Abstract
A rapid, sensitive, and economical pipette tip solid phase extraction (PT-SPE) based on graphene oxide/polypyrrole (GO/PPy) coupled with high performance liquid chromatography (HPLC) was developed for the extraction and determination of sulfonamides (SAs) in honey and milk samples. The PT-SPE procedure involved only 3.0 mg of adsorbent, 3 min adsorptive extraction time, and less than 1.0 mL of solvent consumption (sample, washing, or eluent). Under the optimal conditions, good linearities (0.01–10.00 μg mL−1) were obtained with correlation coefficients (r) > 0.9996. The limits of detection (S/N = 3) were in the range of 1.04–1.50 ng mL−1. Meanwhile, the proposed method was successfully applied for the analysis of SAs in honey and milk with average recoveries within 62.3–109.0% and relative standard deviations less than 11.2%. The results demonstrated that the developed PT-SPE-HPLC method was suitable for the analysis of SAs in complex matrix due to its high sensitivity and accuracy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfonamides (SAs), a group of synthetic antimicrobial drugs, are widely applied in human therapy and used as animals’ growth promoters for preventing bacterial and protozoal diseases (Huertas-Pérez et al. 2016). Their activity is based on the competition of folic acid synthase with p-aminobenzoic acid, which inhibits bacterial multiplication (Mistri et al. 2010). However, the extensive use of SAs as prophylactic veterinary resulted in the persistent presence of antibiotic traces in products of animal origin, which caused a potential threat to human health, such as allergic reactions, carcinogenic potency, and resistance to antibiotics (Dasenaki and Thomaidis 2010). According to European Union (EU), the combined total residues of all substances within the sulfonamide group should not exceed 100 μg kg−1 in milk, muscle, fat, liver, and kidney (EU Regulation 37/2010, 2009). Although none of that has been set for honey, a “zero-tolerance” policy has been implemented recently. The EU regulations (Council Directive 2001/110/EC, 2002) mention that “If possible, honey must, as far as possible, be free from any organic or inorganic matters foreign to its composition.” Therefore, it is of great necessity to develop rapid and sensitive methods for monitoring SA presence in milk and honey.
Sample purification and pretreatment is an essential step before instrumental analysis on account of the complex sample matrices and the low level of target analytes. Solid phase extraction (SPE) has been one of the most widely used methods for sample preparation, substituting the classic liquid-liquid extraction (LLE), due to its merits of low solvent consumption, simple operation, and high performance (Shi, et al. 2014). However, the traditional SPE process still present several disadvantages, such as tedious routine, vast consumption of solvent, and high cartridge cost. Recently, a representative miniaturized SPE mode, based on the use of pipette tip (PT) replacing SPE cartridge, named as pipette tip SPE (PT-SPE) has received widespread attention owing to simplicity, low cost, and remarkable reduction consumption of adsorbent, time, and reagents (Yan et al. 2014b). In previous reports, the PT-SPE as a promising sample pretreatment technique has successfully applied for drug residues analysis, including flavonoids in biological samples (Wang et al. 2017), fluoroquinolones in human urine (de Oliveira et al. 2016), and sulfonamides in milk or water (Sun et al. 2014; Yan et al. 2014a). Yan et al. (2014a) developed a miniaturized graphene-based pipette tip extraction device packed with 3.0 mg graphene to handle 2.0 mL of milk samples for rapid screening of SAs.
Graphene oxide (GO), a unique two-dimensional single-atom layer carbon nanomaterial with extraordinary electronic and mechanical properties, excellent thermal and chemical stability, and ultrahigh theoretical surface area (2630 m2/g), has been abundantly researched in recent years (Rao et al. 2009). As a wonderful adsorbent, GO shows strong affinity to the aromatic compounds owing to its large delocalized π-conjugated structure (Wang et al. 2011). Kazemi et al. used iron oxide functionalized graphene oxide as sorbent for dispersive micro-solid phase extraction of sulfadiazine from milk, honey, and water samples (Kazemi et al. 2016). It is difficult to reach the theoretical surface area of GO because GO sheets tend to stack and agglomerate duo to the strong Van der Waals interaction between them, which is adverse for the adsorption of contaminants (Wang et al. 2012). Besides, the high dispersibility of GO made it difficult to be separated from the sample solution after extraction. Functionalization of GO proved to be a good alternative scheme to resolve these issues due to its plentiful reactive oxygen functional groups. Polypyrrole (PPy) is one of the most widely investigated conducting polymers. Recently, PPy has attracted great attention in separation science because they could adsorb benzenoid compounds by π-π and hydrophobic interactions, hydrogen bonding, and anion-ion exchange characteristics (Chullasat et al. 2017; Tahmasebi et al. 2013). Rahimi et al. applied PPy as an efficient SPE sorbent for the determination of chloramphenicol residue in chicken liver, kidney, and meat with satisfactory results (Rahimi et al. 2017). Based on favorable adsorption properties of GO and PPy, Wang et al. fabricated GO/PPy foam material and designed a syringe assistant PT-SPE strategy for auxin extraction in papaya juice (Wang et al. 2014). The results indicated that the GO/PPy possessed a unique foam-like structure and PT-SPE showed significant capacity for eliminating the interferences from the papaya juice matrix. However, there are some shortcomings in Wang’s work, for example, the synthesis process took about 80 h and manual pressurization with a syringe could cause large error during the extraction process.
Therefore, the purpose of the present work was to improve the synthesis method of GO/PPy and develop a miniaturization of pipette tip solid phase extraction (PT-SPE) based on graphene oxide/polypyrrole (GO/PPy) foam material for efficient extraction of seven SAs from honey and milk. GO/PPy was synthesized through a facile in situ polymerization method and characterized with X-ray diffraction (XRD), Fourier transform infrared (FT-IR), and scanning electron microscopy (SEM). Parameters that affect extraction efficiency including amount of the GO/PPy, sample pH, rotation speed, and the type and volume of eluent solvent were optimized in detail. Finally, the proposed PT-SPE method coupled with high performance liquid chromatography (HPLC) was applied for the analysis of SAs in honey and milk sample.
Experimental
Materials and Reagents
Graphite powder and pyrrole were purchased from the Aladdin Industrial Corporation (Shanghai, China). Hydrogen peroxide (H2O2, 30%), potassium permanganate (KMnO4), concentrated sulfuric acid (H2SO4), and sodium nitrate (NaNO3) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All the other reagents were of analytical grade. The deionized water with a resistivity of 18.2 MΩ cm−1 obtained from a Milli-Q apparatus (Millipore, Bedford, MA, USA) was used throughout the work.
Standards of SAs including sulfathiazole (STZ), sulfapyridine (SPD), sulfamethizole (SMTZ), sulfadoxine (SDX), sulfisoxazole (SIZ), sulfamethoxazole (SMZ), and sulfadimethoxine (SDM) were purchased from the Aladdin Industrial Corporation (Shanghai, China). The chemical structures of seven SAs are shown in Fig. 1. Individual stock solution of the seven SAs was prepared in methanol at a concentration of 1000 μg mL−1. Working standard solutions were freshly prepared by dilution of the stock standard solution with deionized water to different concentration. All of the solutions were stored at 4 °C in the dark.
Instruments and Conditions
XRD spectra (PANalytical B.V., Netherlands) were obtained on an Empyrean diffractometer using Cu-Kα radiation source. Infrared spectra were collected on a NEXUS 670 Fourier transform infrared (FT-IR) spectrometer (Thermo Nicolet Co. Ltd., USA) in the form of KBr pellets. The morphological analysis of the adsorbent was performed using a JEOL JSM-6360 LV scanning electron microscope (SEM; JEOL (Europe) B.V., Belgium). The BSA224S analytical balance (max 220 g, d = 0.1 mg) was made by Sartorius Industrial Weighing Equipment (Beijing, China) Co., Ltd.
An Agilent 1260 HPLC (Agilent Technologies Co. Ltd., USA) system equipped with a quaternary pump, a degasser, a temperature regulator, and a variable wavelength detector was employed for all separations and detections. Separations were performed in a ZORBAX SB-C18 column (150 × 4.6 mm i.d., 5 μm) supplied by Agilent, USA. An injection volume of sample was fixed at 20.0 μL. Optimization of the separation conditions was evaluated based on our previous reports (Li et al. 2015). The mobile phase was a mixture of acetonitrile and acetic acid (0.5%, v/v) in a volume ratio of 25:75, flow rate of 1.0 mL min−1, and column temperature of 25 °C. The SAs were detected at 270 nm in less than 12 min.
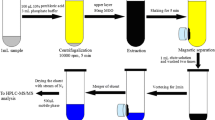
The PT-SPE device was self-assembled in laboratory with common components. Pipette tip possesses a special conical shape with different diameters in two ends, which is similar to a SPE cartridge. The pipette tip cartridge was constructed with 1000- and 200-μL polypropylene pipette tips, as shown in Fig. 2. After washed with acetonitrile and water, then dried, the 200-μL polypropylene pipette tip was loaded with 3.0 mg GO/PPy and degreased cotton was packed as spacers at both ends of the adsorbent. Then, the tip of 1000 μL pipette was cut and the top was inserted into the packed one. The narrowness of the pipette tip and the expansion of the loose GO/PPy material made the adsorbent adequately packed in the column (the height of adsorbent bed was about 0.8 cm), which benefits better extraction performance. Meanwhile, the 2.0-mL centrifuge tube was employed as a container for extracts. Thus, the PT-SPE device was assembled.
Synthesis of GO and GO/PPy Nanocomposite
The modified Hummers method was applied to synthesize GO (Marcano et al. 2010). In the typical synthesis, 1.0 g graphite powder and 1.0 g NaNO3 were dispersed into 33.0 mL H2SO4 (98%) in an ice bath, then 6.0 g KMnO4 was added slowly below 10 °C over 1 h. Then, the ice bath was removed and the solution was heated to 35 °C for 90 min in a water bath. Successively, 40.0 mL of deionized water was slowly added within 15 min and the solution was heated to 95 °C for 35 min. Afterwards, the suspension was further diluted with 100.0 mL of deionized water and then 6.0 mL of H2O2 (30%) was added with another 30 min agitation. Graphite oxide particles were separated by centrifugation before being washed with deionized water to eliminate acid. After that, the gel-like particles were vacuum-dried at 60 °C for 24 h. A dark brown substance of the flaky GO was obtained.
According to Wang’s work with several modifications (Wang et al. 2014), GO/PPy composite was synthesized by in situ polymerization of pyrrole monomer on GO sheets with FeCl3 as an oxidant and water as a solvent. In the procedure, 0.2 g GO was dispersed in 50.0 mL H2O and then 0.80 mL pyrrole was dissolved in 25.0 mL H2O by ultrasound. Then, the GO dispersion was added dropwise to pyrrole solution in an ice bath with vigorous stirring for 2 h. After that, 10.0 g FeCl3·6H2O was dissolved with deionized water (25.0 mL) and then added into the mixture under stirring. In situ polymerization was carried out in an ice bath under N2 for 12 h.
The obtained GO/PPy precipitate was filtered and washed by deionized water and ethanol, respectively. Finally, the black foam-like power was obtained after dried in a vacuum oven at 60 °C for overnight.
Sample Preparation
Honey and milk samples were purchased from a local market. In the present work, the samples were firstly pretreated by acetonitrile for protein precipitation, and then the proposed PT-SPE procedure was employed to extract the analytes and eliminate or reduce matrix effects. The specific operations were as follows:
Six milliliters of acetonitrile was used to treat 1.0 mL (nearly 1.0 g) honey and milk samples. After extracted by vortex and sonication, the sample was centrifuged and the supernatant was collected. Moreover, the fat in the supernatant was removed by 6.0 mL of n-hexane. After that, the obtained extracts were filtrated and evaporated, and the residues were dissolved again with 100 μL methanol and diluted to 1.0 mL with deionized water for further extraction.
For PT-SPE procedure, prior to extraction, the PT-SPE cartridge was pre-conditioned with 1.0 mL methanol and 1.0 mL water, successively. After that, one aliquot of 1.0 mL sample was loaded into the cartridge and the extraction was carried out at 1000 rpm in 3 min. After washed with 1.0 mL of water, the cartridge was dried by centrifugation at high speed. Finally, the analytes were eluted with 0.6 mL methanol and the elution was filtered through a 0.22-μm nylon syringe filter.
Results and Discussion
Characterization of Materials
The surface morphologies of GO/PPy were visualized by SEM. It can be seen from Fig. 3a that GO has scaly wrinkled lamellar structure with large surface area. Figure 3b shows that GO/PPy present three-dimensional, loose, and foam-like structure, which markedly increases the surface area of the prepared material, thus improving the adsorptive performance of GO/PPy. When the magnification was raised, as shown in Fig. 3c, the wrinkled extent was greatly improved after in situ intercalative polymerization due to electron donor and acceptor interactions between the π-electron system in GO and PPy (Shan et al. 2015). The curly, consecutive, and uniform sheet structure indicated that GO was dispersed homogeneously in the PPy without stack and aggregation.
Figure 3d exhibits the FT-IR spectra of GO and the as-prepared GO/PPy composite. The FT-IR spectrum of the GO shows that a broad absorption at 3407 cm−1 corresponds to the O-H stretching vibration. The peak that appear at 1729 cm−1 is assigned to the C=O stretching vibration of the -COOH groups and the band at 1624 cm−1 C=C is due to stretching vibration of the sp2 carbon skeleton. The other two peaks located at 1220 and 1057 cm−1 may be ascribed to C-O-C stretching vibration of epoxy and alkoxy functionalities (Li et al. 2016). For GO/PPy, the presence of the O-H stretching vibration peak at 3410 cm−1 suggests GO was successfully preserved in hybrid GO/PPy material. The peaks at 1543 and 1455 cm−1 belong to antisymmetric and symmetric pyrrole-ring fundamental vibration of PPy, respectively (Li et al. 2012), and the bands located at 1298 and 1168 cm−1 are related to C-N and C-C stretching vibration, respectively. The peaks at 1031 and 901 cm−1 are assigned to C-H in-plane bending mode and C-H out-of-plane vibrations (Yao et al. 2014). These characteristic FT-IR features demonstrate the formation of PPy and successful synthesis of GO/PPy composite.
Figure 3e shows the powder XRD patterns of as-synthesized GO and GO/PPy. It can be seen that a sharp and strong diffraction peak at 2θ = 10.01° is observed in GO, which is corresponding to the (002) reflection of GO nanosheets (Li et al. 2016). For GO/PPy, a broad peak at 2θ = 24.98° is observed, which is well consistent with the XRD pattern of pure polypyrrole, indicating that no additional crystalline phase has been introduced into the composite (Zhang, Zhang et al., 2014a). The (001) diffraction peak of GO/PPy shifted to a lower angle (2θ = 7.13°) compared with pristine GO because the monolayer of water molecules in GO is displaced by the incoming polymer via intercalation (Gu et al. 2010). These results reflect that GO sheets are uniformly dispersed in PPy matrix with negligible π-stacking between themselves.
Optimization of PT-SPE Procedures
For complicated samples, the matrix effect should be evaluated and eliminated carefully when developing an assay. The common pretreatment procedure involved the extraction of analytes and precipitating protein by utilizing an organic solvent for preliminarily purifying the matrix before HPLC (Li et al. 2016), rather than direct MSPE-LC-MS/MS analysis (Gao et al. 2011). However, it was not enough clean to obtain good sensitivity while analyzing the compounds from complex matrix. The extracts after the treatment of SPE were relatively cleaner and could obtain higher extraction efficiency (Van Eeckhaut et al. 2009).
In order to improve the extraction performance of PT-SPE procedures, several factors including the amount of sorbent, sample pH, rotation speed, and the type and volume of elution solvent were optimized in detail. The spiked standard solutions of SAs (2.0 μg mL−1) were employed for the optimization experiments and each test was conducted in triplicates.
The Amount of GO/PPy
The packed amount of GO/PPy is a key parameter affecting extraction efficiency for SAs. Different amounts of the sorbent ranging from 1.0 to 5.0 mg were examined. As shown in Fig. 4a, the extraction recoveries of SAs increased gradually with the increase of the adsorbent amount as expected. When the amount of adsorbent was higher than 3.0 mg, the recovery appeared slightly decline. The reason for the decrease of extraction recovery might lie in that excess adsorbent could aggrandize column pressure and affect the separation, which also could accrete experiment error and result in the waste of adsorbent. Therefore, 3.0 mg GO/PPy was selected as a packing material in the subsequent work. The small dosage of adsorbent is far below the reported PT-SPE technique (Sun et al. 2014; Luo et al. 2016), which is one of the advantages of the present method.
a The effect of the GO/PPy amount on extraction efficiency (conditions: pH 4.0, 3.0 mg GO/PPy, 1000 rpm, 1.00 mL eluent). b The effect of sample pH on extraction efficiency (conditions = 3.0 mg GO/PPy, 2000 rpm, 1.00 mL methanol as eluent). c The effect of elution solvent on extraction efficiency (conditions: pH = 4, 3.0 mg GO/PPy, 2000 rpm, 1.00 mL methanol as eluent). d The effect of the rotation speed on extraction efficiency (conditions: pH 4.0, 3.0 mg). e The effect of the elution volume on extraction efficiency (conditions: pH 4.0, 3.0 mg GO/PPy, 1000 rpm, methanol as eluent)
Sample pH
The sample pH was crucial for the retention of SAs due to its strong effect on the existing forms of SAs and thus the interaction between analytes and sorbent. The effect of sample pH on the extraction efficiency was studied with pH varying from 2.0 to 12.0. The pH of the solution was adjusted using 0.1 and 1.0 M HCl or NaOH. As shown in Fig. 4b, the extraction recovery increased significantly when the sample pH increased from 2.0 to 4.0 and then decreased gradually with further increasing of that. The maximum extraction efficiency for SAs was obtained at the pH value of 4.0. The seven SA molecules contain carboxylic groups and amino groups (pKa1 = 1.3~2.3; pKa2 = 5.0~8.4). At pH 4.0, SA molecules were primarily in protonated and intermediate forms, so the high extraction efficiency might be ascribed to the strong π-π and hydrophobic interactions between SAs and GO/PPy (Marcano et al. 2010). However, with the increase of pH, the deprotonation of SAs would dramatically decrease the π-withdrawing ability and then suppress the π-π electron-donor-acceptor interaction with the π-donor of GO/PPy, so the extraction efficiency was reduced (Ji et al. 2009). When sample pH value was continuously increased to 10.0, the extraction recovery recovered slightly. The possible reason was that the analytes had converted into neutral and anionic forms; therefore, the π-π interaction was significantly diminished and hydrophobic interaction played a dominant role. However, the strong alkaline condition is unbeneficial for extraction, so the extraction efficiency dramatically dropped when pH further increased. According to the result, the pH of 4.0 was employed in the following experiment.
Type of Elution Solvent
The type of eluent also plays a unique role for high extraction efficiency in the PT-SPE process. In this study, several commonly used organic solvents including methanol, acetonitrile, acetone, and ethyl acetate were selected to investigate the effect of eluent on the extraction efficiency of SAs (shown in Fig. 4c). The results indicated that the best desorption yield for the SAs was obtained with methanol as an elution solvent, while the lowest desorption yield was observed when using ethyl acetate as the solvent. SAs are polar substances (Karageorgou et al. 2016), so the polar methanol is beneficial to destroy the interaction between SAs and adsorbent based on Like Dissolves Like Theory. Hence, methanol was selected as the eluent.
Rotation Speed
In the PT-SPE procedure, the rotation speed of centrifuge was a significant factor for evaluating the efficiency of the assay since adsorption-desorption process was carried out by centrifuging. The effect of rotation speed 1000, 2000, 3000, 4000, and 5000 rpm (the corresponding centripetal forces are 70, 280, 630, 1120, and 1740 g) on the extraction efficiency was shown in Fig. 4d. It can be seen that when the rotation speed was 1000 rpm, the extraction recovery of all components could achieve maximum because it is effortless for sample solution to pass through the loose GO/PPy adsorbent effectively. However, the extraction recovery dropped when the rotation speed increased to 2000 rpm for the reduced interaction time is unfavorable for extraction. It is worth noting that the adsorptive extraction procedure could be accomplished in 3 min despite the very low speed, which suggest the high efficiency of interaction between adsorbent and analytes. Based on all these factors, the rotation speed of 1000 rpm was adopted for further studies.
Volume of Elution Solvent
The effect of the eluent volume on extraction efficiency was also investigated in the range of 0.2–1.0 mL (Fig. 4e). The results revealed that most of the analytes could be desorbed from the sorbent with 0.6 mL of methanol.
Method Validation
Under the optimal conditions, the quantitative performance of the suggested GO/PPy-based PT-SPE-HPLC method for the determination of the SAs was validated and the parameters including linearities, limits of detection (LODs), limits of quantification (LOQs), and reproducibility were listed in Table 1. As summarized in Table 1, regression equations with the peak areas against the concentration (0.01–10.00 μg mL−1) for seven SAs exhibited good linearities (r ≥ 0.9996). The obtained LODs (S/N = 3) and LOQs (S/N = 10) were 1.04–1.50 and 3.48–5.05 ng mL−1, respectively.
Precision and accuracy were evaluated by analyzing five repeated extractions of solutions at a concentration of 5.0 μg mL−1 of the analytest. Five parallel analytes over a day gave the intra-day RSDs, and the inter-day RSDs were determined on five consecutive days. The results showed that the RSDs for intra-day and inter-day were less than 1.1 and 1.9%, respectively.
Real Sample Analysis
The established PT-SPE method was applied for the determination of SAs in honey and milk samples to further assess the practicability and reliability of the method. The results showed that honey and milk samples were free of SA residues. To evaluate the trueness of the proposed method, all of the samples were spiked at three concentrations levels (0.1, 0.5, and 2.0 μg mL−1) and then analyzed under optimal conditions. Three replicates were conducted for each concentration level. Figure 5 shows the typical chromatograms of honey and milk samples without and with spiked standards at 2.0 μg mL−1, and no interfering peaks were observed at the retention time of the analytes. As summarized in Table 2, the obtained recoveries of SAs in the spiked honey and milk samples were in the range of 62.3–109.0 and 66.6–106.9% with RSDs less than 11.2 and 10.8%, respectively. The results revealed that the proposed PT-SPE method was sensitive, effective, and reliable for pretreating and screening of SAs at low concentration levels in honey and milk samples.
Comparison of Proposed PT-SPE with Other Methods for Analyzing SAs
The performance of the developed PT-SPE coupled to HPLC method was compared with other analytical techniques for determination of SAs in animal derived food, and the results are summarized in Table 3. It can be seen that the proposed method demonstrated the merits of fewer amount of adsorbent, shorter analysis time, wider linear range, lower LODs, and comparable recoveries. Meanwhile, the modified tip is easily assembled and the extraction process is accessible, cost-efficient, and environmentally friendly with minimum consumption of sample and solvent. Therefore, the proposed PT-SPE-HPLC method showed great potential in extracting and detecting SAs from complex food samples.
Conclusions
The present study demonstrated that GO/PPy as an adsorbent loaded in the pipette tip coupled to HPLC was effective for the extraction and determination of SAs in honey and milk samples. The self-assembled PT-SPE device was freely accessed in laboratory and considerably low cost compared with conventional SPE cartridge, and the procedure was simple and easy-to-handle with the aid of centrifugation. The extraction was performed by repeated aspiration and dispensation of solution, which is beneficial to the sufficient adsorption of analytes onto a sorbent (especially for small amount of sorbent). Compared with the commonly used format, the highlighted advantages of PT-SPE were that the amount of adsorbent used in extraction was only 3.0 mg and adsorptive extraction time was 3 min, which is far lower than that in other methods. In summary, the proposed PT-SPE technique is convenient, fast, and has low consumption of material and solvent for sample pretreatment. Moreover, the proposed method showed good linearities, low LODs, and comparable recoveries for screening SAs in honey and milk samples. However, the minimum sample consumption limited the enrichment factor of the proposed method. To broaden the applications of miniaturized SPE procedure in sample pretreatment, more advanced technology needs to be designed and utilized.
References
Abdallah H, Arnaudguilhem C, Jaber F, Lobinski R (2014) Multiresidue analysis of 22 sulfonamides and their metabolites in animal tissues using quick, easy, cheap, effective, rugged, and safe extraction and high resolution mass spectrometry (hybrid linear ion trap-Orbitrap). J Chromatogr A 1355:61–72
Chullasat K, Nurerk P, Kanatharana P, Kueseng P, Sukchuay T, Bunkoed O (2017) Hybrid monolith sorbent of polypyrrole-coated graphene oxide incorporated into a polyvinyl alcohol cryogel for extraction and enrichment of sulfonamides from water samples. Anal Chim Acta 961:59–66
Commission Regulation (European Union, EU), No 37/2010: 2009, On pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin
Council Directive (2002) 2001/110/EC of 20 December relating to honey. Off J Eur Commun L10:47–52
Dasenaki ME, Thomaidis NS (2010) Multi-residue determination of seventeen sulfonamides and five tetracyclines in fish tissue using a multi-stage LC–ESI–MS/MS approach based on advanced mass spectrometric techniques. Anal Chim Acta 672:93–102
de Oliveira HL, da Silva Anacleto S, da Silva ATM, Pereira AC, de Souza Borges W, Figueiredo EC, Borges KB (2016) Molecularly imprinted pipette-tip solid phase extraction for selective determination of fluoroquinolones in human urine using HPLC-DAD. J Chromatogr B 1033:427–439
Dmitrienko SG, Kochuk EV, Tolmacheva VV, Apyari VV, Zolotov YA (2015) Determination of the total content of some sulfonamides in milk using solid-phase extraction coupled with off-line derivatization and spectrophotometric detection. Food Chem 188:51–56
Gao Q, Luo D, Bai M, Chen Z-W, Feng Y-Q (2011) Rapid determination of estrogens in milk samples based on magnetite nanoparticles/polypyrrole magnetic solid-phase extraction coupled with liquid chromatography–tandem mass spectrometry. J Agr Food Chem 59:8543–8549
Gu Z, Li C, Wang G, Zhang L, Li X, Wang W, Jin S (2010) Synthesis and characterization of polypyrrole/graphite oxide composite by in situ emulsion polymerization. J Polym Sci Pol Phys 48:1329–1335
Guillén I, Guardiola L, Almela L, Núñez-Delicado E, Gabaldón JA (2017) Simultaneous determination of nine sulphonamides by LC-MS for routine control of raw honey samples. Food Anal Method 10:1430–1441
Hoff RB, Pizzolato TM, Peralba MCR, Díaz-Cruz MS, Barceló D (2015) Determination of sulfonamide antibiotics and metabolites in liver, muscle and kidney samples by pressurized liquid extraction or ultrasound-assisted extraction followed by liquid chromatography–quadrupole linear ion trap-tandem mass spectrometry (HPLC–QqLIT-MS/MS). Talanta 134:768–778
Hou X-L, Wu Y-L, Yang T, Du X-D (2013) Multi-walled carbon nanotubes–dispersive solid-phase extraction combined with liquid chromatography–tandem mass spectrometry for the analysis of 18 sulfonamides in pork. J Chromatogr B 929:107–115
Huertas-Pérez JF, Arroyo-Manzanares N, Havlíková L, Gámiz-Gracia L, Solich P, García-Campaña AM (2016) Method optimization and validation for the determination of eight sulfonamides in chicken muscle and eggs by modified QuEChERS and liquid chromatography with fluorescence detection. J Pharmaceut Biomed 124:261–266
Ibarra IS, Miranda JM, Rodriguez JA, Nebot C, Cepeda A (2014) Magnetic solid phase extraction followed by high-performance liquid chromatography for the determination of sulphonamides in milk samples. Food Chem 157:511–517
Ji L, Chen W, Zheng S, Xu Z, Zhu D (2009) Adsorption of sulfonamide antibiotics to multiwalled carbon nanotubes. Langmuir 25:11608–11613
Karageorgou E, Manousi N, Samanidou V, Kabir A, Furton KG (2016) Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem 196:428–436
Kazemi E, Dadfarnia S, Haji Shabani AM, Abbasi A, Rashidian Vaziri MR, Behjat A (2016) Iron oxide functionalized graphene oxide as an efficient sorbent for dispersive micro-solid phase extraction of sulfadiazine followed by spectrophotometric and mode-mismatched thermal lens spectrometric determination. Talanta 147:561–568
Li S, Lu X, Xue Y, Lei J, Zheng T, Wang C (2012) Fabrication of polypyrrole/graphene oxide composite nanosheets and their applications for Cr(VI) removal in aqueous solution. PLoS One 7:e43328
Li Y, Wu X, Li Z, Zhong S, Wang W, Wang A, Chen J (2015) Fabrication of CoFe2O4–graphene nanocomposite and its application in the magnetic solid phase extraction of sulfonamides from milk samples. Talanta 144:1279–1286
Li Y, Li Z, Wang W, Zhong S, Chen J, Wang A-J (2016) Miniaturization of self-assembled solid phase extraction based on graphene oxide/chitosan coupled with liquid chromatography for the determination of sulfonamide residues in egg and honey. J Chromatogr A 1447:17–25
Louppis AP, Kontominas MG, Papastephanou C (2017) Determination of antibiotic residues in honey by high-performance liquid chromatography with electronspray ionization tandem mass spectrometry. Food Anal Method 10:3385–3397
Luo Y-B, Chen X-J, Zhang H-F, Jiang X-Y, Li X, Li X-Y, Zhu F-P, Pang Y-Q, Hou H-W (2016) Simultaneous determination of polycyclic aromatic hydrocarbons and tobacco-specific N-nitrosamines in mainstream cigarette smoke using in-pipette-tip solid-phase extraction and on-line gel permeation chromatography-gas chromatography–tandem mass spectrometry. J Chromatogr A 1460:16–23
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814
Mistri HN, Jangid AG, Pudage A, Shah A, Shrivastav PS (2010) Simultaneous determination of sulfamethoxazole and trimethoprim in microgram quantities from low plasma volume by liquid chromatography–tandem mass spectrometry. Microchem J 94:130–138
Rahimi Z, Shahbazi Y, Ahmadi F (2017) Polypyrrole as an efficient solid-phase extraction sorbent for determination of chloramphenicol residue in chicken liver, kidney, and meat. Food Anal Method 10:955–963
Rao CNR, Sood AK, Subrahmanyam KS, Govindaraj A (2009) Graphene: the new two-dimensional nanomaterial. Angew Chem Int Edit 48:7752–7777
Shan L, Chen X, Tian X, Chen J, Zhou Z, Jiang M, Xu X, Hui D (2015) Fabrication of polypyrrole/nano-exfoliated graphite composites by in situ intercalation polymerization and their microwave absorption properties. Compos Part B-Eng 73:181–187
Shi Z, Hu J, Li Q, Zhang S, Liang Y, Zhang H (2014) Graphene based solid phase extraction combined with ultra high performance liquid chromatography–tandem mass spectrometry for carbamate pesticides analysis in environmental water samples. J Chromatogr A 1355:219–227
Sun N, Han Y, Yan H, Song Y (2014) A self-assembly pipette tip graphene solid-phase extraction coupled with liquid chromatography for the determination of three sulfonamides in environmental water. Anal Chim Acta 810:25–31
Tahmasebi E, Yamini Y, Seidi S, Rezazadeh M (2013) Extraction of three nitrophenols using polypyrrole-coated magnetic nanoparticles based on anion exchange process. J Chromatogr A 1314:15–23
Van Eeckhaut A, Lanckmans K, Sarre S, Smolders I, Michotte Y (2009) Validation of bioanalytical LC–MS/MS assays: evaluation of matrix effects. J Chromatogr B 877:2198–2207
Wang C, Feng C, Gao Y, Ma X, Wu Q, Wang Z (2011) Preparation of a graphene-based magnetic nanocomposite for the removal of an organic dye from aqueous solution. Chem Eng J 173:92–97
Wang J, Tsuzuki T, Tang B, Hou X, Sun L, Wang X (2012) Reduced graphene oxide/ZnO composite: reusable adsorbent for pollutant management. ACS Appl Mater Inter 4:3084–3090
Wang L, Wang M, Yan H, Yuan Y, Tian J (2014) A new graphene oxide/polypyrrole foam material with pipette-tip solid-phase extraction for determination of three auxins in papaya juice. J Chromatogr A 1368:37–43
Wang Z, He M, Jiang C, Zhang F, Du S, Feng W, Zhang H (2015) Matrix solid-phase dispersion coupled with homogeneous ionic liquid microextraction for the determination of sulfonamides in animal tissues using high-performance liquid chromatography. J Sep Sci 38:4127–4135
Wang N, Xin H, Zhang Q, Jiang Y, Wang X, Shou D, Qin L (2017) Carbon nanotube-polymer composite for effervescent pipette tip solid phase microextraction of alkaloids and flavonoids from Epimedii herba in biological samples. Talanta 162:10–18
Yan H, Sun N, Liu S, Row KH, Song Y (2014a) Miniaturized graphene-based pipette tip extraction coupled with liquid chromatography for the determination of sulfonamide residues in bovine milk. Food Chem 158:239–244
Yan H, Yang C, Sun Y, Row KH (2014b) Ionic liquid molecularly imprinted polymers for application in pipette-tip solid-phase extraction coupled with gas chromatography for rapid screening of dicofol in celery. J Chromatogr A 1361:53–59
Yao W, Ni T, Chen S, Li H, Lu Y (2014) Graphene/Fe3O4@polypyrrole nanocomposites as a synergistic adsorbent for Cr(VI) ion removal. Compos Sci Technol 99:15–22
Zhang C, Zhang Z, Li G (2014a) Preparation of sulfonated graphene/polypyrrole solid-phase microextraction coating by in situ electrochemical polymerization for analysis of trace terpenes. J Chromatogr A 1346:8–15
Zhang YD, Zheng N, Han RW, Zheng BQ, Yu ZN, Li SL, Zheng SS, Wang JQ (2014b) Occurrence of tetracyclines, sulfonamides, sulfamethazine and quinolones in pasteurized milk and UHT milk in China's market. Food Control 36:238–242
Acknowledgements
This project was financially supported by the National Natural Science Foundation of China (Nos. 21305128, 21275130 and 21275131) and a project of Natural Science Foundation of Zhejiang Province of China (No. LY17B050002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mengyu Qi declares that she has no conflict of interest. Chunyan Tu declares that she has no conflict of interest. Zhaoqian Li declares that she has no conflict of interest. Weiping Wang declares that she has no conflict of interest. Jianrong Chen declares that he has no conflict of interest. Ai-Jun Wang declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent is not applicable for the nature of this study.
Rights and permissions
About this article
Cite this article
Qi, M., Tu, C., Li, Z. et al. Determination of Sulfonamide Residues in Honey and Milk by HPLC Coupled with Novel Graphene Oxide/Polypyrrole Foam Material-Pipette Tip Solid Phase Extraction. Food Anal. Methods 11, 2885–2896 (2018). https://doi.org/10.1007/s12161-018-1271-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1271-5