Abstract
An effective and sensitive method to determine six cephalosporins (cefadroxil, cephapirin sodium, cefixime, cefuroxime sodium, cefoxitin sodium, and ceftiofur hydrochloride) in milk samples was developed using graphene oxide-reinforced hollow fiber solid-phase microextraction (GO-HF-SPME). After extraction, analytes were desorbed and analyzed using high-performance liquid chromatography-photodiode array detection (HPLC-PDA). The graphene oxide (GO) was dispersed in N,N-dimethyl formamide by ultrasonication and then immobilized into the pores of a hollow fiber (HF). Several factors of GO-HF-SPME experiment, such as the pH of the sample solution, type of organic desorption solvent, pH of the desorption solvent, extraction time, and desorption time, were optimized to achieve the highest extraction efficiency. Under the optimized extraction conditions, the method showed good linear ranges (0.05–10 μg/mL) with correlation coefficients higher than 0.9950, low limits of determination (LODs, 0.01–0.02 μg/mL), and good recoveries (71–108 %) at three different spiked levels. The results demonstrated that GO-HF-SPME would be a promising method for the enrichment of cephalosporins in dairy products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
People have recently been paying increasing attention to noxious substances in their foods, surroundings, and medicines. However, most of these substances exist in intricate sample matrices at trace levels. Therefore, appropriate sample preconcentration techniques are urgently needed to effectively detect them. Globally, milk is one of the most consumed foods and is important for human health. Because it contains essential nutritional components such as proteins, calcium, and saturated fat, it is considered a necessary and inexpensive food. However, its quality can be impaired by the prevalent use of cephalosporin antimicrobials as feed additives in veterinary medicine (Samanidou and Nisyriou 2008). Therefore, the monitoring of antibiotic residues is very important in controlling the safety of milk for human consumption.
In recent years, β-lactam antibiotics have been rapidly developed, where most attention has been focused on cephalosporin antibiotics. Cephalosporins were used for the treatment of infections caused by Gram-positive and Gram-negative bacteria. They function by inhibiting the synthesis of essential structural components of the bacterial cell wall. They are among the safest and most effective broad-spectrum bactericidal antimicrobial agents; therefore, they are the most prescribed (Oliveira et al. 2007). Cephalosporins are divided into four generations—first-, second-, third-, and, recently, fourth-generation compounds—according to the extent of their effect against Gram-negative activity. Third-generation cephalosporins are characterized by a broader antibacterial spectrum in comparison with the first- and second-generation agents because they are resistant to β-lactamase. Fourth-generation agents include molecules with a spectrum similar to those of the third-generation agents but with a markedly increased stability toward hydrolysis by β-lactamase (Pehourcq and Jarry 1998). Each generation exhibits a broader spectrum of activity than the preceding generation. These antibiotics currently hold a large share of the global market and can be considered one of the most important and most frequently used groups of antibiotics (El-Shaboury et al. 2007). Researchers have noted that method development for these antibiotics is substantially more difficult than that for other antibiotics because of the chemical instability of the common β-lactam nucleus and the minor differences in chemical structures among the analogues. Cephalosporin residues constitute a risk to human health because they can cause allergic reactions in hypersensitive individuals and can lead to the emergence of drug-resistant bacteria. Analysis of these residues plays a key role in ensuring food safety (Song et al. 2014).
Some methods have been successfully developed for the determination of cephalosporin antibiotics in biological samples using high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection (Jin et al. 2014; Quesada-Molina et al. 2012; El-Shaboury et al. 2007). At the same time, a solid-phase extraction method was developed for the extraction of several cephalosporin antibiotics from aqueous solutions using carbon nanotubes as the adsorbent (Niu et al. 2007). Moreover, solid-phase microextraction, which allows a large sample volume to be concentrated and purified in a single step, has been widely applied in the analysis of trace levels of analytes in water samples. The sample pretreatment techniques used for the isolation of cephalosporins from various matrices are usually solid-phase microextraction (SPME) and liquid–liquid extraction (Ghidini et al. 2003; Holstege et al. 2002; Moats and Romanowski 1998; Choma et al. 2008; Liu et al. 2007). Since being discovered in 2004 (Novoselov et al. 2004), graphene, a new two-dimensional material comprising a single layer of sp2-hybridized carbon atoms (Geim and Novoselov 2007), has become a topic of intensive research and has gained extensive attention in the materials sciences because of its various unique properties (Lee et al. 2008; Bolotin et al. 2008 ). Graphene has been reported to possess a high theoretical specific surface area (2630 m2/g) (Stoller et al. 2008), which suggests a high sorption capacity. In addition, because of its large delocalized π-electron system, graphene can form strong π–π stacking interactions with benzene rings (Cai et al. 2003). These unique properties make graphene superior for the adsorption of benzenoid compounds (Chen et al. 2010; Zhang and Lee 2011; Zhang et al. 2011). Graphene oxide (GO), a precursor to graphene after reduction, consists of a hexagonal carbon network bearing hydroxyl and epoxide functional groups on its “basal” plane, whereas the edges are mostly decorated by carboxyl and carbonyl groups (Boukhvalov and Katsnelson 2008; Kudin et al. 2008). These high-specific-surface-area functional groups can adsorb cephalosporins in milk samples. Over the past few years, GO has been used as an adsorbent in SPME (Ponnusamy and Jen 2011; Wang et al. 2014), solid-phase extraction (SPE) (Ning et al. 2014; Liu et al. 2011; Luo et al. 2013), liquid-phase microextraction (LPME) (Payán et al. 2010), magnetic solid-phase extraction (MSPE) (Wang et al. 2012; Wu et al. 2011), and dispersive solid-phase extraction (DSPE) (Wen et al. 2014). Because milk is a complex matrix with high protein and fat contents, which often interfere with analytical procedures, emphasis is placed on sample preparation for extracting and determining cephalosporins in milk.
Excellent cleanup efficiency can be achieved with the hollow fiber (HF) playing the role of a filter. The large molecules (proteins and saturated fat) cannot permeate through the wall pores of the HF (Li et al. 2011), which makes it very applicable to complex matrix samples. Moreover, the HF is disposable after each use because of its low cost, which can overcome carry-over problems and enhance the reproducibility.
In view of the above, GO-reinforced HFs were prepared according to previous reports (Song et al. 2013; Ye et al. 2014). The aim of the present study was to develop and validate a simple, rapid, and cost-effective method for the simultaneous determination of the selected cephalosporin antibiotics (cefadroxil, cephapirin sodium, cefixime, cefuroxime sodium, cefoxitin sodium, and ceftiofur hydrochloride), which are widely used antibiotics, in milk samples using a method involving GO-HF-SPME coupled with HPLC. What is more, chromatographic conditions were successfully optimized by gradient elution method (Sun et al. 2014; Hu et al. 2015). The accuracy and precision of the HPLC analysis and method detection limits are described. The SPME optimization and stability of the analytes were also discussed in this study. The method was validated using fresh milk products from a supermarket.
Materials and Methods
Chemicals and Reagents
Cefadroxil (FAD), cephapirin sodium (HAP), cefixime (CFM), cefuroxime sodium (CXM), cefoxitin sodium (FOX), and ceftiofur hydrochloride (EFT) were purchased from Sigma-Aldrich (Germany). N,N-Dimethyl formamide (DMF), acetone, and acetic acid were obtained from the Beijing Chemical Plant (Beijing, China). Chromatographic-grade acetone, acetonitrile, and methanol were purchased from Merck Co. (Darmstadt, Germany). All other reagents were of analytical grade and were used as received, without further purification.
GO was supplied by the XFNano Materials Tech. Co., Ltd. (Nanjing, China). Accurel Q3/2 polypropylene HF membranes (200-μm wall thickness, 600-μm internal diameter (i.d.), 0.2-μm average pore size) were provided by Membrane (Wuppertal, Germany). Ultrapure water (18.2 MΩ cm−1) was obtained from a Milli-Q system (Billerica, MA, USA). All solutions were filtered through 0.22-μm filters (Tianjin, China).
Instrumental and Chromatographic Conditions
All separations were performed on a HPLC (Waters 2695, Waters Technologies, USA) equipped with a photodiode array detector (PDA, Waters 2998, Waters Technologies, USA). A reversed-phase C18 column (5 μm, 150 mm × 4.6 mm i.d.) was used as the separation column. The data were acquired using the Empower software (Waters Technologies, USA). The gradient elution was performed with acetonitrile (solvent A) and a sodium acetate–acetic acid (NaAC-HAC) buffer solution (pH = 3.4, solvent B). The elution program was as follows: 0.00–5 min, solvent A/solvent B (5/95, v/v); 5.00–15.00 min, solvent A/solvent B (25/75, v/v); and 15.00–20.00 min, solvent A/solvent B (5/95, v/v). The flow rate, injection volume, and UV wavelength were 0.8 mL/min, 10 μL, and 254 nm, respectively. All of the mobile phases were filtered using a 0.22-μm filter membrane and degassed for 30 min before use. The centrifuge was purchased from Sigma (3 K15, Germany), and the centrifugations were performed at 25 °C.
Scanning electron micrographs were obtained using an S4800 field emission scanning electron microscope (Hitachi, Japan) operated at 15 kV. Raman spectra (Renishaw, England) were obtained under appropriate conditions with excitation at 633 nm. The laser power was set to 50 %, and backscattered light was collected using a 50-fold objective lens following a 10-s exposure time. The other conditions are listed in the corresponding figures.
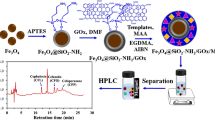
Dispersion of GO
To prepare surfactant-stabilized GO dispersions, 60.0 mg of GO was sonicated in 20 mL of DMF for 2 h, after which GO was evenly dispersed in the DMF at a concentration of 3.0 mg/mL GO.
Preparation of GO-Reinforced HF
The HF was cut manually into segments of 2 cm, ultrasonically cleaned in acetonitrile for 15 min to remove any possible impurities, and then dried in air. To prepare completely immobilize GO onto the HF wall pores, HF segments were immersed in the homogeneous 3.0 mg/mL GO dispersion. GO was then adsorbed onto the wall pores of HF via sonication at room temperature for 2 h. Afterward, ultrapure water was used to remove excess GO from the surface and inner lumen of the HF. Finally, the prepared GO-HF was dried on a filter paper in air.
Preparation of Standard Solutions and Milk Samples
The stock solutions of FAD, HAP, CFM, CXM, FOX, and EFT dissolved in acetonitrile were stored at 4 °C. Working solutions at different concentrations were freshly prepared daily by diluting the stock solutions with ultrapure water. Fresh milk product was purchased from a supermarket in Beijing (China) and was stored at 4 °C. Approximately 0.1 mL of milk, 0.2 mL of HAC, and a buffer solution of NaAC-HAC were added to a total volume of 1.5 mL, and the resulting solution was extracted under the optimized conditions.
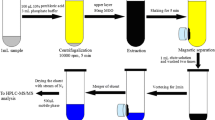
GO-HF-SPME Procedure
The HF was first cleaned with ultrapure water, and the treated GO-HF was then placed into 1.5 mL of standard solution (pH was adjusted to 4.0 using acetic acid) of the corresponding concentration. Next, the solution extraction process was performed for 30 min (the optimum extraction time) at an agitation rate of 1500 rpm. The GO-HF was subsequently removed, and the redundant solvents were absorbed by a filter paper. Finally, the GO-HF was placed in an end-sealed pipette tip with 50 μL of methanol (pH was adjusted to 8.0 using sodium hydroxide) to desorb analytes for 50 min at an agitation rate of 1500 rpm. Fifty microliters of the desorbed solution was drawn out, and the resulting desorption solution was rapidly transferred into a sample bottle for analysis of the target by HPLC. To erase any possible carry-over effect, fresh GO-HF was used in each experiment. Common, unmodified HF was be used to extract analytes under identical conditions using the aforementioned procedure and subsequently compared the enrichment performances of HF and GO-HF.
Results and Discussion
Characterization of GO and GO-HF
GO, HF, and GO-HF were characterized by SEM. Figure 1a shows that the GO sheets assume a layered structure and exhibit wrinkled, thin, and smooth surfaces. As shown in Fig. 1b, the surface of the common HF is multi-aperture. Moreover, the holes were filled with GO after ultrasonication, as shown in Fig. 1c. A comparison of these micrographs indicates that GO was adsorbed onto the wall pores of the HF.
Raman spectroscopy is a perfect measure used to characterize the electronic states and structure of GO. The D and G bands represent disordered sp3 carbon and ordered sp2 crystalline graphite-like structures, respectively. Figure 2 shows that the commercial HF had no absorption peaks, and GO produced a D band (approximately 1336 cm−1) and G band (approximately 1596 cm−1). After HF was modified, GO-HF spectra show D band (approximately 1342 cm−1) and G band (approximately 1587 cm−1), which agreed with the usual features of carbon materials in the Raman spectra. Therefore, the Raman spectra indicate that GO was attached to the HF.
Optimization of the Extraction Procedure
To obtain a high extraction efficiency and enrichment factor, a series of sample solutions spiked with 1.0 μg/mL standard mixture was used to study the extraction performance under different experimental conditions. All of the experiments were performed in triplicate, and the means of the results were used for optimization evaluation.
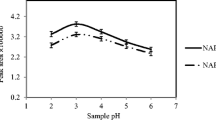
Effect of the Cephalosporin Extract Solution pH
A suitable pH of the sample solution can improve the extraction efficiency and reduce matrix interferences. Hydrophobic and π-π interactions are the two main interactions between the GO and the analytes, and the pH should be adjusted such that the majority of the analytes remain in their molecular form to enhance the extraction efficiency. Therefore, the influence of the pH of the sample solution on the extraction efficiency was studied in the pH range from 2.0 to 12.0. As the results illustrate in Fig. 3, when the pH was greater than 6.0, the amounts of analytes extracted were substantially reduced because of the ionization of the target compounds. However, when the pH was 4.0, more target objects exist in the form of electric neutral molecules and cephalosporin was absorbed more easily by GO, making its peak area the largest. Therefore, the optimum pH of the sample solution was 4.0.
Selection of the Organic Desorption Solvent
The polypropylene membrane and GO are hydrophobic, and low wettability was observed when GO-HF was directly exposed to a sample solution, which led to slow extraction rates and low extraction efficiencies. Solving these deficiencies required improvement of the wettability of the GO-HF. In this study, the wettability of GO-HF was enhanced by conditioning with an organic solvent that was suited to the fiber. Methanol, acetonitrile, and acetone, as three common organic solvents used for desorption, were investigated in the GO-HF-SPME procedure. The experiments demonstrated that methanol produced better results than acetonitrile and acetone, as shown in Fig. 4. The excellent wettability of methanol was demonstrated by its higher extraction efficiency. Thus, methanol was selected for subsequent experiments.
Effect of the pH of the Desorption Solvent
In SPME, mass transfer was promoted by optimal pH conditions in the sample solution. In the present study, desorption was performed under different pH levels ranging from 2.0 to 12.0. As indicated by the results illustrated in Fig. 5, an increase in the peak area was observed in the pH range from 2.0 to 8.0. When the pH was adjusted to 8.0, the peak area reached its maximum and then decreased in the range of 8.0 to 12.0. Therefore, the optimum pH value of desorption solution was selected as 8.0.
Effect of the Extraction Time
The extraction time is a very important parameter of the extraction procedure. The function of the extraction time was examined in the present study. The extraction and desorption of analytes on GO-HF are a dynamic balance; thus, the extraction efficiency usually varies with the extraction time. Therefore, the extraction time was investigated over the range from 10 to 60 min, as shown in Fig. 6a. The peak areas of the analytes were increased as the extraction time was increased from 10 to 50 min. Given the comprehensive effects of the six cephalosporins and the high extraction efficiency, 30 min was used as the extraction time in subsequent experiments. Although this extraction time is relatively long, a large number of samples can be extracted simultaneously because of the simple experimental setup and the low cost of the GO-HF.
Effect of the Desorption Time
After extraction, the HF was transferred to a centrifuge tube containing methanol for desorption via vortex mixing. A series of desorption times was investigated in the range of 10–60 min at room temperature to ensure that all of the cephalosporins were completely desorbed from the GO-HF. As shown in Fig. 6b, the peak areas increased as the desorption time was increased from 10 to 50 min, after which they decreased slightly. This trend may result from the re-absorption of the desorbed analytes during the desorption period. The largest peak areas of the cephalosporins were obtained at a desorption time of 50 min. Therefore, 50 min was chosen as the optimal desorption time.
Comparison Between HF and GO-HF
In this research, the unmodified HF and GO-HF were used separately to extract a 0.2 μg/mL standard solution mixture via the proposed SPME laboratory procedure; the results are compared in Fig. 7. As evident in this figure, the GO-HF resulted in a substantially greater peak area compared to that achieved with the common HF. GO-HF exhibited advantages in the extraction process (curve c), which are attributable to GO, whose high adsorption capacity and efficient extraction or desorption capability resulted in increases in both the amount of analyte extracted and the extraction efficiency. The six target analytes were only slightly detected when no preparation procedure was used (curve b), but they could not be detected using the proposed SPME procedure with unmodified HF (curve a). This result was likely a consequence of the HF lacking any special adsorption ability toward the six target analytes.
Evaluation of the SPME Method
The spiked samples were extracted using an established procedure and analyzed via HPLC-PDA. The performance parameters of the GO-HF-SPME technique, such as the linearity, low limits of determination (LODs), and limits of quantification (LOQs), for the cephalosporins were evaluated under the optimum extraction conditions. As shown in Table 1, the concentration of each of the cephalosporin samples was analyzed three times. The target analytes had satisfactory linear ranges and correlation coefficients. Moreover, the linear regression equations for FAD, HAP, CFM, CXM, FOX, and EFT were y = 9765x + 13391, y = 48571x + 91719, y = 39662x + 146558, y = 28539x + 51176, y = 13747x + 47517, and y = 428113x + 263997, respectively, where y represents the peak area of the cephalosporin and x denotes the concentration of the cephalosporin in the standard solution. The LODs and LOQs of the cephalosporins, which were investigated in standard solutions, were calculated at signal to noise ratios of 3 (S/N = 3) and 10 (S/N = 10), respectively. The LODs were 0.02 μg/mL for FAD, HAP, and FOX and 0.01 μg/mL for CFM, CXM, and EFT. Meanwhile, LOQs of 0.06 μg/mL for FAD, HAP, and FOX and 0.03 μg/mL for CFM, CXM, and EFT were obtained.
The intra-day and inter-day precisions of the instrument were investigated using the GO-HF-SPME technique via the repeated injection of a standard solution. The intra-assay precision was measured for six continuous injections during the same day, whereas the inter-assay precision was measured on three consecutive days. The results are shown in Table 1. The relative standard deviations (RSDs) of the peak areas in the intra-assay precision were 5.5, 5.8, 4.3, 5.7, 3.8, and 4.7 % for FAD, HAP, CFM, CXM, FOX, and EFT, respectively, whereas the RSDs of the peak areas were 8.3, 6.9, 5.9, 6.7, 5.7, and 6.4 % for FAD, HAP, CFM, CXM, FOX, and EFT, respectively, in the inter-assay precision. The RSD values for all of the compounds ranged from 3.8 to 8.3 %, demonstrating that the GO-HF preparation method was quite stable and acceptable.
Analysis of the Cephalosporins in Milk Samples Using GO-HF-SPME
To assess the accuracy of the established analytical method, recoveries at three spiked concentration levels were tested. Three different concentrations of cephalosporin were spiked into the original sample, respectively. Then, three sets of milk samples were extracted using the same method as that previously optimized and analyzed. A summary of the recovery data obtained by HPLC is shown in Table 2. The recoveries ranged from 71 to 108 %, and the RSDs (n = 3) ranged from 2.6 to 8.6 %.
In addition, real blank milk was extracted and analyzed to investigate the specificity of the method. Figure 8 shows typical chromatograms obtained from the analysis of the real blank milk and milk samples containing 1.0 μg/mL cephalosporins after extraction via the proposed procedure. No peaks were observed at the retention times of the corresponding analytes in the blank sample, which indicates that other coexisting species and the sample–matrix components did not interfere with precise analysis of the analytes. Then, the developed method was applied for the detection of cephalosporins in the different batches of milk sample from four manufactures. Contents of fat, protein, and carbohydrate in these milk samples were in the range of 2.0–2.7, 1.6–2.3, and 3.0–4.3 mg mL−1, respectively. Results shown in Table 3 indicated that EFT was found in two batches of milk but could not be quantified.
Comparison of Some Methods Used for Determination of Cephalosporins
The extraction efficiency of the proposed method was compared with other reported sample preconcentration techniques from the angle of detection method, extraction method, the volume of elution solvent, linearity range, relative coefficient, and recovery. As listed in Table 4, the analytical property of the presented method is comparable with most of the listed methods. The GO-HF-SPME required relatively long extraction time and desorption time to obtain the highest extraction performance. Due to that preparation of dispersion of GO, the cleanup function of HF, and dilution of standard solutions take the place of the other, complicated steps were needed ahead of this microextraction. However, the procedure is simple and the extraction and desorption are completed using a vortex mixer. Of elution solvent, 0.05 mL was used in the developed method, making it green and cost-effective. The linear dynamic ranges and correlation coefficients were analogous to those reported sample preconcentration techniques. The large specific surface area and effective adsorption of GO-HF improve the extraction efficiency of this developed method. Thus, the novel mode using GO-HF as a new microextraction medium was useful and high stability.
Conclusions
A new method based on GO-HF-SPME combined with HPLC was successfully developed for the determination of cephalosporins in milk samples. In this research, GO was well dispersed in DMF and adhered to the wall pores of HF via ultrasonic-assisted dispersion. The prepared GO-HF was then applied to SPME. In the present experiments, HF can prevent large molecules, such as proteins, from entering the small pores. The target cephalosporins were first adsorbed by GO-HF and then desorbed by methanol via vortex mixing. Most importantly, the cleanup function of HF and selective adsorption of GO resulted in negligible matrix effects, and the sample required only simple homogenization and dilution with ultrapure water prior to the GO-HF-SPME procedure. Therefore, this method can be directly applied in relatively complicated samples. After the optimization of the extraction conditions for cephalosporins, relatively low limits of detection, effective sample cleanup, and acceptable precisions of repeatability as well as reproducibility were obtained. Therefore, this method is suitable as an alternative to other microextraction methods proposed for the enrichment of cephalosporins. In conclusion, the results of the present experiments showed that GO-HF-SPME coupled with HPLC is a rapid, simple, and cost-effective technique with a low consumption of organic solvent and is suitable for monitoring six types of cephalosporins in milk samples. In addition, the GO-reinforced HF-SMPE method would have potential applications for the concentration of other surrounding pollutants.
References
Bolotin KI, Sikes KJ, Jiang Z, Klima M, Fudenberg G, Hone J, Kim P, Stormer HL (2008) Ultrahigh electron mobility in suspended graphene. Solid State Commun 146(9–10):351–355
Boukhvalov DW, Katsnelson MI (2008) Modeling of graphite oxide. J Am Chem Soc 130(32):10697–10701
Cai Y, Jiang G, Liu J, Zhou Q (2003) Multiwalled carbon nanotubes as a solid-phase extraction adsorbent for the determination of bisphenol a, 4-n-nonylphenol, and 4-tert-octylphenol. Anal Chem 75(10):2517–2521
Chen JM, Zou J, Zeng JB, Song XH, Ji JJ, Wang YR, Ha J, Chen X (2010) Preparation and evaluation of graphene-coated solid-phase microextraction fiber. Anal Chim Acta 678(1):44–49
Choma IM, Kowalski C, Lodkowski R, Burmaczuk A, Komaniecka I (2008) TLC-DB as an alternative to the HPLC method in the determination of cefacetril residues in cow’s milk. J Liq Chromatogr Relat Technol 31(13):1903–1912
Dorival-García N, Junza A, Zafra-Gómez A, Barrón D, Navalón A (2016) Simultaneous determination of quinolone and β-lactam residues in raw cow milk samples using ultrasound-assisted extraction and dispersive-SPE prior to UHPLC-MS/MS analysis. Food Control 60:382–393
El-Shaboury SR, Saleh GA, Mohamed FA, Rageh AH (2007) Analysis of cephalosporin antibiotics. J Pharm Biomed Anal 45(1):1–19
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6(3):183–191
Ghidini S, Zanardi E, Varisco G, Chizzolini R (2003) Residues of beta-lactam antibiotics in bovine milk: confirmatory analysis by liquid chromatography tandem mass spectrometry after microbial assay screening. Food Addit Contam 20(6):528–534
Gros M, Rodríguez-Mozaz S, Barceló D (2013) Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J Chromatogr A 1292:173–188
Holstege DM, Puchsner B, Whitehead G, Galey FD (2002) Screening and mass spectral confirmation of beta-lactam antibiotic residues in milk using LC-MS/MS. J Agric Food Chem 50(2):406–411
Hou XL, Wu YL, Lv Y, Xu XQ, Zhao J, Yang T (2013) Development and validation of an ultra high performance liquid chromatography tandem mass spectrometry method for determination of 10 cephalosporins and desacetylcefapirin in milk. J Chromatogr B 931:6–11
Hu C, He M, Chen BB, Hu B (2015) Simultaneous determination of polar and apolar compounds in environmental samples by a polyaniline/hydroxyl multi-walled carbon nanotubes composite-coated stir bar sorptive extraction coupled with high performance liquid chromatography. J Chromatogr A 1394:36–35
Jin HE, Jin SE, Maeng HJ (2014) Recent bioanalytical methods for quantification of third-generation cephalosporins using HPLC and LC-MS(/MS) and their applications in pharmacokinetic studies. Biomed Chromatogr 28(11):1565–1587
Karageorgou EG, Samanidou VF (2010) Application of ultrasound-assisted matrix solid phase dispersion extraction to the HPLC confirmatory determination of cephalosporin residues in milk. J Sep Sci 33:2862–2871
Karageorgou EG, Samanidou VF, Papadoyannis IN (2012) Ultrasound-assisted matrix solid phase dispersive extraction for the simultaneous analysis of β-lactams (four penicillins and eight cephalosporins) in milk by high performance liquid chromatography with photodiode array detection. J Sep Sci 35:2599–2607
Kudin KN, Ozbas B, Schniepp HC, Prud’homme RK, Aksay IA, Car R (2008) Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett 8(1):36–41
Lee C, Wei XD, Kysar JW, Hone J (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321(5887):385–388
Li J, Zhang HF, Shi YP (2011) Monitoring multi-class pesticide residues in fresh grape by hollow fibre sorptive extraction combined with gas chromatography–mass spectrometry. Food Chem 127(2):784–790
Liu W, Zhang Z, Liu Z (2007) Determination of β-lactam antibiotics in milk using micro-flow chemiluminescence system with on-line solid phase extraction. Anal Chim Acta 592(2):187–192
Liu Q, Shi J, Zeng L, Wang T, Cai Y, Jiang G (2011) Evaluation of graphene as an advantageous adsorbent for solid-phase extraction with chlorophenols as model analytes. J Chromatogr A 1218(2):197–204
Luo YB, Zhu GT, Li XS, Yuan BF, Feng YB (2013) Facile fabrication of reduced graphene oxide-encapsulated silica: a sorbent for solid-phase extraction. J Chromatogr A 1299:10–17
Moats WA, Romanowski RD (1998) Multiresidue determination of β-lactam antibiotics in milk and tissues with the aid of high-performance liquid chromatographic fractionation for clean up. J Chromatogr A 812(1–2):237–247
Nemutlu E, Kır S, Katlan D, Beksac M (2009) Simultaneous multiresponse optimization of an HPLC method to separate seven cephalosporins in plasma and amniotic fluid: application to validation and quantification of cefepime, cefixime and cefoperazone. Talanta 80:117–126
Ning FJ, Peng HL, Li JH, Chen LX, Xiong H (2014) Molecularly imprinted polymer on magnetic graphene oxide for fast and selective extraction of 17 beta-estradiol. J Agric Food Chem 62(30):7436–7443
Niu HY, Cai YQ, Shi YL, Wei FS, Liu JM, Mou SF, Jiang GB (2007) Evaluation of carbon nanotubes as a solid-phase extraction adsorbent for the extraction of cephalosporins antibiotics, sulfonamides and phenolic compounds from aqueous solution. Anal Chim Acta 594(1):81–92
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306(5696):666–669
Oliveira RV, De Pietro AC, Cass QB (2007) Quantification of cephalexin as residue levels in bovine milk by high-performance liquid chromatography with on-line sample clean up. Talanta 71(3):1233–1238
Panahi HA, Mehramizi A, Hosseinifard Z, Moniri E (2013) Synthesis and characterization of new molecular imprinting poly[1-(N,N-bis-carboxymethyl)amino-3-allylglycerol-co-dimethylacrylamide] for selective sorption and determination of cefuroxime sodium in biological and pharmaceutical samples. React Funct Polym 73:132–140
Payán MR, López MÁB, Fernández-Torres R, Mochóna MC, Ariza JLG (2010) Application of hollow fiber-based liquid-phase microextraction (HF-LPME) for the determination of acidic pharmaceuticals in wastewaters. Talanta 82(2):854–858
Pehourcq F, Jarry C (1998) Determination of third-generation cephalosporins by high-performance liquid chromatography in connection with pharmacokinetic studies. J Chromatogr A 812(1–2):159–178
Ponnusamy VK, Jen JF (2011) A novel graphene nanosheets coated stainless steel fiber for microwave assisted headspace solid phase microextraction of organochlorine pesticides in aqueous samples followed by gas chromatography with electron capture detection. J Chromatogr A 1218(39):6861–6868
Quesada-Molina C, Claude B, García-Campaña AM, del Olmo-Iruela M, Morin P (2012) Convenient solid phase extraction of cephalosporins in milk using a molecularly imprinted polymer. Food Chem 135:775–779
Quesada-Molina C, García-Campaña AM, del Olmo-Iruela M (2013) Ion-paired extraction of cephalosporins in acetone prior to their analysis by capillary liquid chromatography in environmental water and meat samples. Talanta, 115:943–949
Rossmann J, Schubert S, Gurke R, Oertel R, Kirch W (2014) Simultaneous determination of most prescribed antibiotics inmultiple urban wastewater by SPE-LC–MS/MS. J Chromatogr B 969:162–170
Samanidou V, Nisyriou S (2008) Multi-residue methods for confirmatory determination of antibiotics in milk. J Sep Sci 31(11):2068–2090
Song XY, Shi YP, Chen J (2013) Carbon nanotubes-reinforced hollow fibre solid-phase microextraction coupled with high performance liquid chromatography for the determination of carbamate pesticides in apples. Food Chem 139:246–252
Song XL, Xu SF, Chen LX, Wei YQ, Xiong H (2014) Recent advances in molecularly imprinted polymers in food analysis. J Appl Polym Sci 131(16):40766
Stoller MD, Park SJ, Zhu YW, An J, Ruoff RS (2008) Graphene-based ultracapacitors. Nano Lett 8(10):3498–3502
Sun WJ, Qu SP, Du ZX (2014) Hollow fiber liquid-phase microextraction combined with ultra-high performance liquid chromatography-tandem mass spectrometry for the simultaneous determination of naloxone, buprenorphine and norbuprenorphine in human plasma. J Chromatogr A 951:157–163
Wang W, Li Y, Wu Q, Wang C, Zang X, Wang Z (2012) Extraction of neonicotinoid insecticides from environmental water samples with magnetic graphene nanoparticles as adsorbent followed by determination with HPLC. Anal Methods 4(3):766–772
Wang L, Zang XH, Wang C, Wang Z (2014) Graphene oxide as a micro-solid-phase extraction sorbent for the enrichment of parabens from water and vinegar samples. J Sep Sci 37(13):1656–1662
Wen YY, Niu ZL, Ma YL, Ma JP, Chen LX (2014) Graphene oxide-based microspheres for the dispersive solid-phase extraction of non-steroidal estrogens from water samples. J Chromatogr A 1368:18–25
Wu Q, Zhao G, Feng C, Wang C, Wang Z (2011) Preparation of a graphene-based magnetic nanocomposite for the extraction of carbamate pesticides from environmental water samples. J Chromatogr A 1218(44):7936–7942
Ye NS, Gao T, Li J (2014) Hollow fiber-supported graphene oxide molecularly imprinted polymers for the determination of dopamine using HPLC-PDA. Anal Methods 6(18):7518–7524
Zhang H, Lee HK (2011) Plunger-in-needle solid-phase microextraction with graphene-based sol–gel coating as sorbent for determination of polybrominated diphenyl ethers. J Chromatogr A 1218(28):4509–4516
Zhang S, Du Z, Li G (2011) Layer-by-layer fabrication of chemical-bonded graphene coating for solid-phase microextraction. Anal Chem 83(19):7531–7541
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Importation and Development of High-Caliber Talents Project of Beijing Municipal Institutions (No. CIT&TCD 201504083), the Beijing Natural Science Foundation (No. 2162008) and the Beijing Municipal Science and Technology Commission (No. Z141100002614020).
Conflict of Interest
Xinwei Chen declares that he has no conflict of interest, and Nengsheng Ye declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Chen, X., Ye, N. Graphene Oxide-Reinforced Hollow Fiber Solid-Phase Microextraction Coupled with High-Performance Liquid Chromatography for the Determination of Cephalosporins in Milk Samples. Food Anal. Methods 9, 2452–2462 (2016). https://doi.org/10.1007/s12161-016-0435-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0435-4