.0 mol L−1 hydrochloric acid. As a result, in the pH range investigated, no obvious peak area changes were observed for the PUHs, which could be attributed to the fact that the PUHs are neutral compounds and their existing forms are unlikely influenced by the pH of the sample solution. Therefore, the pH of the sample solution was not adjusted.
Effect of Salt Addition to the Sample Solution
The addition of salt to the sample solution can decrease the solubility of the analytes and therefore enhance extraction yields due to the salting-out effect. The effect of the concentrations of NaCl on the extraction efficiency was investigated in the range of 0–25 % (w/v) at an interval of 5 % in the sample solution. Based on the results shown in Fig. 2b, the peak areas of the analytes increased when the concentration of NaCl was increased from 0 to 15 % and the peak areas remained nearly constant or decreased when the concentration of NaCl was further increased. Based on the above experimental data, all the subsequent experiments were performed with the addition of NaCl at 15 %.
The Effect of Extraction Time
The extraction time is an important factor in HF-LPME procedure because it influences the analytes’ partition between the sample solution and the membrane (in the lumen of the fiber). The effect of extraction time on the extraction efficiency was studied from 10 to 60 min. As can be seen from Fig. 2c, the peak areas of the PUHs increased by increasing the extraction time up to 30 min, and after that, the signals kept almost constant. This result suggests that the extraction equilibrium could be achieved at about 30 min. Therefore, the extraction time was selected to be 30 min.
Effect of the Stirring Speed
In HF-LPME process, sample solution agitation can continuously update the sample solution outside of the fiber and increase the contacting frequencies between the analytes and the fiber. Thus, the sample solution agitation can reduce the extraction equilibrium time and enhance the extraction efficiency. As shown in Fig. 2d, the influence of the stirring rate at 200, 400, 600, 800, and 1,000 rpm was surveyed. The results showed that the peak areas of the analytes increased with increased stirring speed from 200 to 800 rpm and then remained almost unchanged. On the other hand, when stirring speed was too fast, air bubbles are tending to form outside the fiber membrane and this could affect the extraction accuracy and reproducibility. Hence, 800 rpm was selected for the subsequent experiments.
Effect of the Desorption Condition
After extraction, the hollow fiber was transferred to a centrifuge tube for desorption. In this experiment, three different organic solvents, i.e., acetonitrile, methanol, and acetone, were tested as the desorption solvent. The results showed that the desorption power of acetonitrile was mainly similar to either acetone or methanol. Thus, acetonitrile was selected as the desorption solvent. The volume of acetonitrile was optimized in the range from 30 to 100 μL, and as a result, 75 μL yielded the best desorption result. The desorption time was investigated by vortexing the tube for the time in the range from 1 to 5 min. As a result, there was no obvious difference between the tested desorption times; 1 min was chosen.
Validation of the Method
Based on the above optimization, the analytical performance of the developed method was validated through the determination of linearity, limits of detection (LODs), and repeatability and the result are summarized in Table 1. Calibration curves were established for all the analytes in the concentration range of 10.0–400.0 μg L−1 using six spiked concentrations in PUHs-free milk samples. For each concentration, five replicate extractions and determinations were performed. Good linearity was observed with the correlation coefficients (r) ranging from 0.9911 to 0.9987. The LODs at a signal to noise ratio of 3 (S/N = 3) were in the range from 1.6 to 2.0 μg L−1. The repeatability of the method was tested by five parallel experiments for the spiked samples at the concentration of 30.0 μg L−1 for each of the PUHs under the optimum extraction conditions. The resultant relative standard deviations (RSDs) were from 5.2 to 7.4 %. These results showed that the method had a high sensitivity and good repeatability.
Application in the Real Sample
The method was applied to the analysis of the five PUHs in commercial milk sample bought from Baoding. Figure 3 shows the typical chromatograms of the blank and spiked milk samples. The concentration of isoproturon in the milk sample was found to be 6.7 μg L−1, while the other four PUHs, chlortoluron, diuron, monolinuron, and buturon were not detected out. Moreover, the spiked milk sample with each analytes spiked at the concentrations of 80.0 and 160.0 μg L−1 was analyzed to investigate the accuracy of the method. The recovery of the method was defined as the percentage ratio between the concentration found and concentration spiked of each analyte. The results are shown in Table 2. As a result, the recoveries obtained were between 81.3 and 99.8 % with the RSDs ranging from 5.2 to 7.3 %. These results indicated that the method can be applied to the determination of the herbicide residues in milk samples.
Conclusions
In this study, a new method of graphene reinforced HF-LPME was developed for the enrichment of the five phenylureas in milk sample. The results showed that the method is simple sensitivity, and with an excellent pre-concentration and cleanup efficiency. The method can be a useful alternative approach for the analysis of the phenylurea herbicides in complex samples.
References
Bautista A, Aaron JJ, Mahedero MC, Muñoz de la Peña A (1999) Analusis 27:857
Charrêteur C, Colin R, Morin D, Péron JJ (1998) Analusis 26:8
Chou TY, Lin SL, Fuh MR (2009) Talanta 80:493
Delapena A, Mahedero M, Bautistasanchez A (2003) Talanta 60:279
Elcombe CR, Odum J, Foster JR, Stone S, Hasmall S, Soames AR, Kimber I, Ashby J (2002) Environ Health Perspect 110:363
Escuderos-Morenas ML, Santos-Delgado MJ, Rubio-Barroso S, Polo-Díez LM (2003) J Chromatogr A 1011:143
Es’haghi Z, Golsefidi MA, Saify A, Tanha AA, Rezaeifar Z, Alian-Nezhadi Z (2010) J Chromatogr A 1217:2768
Ferrer I, Barcelo D, Thurman E (1999) Anal Chem 71:1009
Gao S, You J, Zheng X, Wang Y, Ren R, Zhang R, Bai Y, Zhang H (2010) Talanta 82:1371
Gerecke AC, Tixier C, Bartels T, Schwarzenbach RP, Müller SR (2001) J Chromatogr A 930:9
Ghambarian M, Yamini Y, Esrafili A (2012) Microchim Acta 177:271
Karg FP (1993) J Chromatogr A 634:87
Lin H, Sung Y, Huang S (2003) J Chromatogr A 1012:57
Lundén Å, Noren K (1998) Arch Environ Contam Toxicol 34:414
Melo LFC, Collins CH, Jardim ICSF (2005) J Chromatogr A 1073:75
Mou RX, Chen MX, Zhi JL (2008) J Chromatogr B 875:437
Mughari AR, Vázquez PP, Galera MM (2007) Anal Chim Acta 593:157
Novoselov K, Geim A, Morozov S, Jiang D, Zhang Y, Dubonos S, Grigorieva I, Firsov A (2004) Science 306:666
Pedersen-Bjergaard S, Rasmussen KE (1999) Anal Chem 71:2650
Ren Z, Zhang W, Dai Y, Yang Y, Hao Z (2008) Ind Eng Chem Res 47:4256
Ruberu SR, Draper WM, Perera SK (2000) J Agric Food Chem 48:4109
Sagratini G, Manes J, Giardina D, Damiani P, Pico Y (2007) J Chromatogr A 1147:135
Scott S (1993) Analyst 118:1117
Song XY, Shi YP, Chen J (2012) Talanta 100:153
Tamayo FG, Martin-Esteban A (2005) J Chromatogr A 1098:116
Wang W, Li Y, Wu Q, Wang C, Zang X, Wang Z (2012) Anal Methods 4:766
Wu Q, Zhao G, Feng C, Wang C, Wang Z (2011) J Chromatogr A 1218:7936
Wu Q, Feng C, Zhao G, Wang C, Wang Z (2012) J Sep Sci 35:193
Xiao Q, Hu B (2010) J Chromatogr B 878:1599
Yang W, Ratinac KR, Ringer SP, Thordarson P, Gooding JJ, Braet F (2010) Angew Chem Int Ed 49:2114
Zhang H, Du Z, Ji Y, Mei M (2013) Talanta 109:177
Zhao G, Wang C, Wu Q, Wang Z (2011) Anal Methods 3:1410
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 31171698), the Science and Technology Supporting Program of Hebei Province (no. 12396908D), and the Natural Science Foundation of Hebei Province (B2012204028).
Conflict of Interest
Meng Sun declares that she has no conflict of interest. Ranxiao Tang declares that she has no conflict of interest. Qiuhua Wu declares that she has no conflict of interest. Chun Wang declares that he has no conflict of interest. Zhi Wang declares that he has no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, M., Tang, R., Wu, Q. et al. Graphene Reinforced Hollow Fiber Liquid Phase Microextraction for the Enrichment of some Phenylurea Residues in Milk Sample. Food Anal. Methods 7, 1097–1102 (2014). https://doi.org/10.1007/s12161-013-9719-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-013-9719-0