Abstract
An enzyme-linked immunosorbent assay (ELISA) was developed based on a polyclonal antibody for the analysis of thiacloprid in agricultural samples. Thiacloprid hapten was synthesized and conjugated to bovine serum albumin to produce an immunogen and ovalbumin to produce a coating antigen. Polyclonal antibodies were obtained from immunized New Zealand white rabbits. Under optimal conditions (5 % methanol, 0.1 mol/L Na+, pH 5.5), the ELISA showed a 50 % inhibitory concentration (IC50) value of 0.01 mg/L and a limit of detection (IC10) of 0.47 μg/L. No obvious cross-reaction with the other structural analogs of neonicotinoid insecticides showed that the polyclonal antibodies had a high specificity for thiacloprid. The average recoveries from spiked water, soil, pear, and tomato were in the range of 80 to 119 %. The results of the ELISA were confirmed by high-performance liquid chromatography, and the correlation of the results from the two methods had a high correlation coefficient of 0.99 (n = 3) in spiked samples (soil, pear, and tomato). The proposed ELISA could successfully be applied to the determination of thiacloprid residues in agricultural samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thiacloprid is a systemic and broad-spectrum neonicotinoid insecticide against not only sucking insects but also chewing insects and is the second member of Bayer's chloronicotintyl family (Jeschke et al. 2001; Elbert et al. 2001). Because thiacloprid has greater systemic activity, lower acute mammalian toxicity, and no cumulative long-term toxicity, it is widely used in many crops, such as rice, fruits, and vegetables (Liu et al. 2010). Thiacloprid has been attracting interest as a promising insecticide.
Current analytical methods for the determination of thiacloprid residues are high-performance liquid chromatography (HPLC) (Yu et al. 2007; Wang et al. 2011; Seccia et al. 2008; Guzsvány et al. 2007; Wu et al. 2011; Mohan et al. 2010; Peña et al. 2011; Smodiš Škerl et al. 2009). Although these methods have low limits of detection and high precision and sensitivity, they are expensive and require specialized technicians and instrumentation. Therefore, there is growing demand for a simple, cost-effective, sensitive, and selective method for the analysis of thiacloprid residues. Enzyme-linked immunosorbent assay (ELISA) fulfills these requirements, becoming a reliable analytical tool for rapid screening analysis (Hennion and Barcelo 1998). However, there is the disadvantage that ELISA methods are susceptible to interferences from organic solvents and sample matrices (Nunes et al. 1998).
To date, several ELISAs have been developed for neonicotinoids, including imidacloprid, acetamiprid, thiamethoxam, dinotefuran, and imidaclothiz (Li and Li 2000; Wanatabe et al. 2001; Kim et al. 2006; Watanabe et al. 2011 Fang et al. 2011), but thiacloprid ELISA has not been reported. In the paper, the analytical performance and reliability of the newly developed ELISA for the determination of thiacloprid residues were evaluated by HPLC and provided a potential application as a quick and simple screening method in agricultural samples.
Materials and Methods
Materials
Pesticide-grade thiacloprid with a purity of 98.0 % (by HPLC) was obtained from Nanjing Jiangsu Flag Chemical Industry Co., Ltd. (Jiangsu, China). The pesticide standards used for the cross-reactivity studies were supplied by Jiangsu Qizhou Chemical Group Co., Ltd. (Jiangsu, China). Bovine serum albumin (BSA), ovalbumin (OVA), Freund's complete and incomplete adjuvants, goat anti-rabbit IgG–horseradish peroxidase, o-phenylenediamine (OPD), and polyoxyethylene sorbitan monolaurate (Tween-20) were purchased from Sigma Chemical Co. (Shanghai, China). 3-Mercaptopropionic acid (3-MPA) was from A Johnson Matthey Company. All reagents and solvents were analytical grade. The polyclonal antibodies were obtained from immunized New Zealand white rabbits. The rabbits had free access to drinking water and commercial standard laboratory diet (CZZ, Nanjing, China). They were housed according to the EEC 609/86 directives regulating the welfare of experimental animals.
Instruments
Nuclear magnetic resonance (NMR) spectrum was recorded on a DRX 500 spectrometer (Bruker, Germany). Mass spectral (MS) data were obtained with a LC-MSQDECA (Finigan, USA). Ultraviolet spectra were recorded on a DU 800 spectrophotometer (Beckman, USA). Ninety-six-well polystyrene microplates (MaxiSorp) were purchased from Nunc (Roskilde, Denmark). Absorbances were read with an Infinite M200 microtiter plate reader (Tecan, Switzerland) at 490 nm, and the ELISA plates were washed with a Wellwash Plus (Thermo, USA). Thiacloprid was detected using Agilent 1200 HPLC chromatography (Agilent, USA).
Buffers and Solutions
Phosphate-buffered saline (PBS, 0.01 mol/L, pH 7.4), carbonate-buffered saline (CBS, 0.05 mol/L, pH 9.6), and phosphate-buffered saline containing 0.05 % Tween-20 (PBST) were used. The substrate solution contained 0.025 mol/L of citrate and 0.062 mol/L of sodium phosphate, pH 5.4. The OPD solution contained 0.4 mg/mL OPD and 0.012 % H2O2 in the substrate solution.
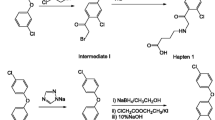
Hapten Synthesis
The hapten synthetic route was illustrated in Fig. 1. A mixture of 1.12 g (4 mmol) of thiacloprid, 0.42 g (4 mmol) of 3-MPA, and 0.45 g of KOH (8 mmol) in 20 mL of DMSO was stirred while the temperature was gradually increased to 100 °C and maintained for 2 h. Water (50 mL) was added after the reaction mixture was cooled to room temperature. The solution was adjusted to pH 3 using 2 mol/L HCl and extracted with dichloromethane (3 × 30 mL). The organic extract was washed with water (3 × 30 mL), dried over anhydrous sodium sulfate, and evaporated under reduced pressure. The residue was purified with silica gel chromatography. The product was characterized by electrospray ionization mass spectrometry (ESI-MS) and NMR: ESI-MS, m/z, 345 [M + Na]+. 1H-NMR (400 MHz, DMSO-d6); δ, 2.72–2.74 (t, 2H, CH2COO), 3.37–3.41 (t, 2H, SCH2), 3.53–3.57 (t, 2H, SCH2), 4.00–4.04 (t, 2H, NCH2), 4.66 (s, 2H, CH2N), 7.27–7.29 (d, J = 8.0, 1H, CH), 7.61–7.63 (d, J = 8.4, 1H, CH), 8.45 (s, 1H, N=CH).
Synthesis and Identification of Hapten–Protein Conjugates
A hapten was coupled to BSA using the active ester method (Zeng et al. 2006) to produce an immunogen and was conjugated with OVA via the mixed anhydride method to produce a coating antigen (Zhang et al. 2008). The conjugates were dialyzed against PBS over 72 h at 4 °C and stored at −20 °C. The conjugates were confirmed by UV–vis spectroscopy. The number of hapten molecules per molecule of protein (hapten density) of conjugate was estimated directly by the molar absorbance at 280 nm (Lu et al. 2009).
Immunization and Antibody Preparation
Two male New Zealand white rabbits weighing approximately 2 kg were immunized with hapten–BSA to raise polyclonal antibodies according to the method described previously (Zeng et al. 2006). The rabbits were bled after 8 days of the last injection. The blood was coagulated for 1 h at 37 °C and 12 h at 4 °C. The antibody was purified using salting out with caprylic acid–ammonium sulfate (Wengatz et al. 1998) and stored at −20 °C after freeze-drying.
Noncompetitive and Indirect Competitive ELISA
Microplates were coated overnight at 4 °C with 100 μL/well of the coating antigen diluted 1:2,000 (v/v) in CBS. The plates were washed three times with PBST. PBS (200 μL/well) containing 1 % OVA was added and incubated for 30 min at 37 °C. After another washing step with PBST, 100 μL/well of antibody (1 mg/L) in PBS was added and incubated for 1 h at 37 °C. Following a further wash, 100 μL/well of a diluted (1:3,000) goat anti-rabbit IgG–horseradish peroxidase was added and incubated for 1 h at 37 °C. After the plates were washed again, 100 μL/well of the OPD solution was added and incubated for 15 min at 37 °C. Finally, 2 mol/L of sulfuric acid (50 μL/well) was added, and the absorbance was measured at 490 nm.
The indirect competitive ELISA method was performed by blocking with sample or standard (50 μL/well) followed by addition of the diluted antibody (50 μL/well). The plates were shaken for 1 min and then processed as indicated above.
ELISA Optimization
The experimental parameters, including organic solvent, ionic strength, and buffer pH value, were studied sequentially to improve the sensitivity of the ELISA. Evaluation of the ELISA was based on the 50 % inhibition concentration (IC50) and the coefficient of determination (R 2) of a four-parameter logistic equation (Qian et al. 2009). Competitive curves were run in PBS solutions containing different concentrations of methanol (from 0 to 40 %, v/v), concentrations of Na+ (0.1, 0.2, 0.3, 0.4, 0.5, and 0.6 mol/L), and pH values (4.5, 5.5, 6.5, 7.5, 8.5, and 9.5) to determine the effects of the solvent, ionic strength, and pH, respectively.
Cross-Reactivity
Under the optimum conditions, cross-reactivity was studied using standard solutions of thiacloprid and some of its analogs. Cross-reactivity values were calculated as follows:
Recovery
The ELISA was applied to the determination of thiacloprid in water, soil, pear, and tomato samples. Tap water and prefiltered paddy water samples were spiked with thiacloprid standards at 0.005, 0.01, and 0.1 mg/L. The samples were directly analyzed by ELISA.
Soil sample (10 g) was spiked with thiacloprid at 0.5, 0.1, and 1 mg/kg. It was mixed with 10 mL PBS containing 50 % methanol, submitted to ultrasonic extraction for 10 min, and then centrifuged for 10 min at 4,000 rpm. The 1 mL of supernatant was diluted ten times with PBS and analyzed by ELISA.
Pear and tomato samples (10 g) were grinded, spiked with thiacloprid at various levels (0.025–1 mg/kg). They were mixed with 10 mL PBS containing 25 % methanol, submitted to ultrasonic extraction for 10 min, and then centrifuged for 10 min at 4,000 rpm. The 1 mL of supernatant was diluted with PBS and analyzed by ELISA. The recoveries and relative standard deviation (RSD) were calculated.
Evaluation of the Assay with HPLC
Soil, pear, and tomato samples were extracted according to Yu et al. (2007) and measured by HPLC with Eclipse XDB-C18 (250 × 4.6 mm I.D., 5 μm) at a wavelength of 245 nm. The mobile phase was 70:30 (v/v) water (0.2 % phosphoric acid)/acetonitrile. The flow rate was 1.0 mL/min and the column temperature was 30 °C. The measured results were compared with the ELISA results.
Data Analysis
Standards and samples were run in triplicate wells, and the mean absorbance values were processed. Standard curves were obtained by plotting absorbance against the logarithm of analyte concentration and fitted to a four-parameter logistic equation:
where A is the asymptotic maximum, B is the curve slope at the inflection point, C is the x value at the inflection point (corresponding to the analyte concentration that reduces absorbance to 50 % of the maximum, IC50), and D is the asymptotic minimum.
Results and Discussion
Identification of Conjugates
UV–vis spectra showed qualitative differences between the conjugate and the corresponding carrier protein and showed that the carrier protein and hapten had been coupled successfully. The molar ratios were estimated as 12:1 and 6:1 for immunogen and coating antigen, respectively.
Optimization of the ELISA
Organic solvent, ionic strength, and buffer pH value were investigated to optimize the ELISA (Fig. 2). Methanol significantly influenced the sensitivity of the ELISA. As shown in Fig. 2a, a low concentration of methanol resulted in a lower IC50. When methanol concentration in PBS was 0 to 5 %, no significant effect on the IC50 was detected. To ensure the analyte solubility, 5 % methanol was selected for the subsequent assays.
Ionic strength strongly influenced ELISA performance. As shown in Fig. 2b, it was observed that the IC50 was lowest when the Na+ concentration was 0.1 mol/L. Then, the value diminished slightly when the salt concentration was increased.
Figure 2c showed that varying the pH from 4.5 to 9.5 did not largely affect the IC50. However, the assay was more sensitive and had lower IC50 under pH 5.5.
Sensitivity and Specificity of the Assay
A competitive standard curve for thiacloprid (Fig. 3) was obtained under the optimized conditions. The limit of detection (LOD, IC10) and the sensitivity (IC50) of the ELISA were 0.47 μg/L and 0.01 mg/L, respectively. The linear working range determined as the concentration resulting in 20–80 % inhibition was 1.82–106.20 μg/L.
The values of cross-reactivity of the antibody with thiacloprid and related analogs were showed in Table 1. The highest cross-reactivity was found to be acetamiprid (0.72 %), due to the same structure of the =N–CN moiety between acetamiprid and thiacloprid. The values of cross-reactivity for other compounds were less than 0.23 %. Therefore, the results indicated that the assays developed from the antibody had a high specificity to thiacloprid.
Matrix Effects
Soil, tomato, and pear were employed to study matrix effects. Samples were diluted (5-, 10-, and 20-fold) with PBS containing 5 % methanol. The matrix effects were determined by comparing thiacloprid standard curves prepared in matrix extract and those standard curves prepared in matrix-free PBS solution. Results (Fig. 4) showed that different sample matrices had different effects on the sensitivity of the optimized ELISA. Matrix effects of soil and tomato samples were reduced to acceptable levels when samples were diluted tenfold. As for pear sample matrix dilution from 5- to 20-fold, the interference had no effects on the sensitivity of the ELISA. Considering that too much dilution would eventually reduce the limit of quantitation of the sample, tenfold dilution (soil, tomato) and fivefold dilution (pear) were finally selected for subsequent ELISA tests.
Analysis of Spiked Samples
Soil, pear, and tomato samples were spiked with thiacloprid at three levels and directly analyzed by the ELISA without any pretreatment other than dilution with PBS. Table 2 gives the results obtained for each spiked sample determined three times at the best dilution factors as previously described. The recoveries were between 80 and 119 % for soil, pear, and tomato. In water samples, no significant matrix interference was observed, with recoveries ranging from 98 to 109 %. Also, no false-negative result was obtained. Because all RSDs were below 10 %, precision obtained for all samples met the requirement for a residue method. This indicated that the established ELISA was a potential screening tool for thiacloprid residue determination.
Validation of the Assay with HPLC
Three samples were analyzed by the ELISA and HPLC. The results were given in Fig. 5, and good correlation was obtained between ELISA (Y) and HPLC (X) with the linear regression equation of Y = 0.9391X − 0.0078 (R 2 = 0.99, n = 3). These results suggested that thiacloprid in the samples could be simply, rapidly, and accurately detected by the ELISA.
Conclusion
An indirect competitive ELISA for thiacloprid was developed and successfully detected thiacloprid residue in agricultural samples. The antibody showed high sensitivity and specificity, with the IC50 value of 0.01 mg/L and LOD value of 0.47 μg/L. The cross-reactivity for some analogs was below 0.72 %. The matrix interference was eliminated arising from three samples via simple dilution of the crude extracts. Therefore, thiacloprid residue in agricultural samples can be directly determined only giving extraction with methanol and adjustment of methanol concentration in a sample with PBS. The assay results showed good precision and accuracy and were suitable for residue analysis. The method was also validated by HPLC with good correlation. The proposed ELISA could be a feasible quantitative/screening method for thiacloprid in agricultural samples due to its high sensitivity and simplicity, rapidity, lower expenses, and high sample throughput.
References
Elbert A, Buchholz A, Ebbinghaus-Kintscher U, Erdelen C, Nauen R, Schnorbach HJ (2001) Pflanzenschutz-Nachr Bayer (Engl Ed) 54:185
Fang S, Zhang B, Ren KW, Cao MM, Shi HY, Wang MH (2011) J Agric Food Chem 59:1594
Guzsvány V, Madžgalj A, Trebše P, Gaál F, Franko M (2007) Environ Chem Lett 5:203
Hennion MC, Barcelo D (1998) Anal Chim Acta 362:3
Jeschke P, Moriya K, Lantzsch R et al (2001) Pflanzenschutz-Nachr Bayer (Engl Ed) 54:147
Kim HJ, Shelver WL, Hwang EC, Xu T, Li QX (2006) Anal Chim Acta 571:66
Li K, Li QX (2000) J Agric Food Chem 48:3378
Liu LD, Hou ZG, Yin LD, Chen C, Lu ZB, Xie WM (2010) J Anhui Agric Sci 38:12556
Lu XQ, Zhang BH, Shi HY, Wang MH (2009) Food Agric Immunol 20:147
Mohan C, Kumar Y, Madan J, Saxena N (2010) Environ Monit Assess 165:573
Nunes GS, Toscano IA, Barceló D (1998) Trends Anal Chem 17:79
Peña A, Rodríguez-Liébana JA, Mingorance MD (2011) Chemosphere 84:464
Qian GL, Wang LM, Wu YR et al (2009) Food Chem 117:364
Seccia S, Fidente P, Montesano D, Morrica P (2008) J Chromatogr A 1214:115
Smodiš Škerl MI, Velikonja Bolta Š, Baša Česnik H, Gregorc A (2009) Bull Environ Contam Toxicol 83:374
Wanatabe S, Ito S, Kamata Y et al (2001) Anal Chim Acta 427:211
Wang C, Guan WB, Zhang HY (2011) Bull Environ Contam Toxicol 87:440
Watanabe E, Baba K, Miyake S (2011) Talanta 84:1107
Wengatz I, Stoutamire DW, Gee SJ, Hammock BD (1998) J Agric Food Chem 46:2211
Wu QH, Li Z, Wang C, Wu CX, Wang WN, Wang Z (2011) Food Anal Methods 4:559
Yu YL, Wu JL, Stahler M, Pestemer W (2007) J Environ Sci 19:205
Zeng DY, Shi HY, Li B, Wang MH, Song BA (2006) J Agric Food Chem 54:8682
Zhang Q, Wu YR, Wang LM, Hu BS, Li PW, Liu FQ (2008) Anal Chim Acta 625:87
Acknowledgments
This work was supported by the project of Graduates' Research Innovative Programs from Colleges and Universities of Jiangsu Province (no. CXZZ11-0675), the National “863” High-Tech Research Program of China (no. 2011AA100806), and the Special Fund for Agro-scientific Research in the Public Interest (no. 201203022).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Z., Li, M., Shi, H. et al. Development and Evaluation of an Enzyme-Linked Immunosorbent Assay for the Determination of Thiacloprid in Agricultural Samples. Food Anal. Methods 6, 691–697 (2013). https://doi.org/10.1007/s12161-012-9464-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-012-9464-9