Abstract
An indirect, competitive enzyme-linked immunosorbent assay (ic-ELISA) for the detection of difenoconazole was developed. Two haptens were designed and successfully synthesized. Hapten 1 had a particular moiety of difenoconazole, while hapten 2 had its full structure. The polyclonal antibodies against hapten-protein conjugates were prepared by immunizing rabbits. After optimization of the conditions, the detection limit (IC15) and sensitivity (IC50) were 4.58 and 29.10 μg L−1, respectively. The cross-reactivities of the antibody with 11 triazole fungicides were all less than 0.1%, which showed that the antibody had excellent specificity. The recoveries of difenoconazole from the spiked samples ranged from 89.70 to 102.31% with good accuracy. The matrix effect was easily removed using a simple, rapid, and efficient extraction method on fruits and vegetables. The detection limit was all 229 μg kg−1 in fruits and vegetables. To validate the ic-ELISA, samples were spiked with difenoconazole at three different concentrations and simultaneously analyzed using high-performance liquid chromatography (HPLC). The results showed a good correlation between the ic-ELISA and HPLC data (R 2 = 0.9970). As a result, the developed immunosorbent assay is suitable for the quantitative determination of difenoconazole in fruits and vegetables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Difenoconazole is a broad-spectrum and systemic 1,2,4-triazole fungicide that controls foliar, seed, and soil-borne diseases in several fruits, vegetables, and crops (Thom et al. 1997). It acts by interfering with ergosterol biosynthesis in fungi through inhibition of the C-14-demethylation of sterols, which leads to morphological and functional changes in the fungal cell membrane (European Food Safety Authority 2013). As a result, it has been widely used worldwide, although its intensive application may have led to undesirable side effects on human health and the environment (Kesavachandran et al. 2009 and Mu et al. 2013). A systematic investigation determined that difenoconazole could bind human serum albumin (HSA) through the hydrophobic effect, electrostatic interactions, and hydrogen bonding, as demonstrated by spectroscopic and molecular modeling (Li et al. 2013). Difenoconazole has been associated with an increase in the incidence of hepatocellular adenomas and carcinomas in a group of male and female mice following long-term dietary exposure (Li et al. 2012). The dissipation of difenoconazole under field conditions and its residues in apples and soils were studied to evaluate its safety (Guo et al. 2010 and Wang et al. 2012). There is an urgent need to establish a fast, simple detection method for difenoconazole.
Current testing methods for difenoconazole have mainly focused on chromatographic methods with different pretreatments and detector systems (Kong et al. 2012). A gas chromatography (GC) method for detecting difenoconazole residues was developed using a solid-phase extraction (SPE) cleanup in Chinese cabbage and soil (Wang et al. 2008). Liquid chromatography-tandem mass spectrometry (LC-MS/MS) with ethyl acetate extraction was used for simultaneous analysis of fipronil (and its metabolites) and difenoconazole residues in okra (Sandip et al. 2015). Pipette tip-based molecularly imprinted polymer monolith microextraction (PT-MIPMME) followed by high-performance liquid chromatography with a diode array detector (HPLC-DAD) was developed as an analytical method for the sensitive and selective determination of difenoconazole in tap water and grape juice (Du et al. 2014). The advantages of these methods were sensitivity and accuracy, although they require expensive instrumentation and specialized personnel and are time-consuming (Zhang et al. 2015, Farajzadeh et al. 2014 and Wang et al. 2011).
Enzyme-linked immunosorbent assays (ELISA) have received wide attention as a rapid detection method due to its sensitivity, rapidity, and reliability and to its successful applications in pesticide and veterinary detections (Macro et al. 1995a, b). To the best of our knowledge, there is no ELISA method reported for difenoconazole. In this study, two haptens were successfully synthesized, and the polyclonal antibodies of difenoconazole were prepared. After optimization of its conditions, ic-ELISA showed excellent specificity and good accuracy for the detection of difenoconazole in foods.
Materials and Methods
Reagents and Apparatus
Bromoacetyl chloride, 3,4′-dichlorodiphenyl ether, [BMIM]Cl, 4-aminobutyric acid, 1,2,4-triazole sodium, ethyl chloroacetate, myclobutanil, triadimenol, tebuconazole, bitertanol, triadimefon, diniconazole, flusilazole, flutriafol, and propiconazole were purchased from Sigma-Aldrich (St. Louis, MO). Epoxiconazole and azaconazole were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Keyhole limpet hemocyanin (KLH), bovine serum albumin (BSA), ovalbumin (OVA), horseradish peroxidase (HRP), N-hydroxysuccinimide (NHS), N,N-dicyclohexyl-carbodiimide (DCC), and 3,3′,5,5′-tetramethylbenzidine (TMB) were obtained from Merck (Darmstadt, Germany). Double-distilled water was prepared by a Millipore Milli-Q water system (Millipore, USA). Protein A-Sepharose 4B was purchased from Amersham Biosciences (Uppsala, Sweden). The polystyrene 96-well microplates were purchased from Nunc (Rockilde, Denmark). Immunoassay absorbances were recorded using a Multiskan Spectrum in dual-wavelength mode (450–650 nm) from Thermo (Vantaa, Finland).
Solutions
The solutions involved in these experiments were phosphate-buffered saline (PBS, pH 7.4), phosphate-buffered saline with 0.05% Tween 20 (PBST), coating buffer (CB, pH 9.6), blocking buffer (0.5% skimmed milk powder in PBS), and terminating buffer (1.25 mol L−1 H2SO4).
Synthesis of Hapten 1
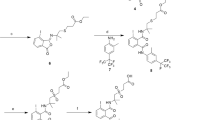
The synthetic route to hapten 1 is shown in Fig. 1. A 100-mL solution containing 6.990 g (0.04 mol) of [BMIM]Cl and 12.975 g (0.08 mol) of ferric chloride was stirred in a three-necked flask for 12 h at room temperature. Bromoacetyl chloride (7.0 mL, 0.084 mol) and 3,4′-dichlorodiphenyl ether (10 mL, 0.054 mol) were added to the above solution, and the mixture was heated at 60 °C for 5 h. The reactant solutions were washed with cyclohexane. The crude products were purified using column chromatography in petroleum ether/ethyl acetate (12:1, v/v). After removal of the solvent by vacuum, intermediate I, 2-bromo-1-(2-chloro-4-(4-chlorophenoxy)phenyl)ethanone, was synthesized. The product was identified by mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy. MS (ESI): found m/z: 359.76 [M]+, calculated m/z for C14H9BrCl2O2: 360.03. 1H NMR (400 MHz, CDCl3) δ (ppm) are as follows: 7.65 (1H, d, ph-H), 7.37–7.39 (2H, m, ph-H), 7.00–7.03 (3H, m, ph-H), 6.91–6.93 (1H, dd, ph-H), 4.71 (1H, s, −CH2−), 4.52 (1H, s, −CH2−).
Sodium bicarbonate (5.29 mg, 0.063 mmol), potassium iodide (10.46 mg, 0.063 mmol), intermediate I (22.62 mg, 0.063 mmol), and 4-aminobutyric acid (9.73 mg, 0.094 mmol) were added to 4 mL of DMF and stirred for 5 h at 70 °C. The crude products were extracted, and after removal of the solvent by vacuum, hapten 1 was obtained as a white powder. MS (ESI): found m/z: 382.29 [M]+, calculated m/z for C18H17Cl2NO4: 382.24. 1H NMR (400 MHz, CDCl3) δ (ppm): 7.65 (1H, d, ph-H), 7.36–7.39 (2H, m, ph-H), 7.00–7.03 (3H, m, ph-H), 6.90 (1H, dd, ph-H), 3.78 (2H, s, −CH2−), 2.54 (2H, t, −CH2−), 2.36 (2H, t, −CH2−), 1.81 (2H, m, −CH2−).
Synthesis of Hapten 2
The synthetic route of hapten 2 is shown in Fig. 1. A solution containing 1.23 g (3.40 mol) of intermediate I and 0.26 g (2.80 mol) of 1,2,4-triazole sodium in 7 mL of isobutanol was stirred for 12 h at 110 °C. The crude products were purified by column chromatography in petroleum ether/ethyl acetate (1:1, v/v). After solvent evaporation, intermediate II, 1-(2-chloro-4-(4-chlorophenoxy)phenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone, was synthesized. The products were identified by mass spectrometry with ESI: m/z 347.02 [M-H]−, calculated for C16H11Cl2N3O2, Mr = 348.18. Intermediate II (99.82 mg, 0.287 mmol) and sodium borohydride (28.12 mg, 0.743 mmol) were added to ethanol and stirred for 10 min at room temperature. The crude products were dissolved in 3 mL of DMF with potassium iodide (47.45 mg, 0.286 mmol) and ethyl chloroacetate (46 μL, 0.432 mmol). The mixture was heated to 110 °C for 12 h. After the pH was adjusted to 9.3 using a 10% sodium hydroxide solution, the mixture was stirred for 4 h at 50 °C. The solution was removed by rotary evaporation and the pH was adjusted to 3.0 using hydrochloric acid. The crude products were extracted and purified by column chromatography in petroleum ether/ethyl acetate solution (1:3, v/v). Hapten 2 was identified by mass spectrometry with ESI: found m/z: 408 [M]+, calculated m/z for C18H15Cl2N3O4: 408. 1H NMR (400 MHz, CDCl3) δ (ppm): 8.23 (1H, s, Tr-H), 7.93 (1H, s, Tr-H), 7.56 (1H, d, ph-H), 7.33–7.35 (2H, m, ph-H), 7.03 (1H, d, ph-H), 6.97 (2H, m, ph-H), 6.83 (1H, dd, ph-H), 4.70–4.83 (2H, q, −CH2−), 4.10 (1H, m, −CH2−), 3.93 (1H, m, −CH2−), 3.10 (1H, t, −CH−).
Preparation of Hapten-Protein Conjugates
The method for the preparation of protein conjugates was similar to previous studies (Macro et al. 1995a, b). Using the active ester method, the two haptens were coupled to KLH or BSA to prepare four immunogens. The two haptens were coupled to OVA for use as coating proteins using the mixed anhydride method.
Antibody Production
The polyclonal antibodies were produced in rabbits with immunogens according to previous reports (Bertold et al. 1995 and Sun et al. 2009). Two male New Zealand white rabbits were each injected subcutaneously six times with each conjugate (1 mg mL−1) at 2-week intervals. The first injection used a mixture of 1 mL of the conjugate and 1 mL of complete Freund’s adjuvant. The subsequent boost injections were the same as the first injection, except that incomplete adjuvant was used instead of complete adjuvant. The rabbits were bled for 10 days when the final injection was finished, and the antisera were purified by protein A-Sepharose 4B affinity chromatography.
Indirect Competitive ELISA
The rapid tests employed 96-well plates that had been coated overnight with a purified coating antigen (1 μg per well in 100 μL of 50 mM CB, pH 9.6). The coated plates were washed three times using PBST, and nonspecific antibody binding was blocked by applying 200 μL per well of 0.5% skimmed milk powder in PBS for 1 h the next day. The plates were washed three times using PBST, and the assay was performed by adding 50 μL of the difenoconazole standard in PBS solution and 50 μL of the purified polyclonal antibody diluted in PBS to each well for 1 h. The plates were washed and then incubated for 0.5 h with 100 μL per well of goat anti-rabbit IgG-HRP diluted 1:20,000 in PBS. After the wells were washed five times with PBST, 100 μL of TMB peroxide-based substrate solution was added to each well. Color development was stopped after 20 min by adding 50 μL of 1.25 M H2SO4, and the absorbance was read.
Optimization of ic-ELISA
The ODmax and IC50 were used as evaluation standards to optimize the various experimental conditions. The ideal ODmax value was chosen as between 0.8 and 1.2, and the IC50 value was minimized, representing higher sensitivity.
The assay conditions of the indirect competitive ELISA, including the concentrations of the coating antigen (0.05, 0.10, or 0.50 μg well−1), the antibody dilution (1:1500, 1:2000, or 1:3000), the blocking solution (0.5 or 1.0% dried skim milk or 0.5 or 1.0% OVA), the ionic strength (10, 30, or 50 mmol L−1), and the pH value of the sample dilution buffer (5.7, 7.4, or 8.5), were optimized.
Cross-Reactivity Determination
To determine the specificity of the difenoconazole antibody, 11 other triazole fungicides (myclobutanil, triadimenol, tebuconazole, bitertanol, triadimefon, diniconazole, epoxiconazole, azaconazole, flusilazole, flutriafol, and propiconazole) were investigated to assess the cross-reactivity.
The cross-reactivity (CR) was calculated as follows:
Sample Preparation for ELISA
Fruits (tangerines, apples, pears, bananas, and papayas) and vegetables (broccoli, cabbage, tomatoes, cucumbers, and celery) were purchased from local markets. To accurately represent the actual condition of the difenoconazole residues, only the edible parts were chosen to be crushed and maintained at 4 °C without any further treatment. Before conducting the spiking and recovery studies, all the samples were characterized without difenoconazole by HPLC. All the samples were prepared for immunoassay using the same extraction procedure: 5 g of the sample was spiked with difenoconazole at three different levels (500, 2500, 25,000 μg kg−1), extracted with 10 mL of hexane, and mixed for 5 min in a rotary shaker. Two milliliters of the clear supernatant was dried using nitrogen blowing, and 1 mL of methanol was added to redissolve it. The solutions were then diluted 50-fold with 0.01 M PBS for analysis (to remove any matrix effects).
HPLC Analysis of Difenoconazole
To validate the ELISA method, the samples were also analyzed using HPLC. The difenoconazole extraction procedure for HPLC was as follows: the samples (1 g) were spiked with difenoconazole at three different levels (10, 50, and 500 μg kg−1), extracted with 1 mL of hexane, and mixed for 5 min using a rotary shaker; the clear supernatant was directly used for HPLC analysis. A Shimadzu HPLC system was equipped with an LC-10AT pump and an ultraviolet detector (230-nm emission wavelength). A C18 column (5 μm, 15 cm × 4.6 mm) was used with a solution of water and acetonitrile (30:70) as the mobile phase at a flow rate of 1.0 mL min−1. The injection volume was 20 μL, and the separation was performed at a column temperature of 40 °C.
Results and Discussion
Hapten Design and Synthesis
Difenoconazole is one of the 1,2,4-triazole fungicides. Aside from its common triazole moiety, it also has a special group, a substituted phenyl ether phenyl moiety. Considering these two aspects, two haptens were designed. Hapten 1 had only the special group and was prepared from intermediate I, 2-bromo-1-(2-chloro-4-(4-chlorophenoxy)phenyl)ethanone, and 4-aminobutyric acid, after acylation of 3,4′-dichloro-diphenylether with [BMIM]Cl-FeCl3 as the catalyst. In the preparation of hapten 2, intermediate I was reacted with 1,2,4-triazole sodium to synthesize intermediate II. After the reduction of intermediate II in the presence of sodium borohydride, it was reacted with chloroacetic acid ethyl ester and then hydrolyzed under basic conditions to produce hapten 2. The two haptens were conjugated to BSA or KLH to prepare the immunogens, and the polyclonal antibodies were obtained by immunizing the rabbits.
Effect on the Titer and Affinity of the Antisera
The titer and inhibition of the antisera were monitored by ic-ELISA after immunization. The screening of the coating antigens (Hapten1-OVA, Hapten2-OVA) and the affinity of the antisera were studied, and the results are shown in Table 1. It was found that the antisera performed best with hapten1-KLH in NO. Three rabbits give a titer of 64,000 and 86% inhibition with hapten1-OVA as the coating antigen. Unfortunately, the antisera from the hapten 2 immunogen did not yield ideal results, possibly because of inefficient conjugation between the hapten and the carrier protein. After purification using a protein A-Sepharose 4B affinity column, the concentration of the antibody was calculated to be 2.12 mg mL−1.
Optimization of ic-ELISA
The indirect competitive ELISA was optimized using a chessboard titration. The optimized condition was chosen according to the lowest IC50 with an absorbance between 0.8 and 1.2 for the control sample in color development, and the results are shown in Table 2. A coating antigen concentration of 0.05 μg per well and an antibody dilution factor of 2000 were the best among all the conditions tested. Furthermore, the chosen blocking solution was 0.5% dried skim milk, the optimal ionic strength of the PBS buffer was 10 mmol L−1, and the optimal pH of the sample dilution buffer was 7.4.
A seven-point calibration curve was constructed using a stepwise dilution of the difenoconazole standard solution under the optimal conditions shown in Fig. 2. The average IC50 (sensitivity) was 29.10 μg L−1, and the average IC15 (limit of detection) was 4.58 μg L−1.
Assay Specificity
The cross-reactivity of the antibody was investigated between difenoconazole and the other 11 triazole fungicides (myclobutanil, triadimenol, tebuconazole, bitertanol, triadimefon, diniconazole, epoxiconazole, azaconazole, flusilazole, flutriafol, and propiconazole) as shown in Table 3. The results showed that the cross-reactivities for the other 11 triazole fungicides were all less than 0.1%. No cross-reactivity was observed with these compounds, which indicated that the antibody had good specificity for difenoconazole. The main reason was that the antibody was obtained from hapten 1, which has the special group of difenoconazole, a substituted phenyl ether phenyl moiety, while the other fungicides did not have the corresponding group.
Matrix Effects
Matrix effects could affect the binding reaction between the antibody and the analyte, leading to reductions in the sensitivity and reliability of the assay. Usually, matrix effects can be removed by diluting sample extracts in the appropriate buffers. Five fruits (tangerines, apples, pears, bananas, and papayas) and five vegetables (broccoli, cabbage, tomatoes, cucumbers, and celery) were chosen as the test samples to study the matrix influence. Difenoconazole standard inhibition curves were prepared in these samples and in PBS as a control. Different dilution folds (5, 10, or 50) in PBS were tested for reducing matrix effects, and the results are shown in Fig. 3. Taking the apple sample as an example, it was shown that at a 50-fold dilution, the sample curves agreed best with the standard curve. As a result, 50-fold dilution was thought to eliminate the matrix effect and could be analyzed using the standard curve. After accounting for a 50-fold dilution in the pretreatment process, the detection limit was 229 μg L−1 in samples.
Recovery Study
Difenoconazole is widely used in fruit and vegetable planting, so five fruits (tangerines, apples, pears, bananas, and papayas) and five vegetables (broccoli, cabbage, tomatoes, cucumbers, and celery) were chosen to assess the accuracy of the developed ELISA through recovery studies. Ten samples were all fortified with difenoconazole at three different levels (10, 50, 500 μg kg−1) and analyzed using the established indirect competitive ELISA. Each sample was evaluated at least three times to verify the repeatability of the assay. The results are shown in Table 4. It was found that the recovery was between 89.70 and 102.31% and that the coefficient of variation was less than 13.11%. These results showed that the developed ELISA was accurate and repeatable.
Validation of the Indirect Competitive ELISA
The comparison of ic-ELISA in the determination of difenoconazole in food samples was studied in the above foods, which were analyzed using HPLC. The obtained results are shown in Fig. 4. The ELISA and HPLC data agreed well, with the slope of a linear fit of 1.015 and a correlation coefficient of 0.9970. This demonstrated that the developed ic-ELISA was well correlated with the HPLC results for these samples and that it could be applied for the accurate determination of difenoconazole in fruits and vegetables.
Comparison of the Developed ic-ELISA with the Other Analytical Methods for the Detection of Difenoconazole
Other analytical methods for the detection of difenoconazole are listed in Table 5. It was found that the LOD value for the LC and GC methods had a lower detection capability than that of the proposed method, and the LOD value of surface-enhanced Raman spectroscopy (SERS) of the proposed method was much higher than that of all the other methods (mg L−1 vs μg L−1). For ic-ELISA and SERS, the best advantages are a simple pretreatment and rapid detection. As a result, the developed ic-ELISA is more suitable for the determination of difenoconazole because of its convenient pretreatment and good sensitivity.
Conclusions
In summary, this is the first report on developing a simple, rapid, and sensitive ic-ELISA method for the detection of difenoconazole in food samples. The polyclonal antibody showed a high specificity for difenoconazole. The sensitivity (IC50) was 29.10 μg L−1, and the detection limit (IC15) was 4.58 μg L−1. The recoveries were 89.70–102.31% in fruits and vegetables, and the coefficient of variation was less than 13.11%. The ic-ELISA results agreed and were strongly correlated with the HPLC analysis. Therefore, ic-ELISA can be used as a quantitative tool for the rapid screening of difenoconazole residues for quality control in foods.
References
Bertold H, Andrea D, Karl K et al (1995) Immunochemical techniques: antibody production for pesticide analysis. A review. Anal Chim Acta 311:393–405. doi:10.1016/0003-2670(95)00148-S
Du T, Cheng J, Wu M, Zhou M, Cheng M (2014) An in situ immobilized pipette tip solid phase microextraction method based on molecularly imprinted polymer monolith for the selective determination of difenoconazole in tap water and grape juice. J Chromatogr B 951:104–109. doi:10.1016/j.jchromb.2014.01.030
European Food Safety Authority (2013) Reasoned opinion on the modification of the existing MRLs for difenoconazole in various crops. EFSA J 11(3):3149. doi:10.2903/j.efsa.2013.3149
Farajzadeh MA, Mogaddam MRA, Ghorbanpour H (2014) Development of a new microextraction method based on elevated temperature dispersive liquid–liquid microextraction for determination of triazole pesticides residues in honey by gas chromatography-nitrogen phosphorus detection. J Chromatogr A 1347:8–16. doi:10.1016/j.chroma.2014.04.067
Guo C, Li J, Guo B, Wang H (2010) Determination and safety evaluation of difenoconazole residues in apples and soils. Bull Environ Contam Toxicol 85:427–431. doi:10.1007/s00128-010-0104-z
Hingmire S, Oulkar DP, Utture SC, Ahammed Shabeer TP, Baerjee K (2015) Residue analysis of fipronil and difenoconazole in okra by liquid chromatography tandem mass spectrometry and their food safety evaluation. Food Chem 176:145–151. doi:10.1016/j.foodchem.2014.12.049
Kesavachandran CN, Fareed M, Pathak MK, Bihari V, Mathur N, Srivastava AK (2009) Adverse health effects of pesticides in agrarian populations of developing countries. Rev Environ Contam Toxicol 200:33–52. doi:10.1007/978-1-4419-0028-9
Kong Z, Dong F, Xu J, Liu X, Zhang C, Li J, Li Y, Chen X, Shan W, Zheng Y (2012) Determination of difenoconazole residue in tomato during home canning by UPLC-MS/MS. Food Control 23:542–546. doi:10.1007/s00128-010-0104-z
Li J, Dong F, Cheng Y, Liu X, Xu J, Li Y, Chen X, Kong Z, Zheng Y (2012) Simultaneous enantioselective determination of triazole fungicide difenoconazole and its main chiral metabolite in vegetables and soil by normal-phase high-performance liquid chromatography. Anal Bioanal Chem 404:2017–2031. doi:10.1007/s00216-012-6240-z
Li Y, Ma X, Lu G (2013) Systematic investigation of the toxic mechanism of difenoconazole on protein by spectroscopic and molecular modeling. Pestic Biochem Physiol 105:155–160. doi:10.1016/j.pestbp.2012.12.010
Macro MP, Gee S, Hammock BD (1995a) Immunochemical techniques for environmental analysis I. Immunosensors. Trends Anal Chem 14:341–350. doi:10.1016/0165-9936(95)97062-6
Macro MP, Gee S, Hammock BD (1995b) Immunochemical techniques for environmental analysis II. Antibody production and immunoassay development. Trends Anal Chem 14:415–425. doi:10.1016/0165-9936(95)90920-I
Mu X, Pang S, Sun X, Gao J, Chen J, Chen X, Li X, Wang C (2013) Evaluation of acute and developmental effects of difenoconazole via multiple stage zebrafish assays. Environ Pollut 175:147–157. doi:10.1016/j.envpol.2012.12.029
Sandip H, Dasharath PO, Sagar CU, Shabeer TPA, Banerjee K (2015) Residue analysis of fipronil and difenoconazole in okra by liquid chromatography tandem mass spectrometry and their food safety evaluation. Food Chem 176:145–151. doi:10.1016/j.foodchem.2014.12.049
Sun J, Liu B, Zhang Y, Wang S (2009) Development of an enzyme-linked immunosorbent assay for metolcarb residue analysis and investigation of matrix effects from different agricultural products. Anal Bioanal Chem 394(8):2223–2230. doi:10.1007/s00216-009-2911-9
Thom E, Ottow JCG, Benckiser G (1997) Degradation of the fungicide difenoconazole in a silt loam soil as affected by pretreatment and organic amendment. Environ Pollut 96:409–414. doi:10.1016/S0269-7491(97)00037-7
Wang C, Wu Q, Wu C, Wang Z (2011) Application of dispersion–solidification liquid–liquid microextraction for the determination of triazole fungicides in environmental water samples by high-performance liquid chromatography. J Hazard Mater 185:71–76. doi:10.1016/j.jhazmat.2010.08.124
Wang K, Wu JX, Zhang HY (2012) Dissipation of difenoconazole in rice, paddy soil, and paddy water under field conditions. Ecotoxicol Environ Saf 86:111–115. doi:10.1016/j.ecoenv.2012.08.026
Wang ZH, Yang T, Qin DM, Gong Y, Ying J (2008) Determination and dynamics of difenoconazole residues in Chinese cabbage and soil. Chin Chem Lett 19:969–972. doi:10.1016/j.cclet.2008.04.028
Xiong JF, Wu RM, Guo P, Liu MH, Wang XB, Ji WD, Yan LY (2016) Rapid detection of difenoconazole residues in celery by surface-enhanced Raman spectroscopy. Mod Food Sci Technol 32(4):283–287. doi:10.13982/j.mfst.1673-9078.2016.4.045
Zhang ZY, Jiang WN, Jian Q (2015) Residues and dissipation kinetics of triazole fungicides difenoconazole and propiconazole in wheat and soil in Chinese fields. Food Chemistry 168:396–403. doi:10.1016/j.foodchem.2014.07.087
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was funded by the National Natural Science Foundation of China (Project No. 31301462) and the Ministry of Science and International Science and Technology Cooperation Program of China (Project No. 2014DFR30350).
Conflict of Interest
Bing Liu declares that she has no conflict of interest. Jiuhui Feng declares that she has no conflict of interest. Xiao Sun declares that she has no conflict of interest. Wei Sheng declares that she has no conflict of interest. Yan Zhang declares that she has no conflict of interest. Shuo Wang declares that he has no conflict of interest.
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of laboratory animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Liu, B., Feng, J., Sun, X. et al. Development of an Enzyme-Linked Immunosorbent Assay for the Detection of Difenoconazole Residues in Fruits and Vegetables. Food Anal. Methods 11, 119–127 (2018). https://doi.org/10.1007/s12161-017-0983-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0983-2