Abstract
To develop a broad-specificity immunoassay for organophosphorus pesticides (OPs), a broad-specificity monoclonal antibody (MAb) for OPs against a generic hapten, O,O-diethyl O-(3-carboxyphenyl) phosphorothioate with the carboxy group in the meta position of the benzene ring, was produced. Eight haptens were prepared and covalently attached to ovalbumin (OVA) for use as coating antigens, and the optimum coating antigen was selected. Then, a sensitive and broadly class selective competitive indirect enzyme-linked immunosorbent assay (ciELISA) based on the MAb and the optimum coating antigen (hapten H-OVA, possessing an O,O-dimethyl generic structure and linked through a linear spacer arm) was developed and optimized. The MAb developed in this study showed quite different cross-reactivity and selectivity compared to previously produced anti-OPs broad-specificity MAbs. Specifically, the MAb showed high and uniform sensitivity to seven O,O-diethyl OPs and six O,O-dimethyl OPs. With the optimum ciELISA, the IC50 values of the 13 OPs were determined as 23.1∼151.2 ng mL−1. The average IC50 and coefficient of variation (CV) for the IC50 values of the 13 OPs were 74.6 ng mL−1 and 33.9 %, respectively. For the recovery study, a QuEChERS approach based on dispersive solid-phase extraction (d-SPE) was implemented to decrease the matrix effects of vegetable and fruit samples. The recoveries of six representative OPs from the spiked samples ranged from 89.4 to 135.5 %; the CV ranged from 3.5 to 15.7 %. The ciELISA was also applied to real samples, followed by confirmation with gas chromatography–tandem mass spectrometry (GC–MS/MS) analysis. The results demonstrated that the ciELISA is suitable for monitoring OP contamination in vegetable and fruit samples.

Development of a MAb-based immunoassay for determination of organophosphorus pesticides pretreated with QuEChERS
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus pesticides (OPs) are the most widely used pesticides in agriculture because of their effective broad-spectrum insecticidal activity and relatively low stability under natural conditions [1–3]. However, OP contaminant residues, which can result in fatal consequences, have been broadly reported in food and environmental samples [4, 5]. The residues have high toxicity in humans and animals, even at very low concentrations [3, 6]. Thus, there is an increasing demand for broader and stricter monitoring of these residues to improve food safety and protect the ecosystem.
Over the past few decades, numerous analytical methods have been developed to determine the presence of OP residues in food and water samples. Among these methods, gas chromatography–mass spectrometry (GC/MS) [7, 8] and high-performance liquid chromatography–mass spectrometry (HPLC/MS) [9, 10] have been widely used due to their sensitivity and reliability. However, these classical methods require complex and expensive instrumentation and laborious and tedious pretreatment of samples and are not conducive to large-scale screening studies. As an alternative, antibody-based immunoassays such as the enzyme-linked immunosorbent assay (ELISA) have proven to be rapid, sensitive, and a cost-effective analytical tool for routine monitoring [11–14]. Recently, broad-specificity immunoassays have emerged to determine a group of similar pesticides because if the total quantity of pesticides in a sample is less than the maximum residue limit of the pesticides, we do not need to further monitor these pesticides [15]. In the past few years, many broad-specificity immunoassays have been reported [16–23]. However, the detection of multiple OPs always has the problem that either the detection spectrum is not broad enough or the detection sensitivity is not uniform.
For the application of the immunoassay technique, the matrix effects of prepared food samples are always the major challenge because the co-extracted matrix contained chlorophyll, lipids, sterols organic acids, sugars, and other components that have serious effects on the analysis results. The complicated food matrix can cause false positives by lowering the intensity of color development or by interfering with the antigen–antibody binding. These types of matrix effects can often be reduced by employing a large dilution of samples [15, 24] or pretreating the samples with solid-phase extraction (SPE) [25, 26]. However, large dilution of the samples resulted in significantly reduced sensitivity [15, 24], and the SPE procedure usually included pretreatment of the sorbent, elution, and solvent evaporation steps, that made it time-consuming, labor-intensive, expensive, and wasteful [27]. As an alternative, the QuEChERS approach based on dispersive solid-phase extraction (d-SPE) demonstrated some advantages, such as its simplicity, minimum steps (SPE steps, including pretreatment of the sorbent, elution, and solvent evaporation, were combined into one d-SPE cleanup step), and effectiveness for cleaning-up complex samples, and it has been successfully applied in the pretreatment of samples for the GC/MS and LC/MS analysis of pesticides in various types of food samples [27–32]. The QuEChERS approach uses less sorbent, smaller amounts of sample, and less equipment, which saves time, effort, and money by comparison with traditional SPE [32].
In the present study, a monoclonal antibody (MAb) with broad specificity and uniform sensitivity to O,O-diethyl and O,O-dimethyl OPs was produced. The optimum coating antigen was selected and used to develop the heterologous competitive indirect enzyme-linked immunosorbent assay (ciELISA). For the recovery study, the QuEChERS approach was performed to decrease the matrix effects in preparation for the immunoassays; OPs were spiked and determined in cabbage, cucumber, tomato, lettuce, apple, and peach samples. Furthermore, the ciELISA that was developed was applied to real samples, followed by confirmation with gas chromatography–tandem mass spectrometry (GC–MS/MS) analysis.
Materials and methods
Materials and instruments
Pesticides were purchased from National Standards (China). N-Hydroxysuccinimide (NHS), N,N′-dicyclohexylcarbodiimide (DCC), bovine serum albumin (BSA), ovalbumin (OVA), peroxidase-labeled goat anti-mouse immunoglobulin ((IgG-HRP), complete Freund’s adjuvants, incomplete Freund’s adjuvants, and TMB were purchased from Sigma Chemical Co. (St. Louis, USA). Dimethylformamide (DMF), n-hexane, and other chemical reagents were purchased from Aladdin Chemistry Co., Ltd. (China). Preparative TLC plates (silica gel F254, 0.2 mm) were purchased from Qingdao Yuyuan Chemical Co., Ltd. (Qingdao, China). Bondesil-primary secondary amine (PSA), C18, and graphitized carbon black (GCB) were purchased from Silibase (Shenzhen, China). Polystyrene 96-well microtiter plates were purchased from Nunc (Roskilde, Denmark). A gas chromatograph–tandem mass spectrometer (GC/MS-TQ8040) was purchased from Shimadzu (Japan). Ultraviolet–visible (UV–vis) spectra were recorded on the K5600 MicroSpectroPhotoMeter (Kaiao, China). Nuclear magnetic resonance (NMR) spectra were obtained using the DRX-600 NMR spectrometer (Bruker, Germany–Switzerland). ELISA plates were washed with the KHB ST-36W plate washer (Shanghai, China), and well absorbance was determined with the Model 680 plate reader (Bio-Rad, USA).
Synthesis and characterization of haptens
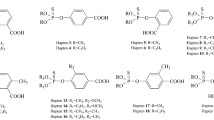
Eight haptens were synthesized to prepare the immunogen or coating antigens. The structures of these haptens are shown in Fig. 1.
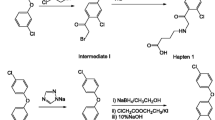
The synthetic routes for hapten A and hapten G are illustrated in Fig. 2. The procedure for the synthesis of hapten A is as follows.
-
Step I
Methyl 4-hydroxybenzoate (1). A solution of 5 g of 4-hydroxybenzoic acid in 20 mL of H2SO4/MeOH (1:10, v/v) was refluxed for 10 h. Then, the mixture was concentrated under reduced pressure. The residue was dissolved in 50 mL of ethyl acetate and adjusted to pH 8.0 with 10 % Na2CO3. The organic phase was dried over anhydrous Na2SO4 and concentrated under reduced pressure to give 5 g of product (1).
-
Step II
O,O-Diethyl O-[(4-methyloxycarbonyl)phenyl]-phosphorothioate (2). A solution of 1 (806 mg, 5.3 mmol) in 20 mL of acetonitrile was added dropwise to a stirred mixture of O,O-diethyl phosphorochlorodithioate (1.2 g, 6.4 mmol) with finely ground K2CO3 (5 g) and 20 mL of acetonitrile. After stirring for 1.5 h at room temperature, the mixture was filtered and the solvent was removed under reduced pressure to give a pale yellow oil (2, 1.6 g).
-
Step III
O,O-Diethyl O-(4-carboxyphenyl)phosphorothioate (hapten A). All of 2 (1.6 g) was added to 40 mL of an absolute ethanol:1 M KOH (3:2) solution and stirred at room temperature for 30 min. Subsequently, the reaction mixture was added to 60 mL of water and washed three times with 30 mL of ethyl acetate. Then, the water phase was adjusted to pH 3.0 with HCl, and white flocs were generated. Subsequently, 100 mL of water was added and filtered to provide a white powder product. The product was washed with 100 mL of water and filtered to acquire white crystals. Finally, the crystals were dried by air drying in an oven (65 °C) to give 1.21 g of hapten A.
The synthetic routes for hapten B–F are the same as for hapten A. Thin-layer chromatography (TLC) was used to monitor the progress of the reactions. Hapten C and F required more time than other haptens to complete the reaction of step II and step III. Using this synthetic strategy, pure products were obtained without column chromatographic purification.
The procedure for the synthesis of hapten G is as follows. A solution of 690 mg (12.3 mmoL) of KOH and 670 mg of (6.5 mmoL) 4-aminobutyric acid in 5 mL of MeOH was added dropwise to a stirred solution of O,O-diethyl phosphorochlorodithioate (1.0 g, 5.3 mmoL) in 5 mL of MeOH (cooled in an ice-water bath). After stirring for 2 h, the mixture was filtered, and the solvent was removed under reduced pressure. The residue was transferred to the separatory funnel with 20 mL of 5 % NaHCO3 and washed several times with n-hexane. Then, the aqueous phase was adjusted to pH 3.0 with HCl, and white flocs were generated. Subsequently, the sediment was extracted three times with 20 mL of dichloromethane. The extracts were dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the colorless oil (1.17 g).
Hapten H was synthesized and characterized as previously described [16]. The structures of the haptens were verified by 1H-NMR. The yields and 1H-NMR results for the haptens are shown in Table S1 (see Electronic Supplementary Material, ESM).
Preparation of hapten–protein conjugates
Hapten B was covalently attached to BSA for use as an immunogen. All of the eight haptens presented in Fig. 1 were attached to OVA for use as coating antigens. The active ester method was used to prepare hapten–protein conjugates [33]. The UV–vis spectrophotometer was used to confirm the structures of the final conjugates.
Production of monoclonal antibodies
Hapten B-BSA was used as an immunogen, and five BALB/C mice were injected. Procedures for the production of monoclonal antibodies, i.e., immunization, cell fusion, hybridoma selection, cloning, and ascites fluid preparation, were similar to the previous study [34]. One week after the last boost, serum was collected from the caudal vein of each mouse, and antisera titers were determined by noncompetitive indirect ELISA using both the homologous and heterologous coating antigens (hapten B-OVA and hapten C-OVA). Subsequently, the antisera were tested against four OPs (1 μg mL−1 parathion, diazinon, parathion-methyl, and fenthion) by ciELISA based on hapten B-OVA and hapten C-OVA. The mouse was selected and sacrificed for monoclonal antibody production. Hapten C-OVA was used in noncompetitive and competitive ELISAs for hybridoma screening. Four OPs (1 μg mL−1 parathion, diazinon, parathion-methyl, and fenthion) were used as analytes in ciELISAs to select the hybridoma cells. After three times subcloning of the selected hybridoma cells, the ascites fluids were produced and then purified by ammonium sulfate precipitation. The broad specificity and sensitivity of the selected MAbs were compared by ciELISA (hapten C-OVA was used as the coating antigen, and 20 OPs were used as analytes). The Mab that showed a higher sensitivity to increased amounts of OPs was selected for further study. The isotype of the chosen MAb was determined with a mouse monoclonal antibody isotyping kit (Roche Applied Science, USA), according to the instructions of the manufacturer.
Selection of the coating antigens
All of the hapten-OVA conjugates were tested for plate coating, and ciELISAs were performed to select coating antigens using parathion, diazinon, parathion-methyl, and fenthion as analytes. The procedures were as follows: microtiter plate was coated with hapten-OVA in 100 μL of phosphate-buffered saline (PBS; 10 mM, pH 7.4) and incubated at 4 °C overnight. The plates were washed three times with PBST (10 mM PBS containing 0.05 % Tween 20 at pH 7.4) and blocked with 1 % skim milk in 1× PBS (200 μL per well) at 37 °C for 1 h. After washing three times, 50 μL of analytes and 50 μL of ascites dissolved in PBS (10 mM, pH 7.4) were added to each well of the blocked plate. After 1 h incubation at 37 °C and three washes, 100 μL per well of diluted (1/5000) goat anti-mouse IgG-HRP was added. The plate was incubated for 1 h at 37 °C, followed by six washes. Finally, 100 μL per well of TMB solution was added. After incubation for 15 min, the reaction was stopped by 50 μL of 2 M H2SO4. The absorbance was read at 450 nm by a microplate reader. The coating antigen that showed the highest sensitivity to the analytes was selected as the optimum coating antigen and used for the further study.
ciELISA optimization
Optimal ciELISA conditions, such as the ionic strength, pH, and concentration of methanol, were determined. The maximal absorbance (A max) and IC50 values were used as criteria to evaluate immunoassay performance. The values of A max/IC50 were used to estimate the influence on ELISA [35]. The working solution that resulted in the highest ratio of A max/IC50 for the ciELISA was selected as the optimized condition. The MAb and coating antigen concentrations for ciELISA were determined by checkerboard protocol.
Cross-reactivity study
Thirteen O,O-diethyl OPs and 12 O,O-dimethyl OPs were tested for cross-reactivity using the optimum ciELISA procedure. The cross-reactivity (CR) was determined by assigning the IC50 of the parathion to be 100 % compared with the IC50 of the other analytes.
Pretreatment of vegetable and fruit samples for ciELISA
The preparation procedure for the vegetable and fruit samples (cabbage, cucumber, lettuce, tomato, apple, and peach) was as follows. First, the sample was finely homogenized, and 10 g was weighed into a 50-mL polypropylene centrifuge tube. Then, 10 mL of acetonitrile (MeCN) was added, and the tube was shaken vigorously for 1 min. After that, 4 g of anhydrous magnesium sulfate and 1 g of NaCl were added, and the tube was shaken vigorously for 1 min, followed by centrifuging for 5 min at 6000 rpm. One milliliter of the upper layer (organic phase) was transferred to the appropriate d-SPE tube. Different sorbents (PSA, C18, and GCB) were tested to decrease the matrix effects of ciELISA. The combinations of different sorbents used for d-SPE are shown in Table S2 (see ESM). After the extract was added, the tube was capped and shaken for 30 s followed by centrifuging for 3 min at 10,000 rpm. A portion of the upper layer (500 μL) was transferred to a 15 × 100-mm glass tube and evaporated at 45 °C under a stream of nitrogen. The residue was redissolved with 5 % MeOH water before analysis by ciELISA.
Analysis of the spiked samples by ciELISA
Three O,O-diethyl OPs (quinalphos, diazinon, parathion) and three O,O-dimethyl OPs (fenthion, fenitrothion, parathion-methyl) were used for recovery study. On the one hand, the six OPs were spiked on vegetables and fruits (cabbage, cucumber, tomato, lettuce, apple, and peach) at two concentrations (200 and 100 ng g−1) on 10 g samples in 50-mL centrifuge tubes. On the other hand, pesticide mixtures were also used for recovery study on spiked cucumbers. Three types of pesticide mixtures (40 ng g−1 of a mixture of quinalphos, diazinon, and parathion; 40 ng g−1 of a mixture of fenthion, fenitrothion, and parathion-methyl; 20 ng g−1 of a mixture of the six OPs) were applied in the recovery study. After being spiked with OPs, the samples were thoroughly mixed for 1 min and incubated for 1 h so that the pesticides would be fully mixed with the samples. The following extraction and d-SPE procedure were the same as the approach described above. To ensure the appropriate measuring results, the final residues of the six vegetable and fruit samples were diluted the same number of times before being detected by the optimized ciELISA.
Analysis of OPs by GC–MS/MS
For GC–MS/MS analysis, samples were pretreated by the QuEChERS method. Briefly, a vegetable or fruit sample was finely homogenized, and 15 g was weighed into a 50-mL polypropylene centrifuge tube. Then, 15 mL of acetonitrile, containing 0.1 % (v/v) of acetic acid, was added, and the tube was shaken vigorously for 1 min. Then, 6 g of anhydrous magnesium sulfate and 1.5 g of NaCl were added, and the tube was shaken vigorously for 1 min, followed by centrifuging for 5 min at 6000 rpm. Two milliliters of the upper layer (organic phase) was transferred to a d-SPE tube (containing 100 mg of PSA, 100 mg of C18, and 24 mg of GCB). After the extract was added, the tube was capped and shaken for 30 s, followed by centrifuging for 3 min at 10,000 rpm. The supernatant was filtered through a 0.22-μm organic filter before being transferred into an autosampler vial for its injection into the GC–MS/MS system.
A GC–MS/MS for analysis of 15 selective OPs (parathion, parathion-methyl, quinalphos, diazinon, phorate, disulfoton, dimethoate, chlorpyrifos-methyl, pirimiphos-methyl, fenthion, phenthoate, fenitrothion, phosalone, azinphos-methyl, and triazophos) was developed. A Shimadzu GC/MS-TQ8040 was used for the instrumental analysis of OPs. The GC separation was performed on an Rtx-5MS column (30 m × 0.25 mm × 0.25 μm film thickness). The injector temperature was set at 250 °C. The injection volume was 1.0 μL in the splitless mode. At 0.75 min, the split mode was switched on with a split flow of 50 mL per minute. At 2 min, the gas saver was turned on at a flow of 20 mL per minute. The initial temperature of the column oven was 95 °C (hold for 1.5 min). This temperature was increased at a rate of 20 °C per minute up to 190 °C; next, the temperature was increased to 230 °C at a rate of 5 °C per minute; and finally, the temperature was increased to 290 °C at a rate of 25 °C per minute (hold for 5 min). The mass spectrometric detector was operated in the electron ionization mode at an ionizing energy of 70 eV. The temperature of ion source and the MS transfer line was 230 °C. The calibration curves were evaluated with matrix-matched standard calibrations in blank extracts of cucumber at concentrations of 20, 50, 100, 200, and 400 ng mL−1.
Results and discussion
Selection and synthesis of haptens
For a broad-specificity immunoassay, the hapten should possess a group that is similar to the target analytes [36]. In the past few decades, numerous haptens were designed and synthesized to produce broad-specificity antibodies for OPs [15–23]. These reports have demonstrated that the spacer arms of haptens always play an important role in antibody production. For example, the polyclonal antisera, which were produced by a generic thiophosphate hapten linked though a linear spacer arm, have a high response to the linear OPs [20]; the MAbs produced by a generic thiophosphate hapten linked though an aromatic ring developed sensitivity-improved ELISAs for OPs because most of the commonly used OPs have an aromatic ring [15, 19, 23, 37]. Specifically, the MAb produced by the O,O-diethyl thiophosphate hapten linked though an aromatic ring with the carboxy group in the para position could recognize many OPs, especially those tending to form coplanar structures with the benzene ring. However, the results of CR studies showed that the MAb lacked a uniform response to OP analytes [23]. Another study demonstrated that the MAb produced by the hapten with the carboxy group in the para position and an additional substituent in the meta position of the benzene ring showed improved sensitivity to the OPs with substituents in the meta positions, such as diazinon, chlorpyrifos, and fenitrothion [15]. In addition, the MAbs produced by the hapten with the carboxy group in the meta position and an additional substituent in the ortho position of the benzene ring could recognize both O,O-diethyl OPs and O,O-dimethyl OPs, but had a low response to diazinon [37]. We thus propose that the MAbs produced by haptens with the carboxy group in the meta position have more uniform specificity to the OPs than the MAbs produced by haptens with the carboxy group in the para position because of more differences in the chemical structure between the haptens and the OPs. Moreover, we think that the additional substituent in the benzene ring is not conductive to promotion of the broad spectrum of antibodies due to the enhanced specificity of the haptens. Therefore, the O,O-diethyl thiophosphate hapten linked through aromatic rings with the carboxy group in the meta position was selected as the generic hapten (hapten B) for preparation of the immunogen.
The three-step synthetic method for hapten A-F, without any severe conditions or purification procedures by column chromatography, provided satisfactory purity and yields of the products. The yields and 1H-NMR results for the haptens are shown in Table S1 in the ESM. Using the active ester method, hapten B was covalently attached to BSA for use as an immunogen, and all of the haptens presented in Fig. 1 were attached to OVA for use as coating antigens. The hapten conjugates were confirmed by UV–vis spectrophotometry. As shown in ESM Fig. S1, the UV–visible spectra of all of the hapten conjugates are significantly changed compared to the carrier protein, which demonstrated the successful linkage.
Production and selection of monoclonal antibodies
The noncompetitive indirect ELISA results showed that antisera from the three times immunized mice could recognize both homologous and heterologous coating antigens well and that antiserum M1 was the best one (see ESM Fig. S2a and Fig. S2b). The ciELISA results demonstrated that antiserum M1 could recognize the four analytes well based on the heterologous coating antigen hapten C-OVA but that it had low sensitivity when homologous coating antigen hapten B-OVA was used (see ESM Fig. S2c). Therefore, hapten C-OVA was used for the following hybridoma cell screening. After cell fusion, hybridoma cells were screened by noncompetitive ELISA, and 98 of 576 cells exhibited a high response to the coating antigen hapten C-OVA. The following ciELISA results showed that nine cells have high sensitivity for the four test OPs, and the best three (3A7, 3B3, 4C3) cells were subcloned and used to produce ascites fluids. Broad specificity and sensitivity of the three MAbs were compared (see ESM Fig. S3), and the results demonstrated that all three MAbs showed broad specificity and sensitivity for the 20 analytes (1 μg mL−1 O,O-diethyl OPs and O,O-dimethyl OPs). Specifically, MAb 3A7, with the higher sensitivity to more OPs, was chosen for further study. The isotype of 3A7 was determined as IgG1 with a κ light chain.
Coating antigen selection
MAb 3A7 could recognize all of the coating antigens and showed high affinity for hapten B-OVA and hapten E-OVA while showing a middle affinity for hapten A-OVA, hapten C-OVA, hapten D-OVA, hapten G-OVA, and hapten H-OVA and low affinity for hapten F-OVA (ESM Fig. S4a). For the ciELISAs, the MAb showed the lowest sensitivity to the four OPs when hapten B-OVA or hapten E-OVA was used. Using hapten A-OVA or hapten G-OVA, the MAb showed high sensitivity to fenthion, but low sensitivity to the rest of the OPs. The MAb showed high sensitivity to all four analytes when hapten C-OVA, hapten D-OVA, or hapten H-OVA was used, and hapten H-OVA showed the highest sensitivity (ESM Fig. S4b). Thus, hapten H-OVA was selected to develop the heterologous ciELISA.
ciELISA optimization
ciELISA was optimized using hapten H-OVA as the homologous coating antigen. The effects of the conditions generated by the ionic strength, pH, and methanol concentration on the A max and IC50 values of parathion were evaluated in this study. As illustrated in Fig. S5a (see ESM), 5× PBS buffer led to the highest A max/IC50 value. And the results illustrated in ESM Fig. S5b and Fig. S5c indicated that A max/IC50 reached the highest value with pH 7.4 and 2.5 % methanol. According to the results of the checkerboard protocol, the optimum concentration of coating antigen was 1 μg mL−1 and the dilution of MAb was 1:7500.
Cross-reactivity study
Twenty-five commonly used OPs were used in the specificity and cross-reactivity (CR) study. The results of the CR study determined by the optimized ciELISA based on the MAb 3A7 and hapten H-OVA are shown in Table 1. The MAb showed high and uniform sensitivity to seven O,O-diethyl OPs and six O,O-dimethyl OPs. The 50 % inhibition values (IC50) of the 13 OPs were determined as 23.1∼151.2 ng mL−1, and the average IC50 and coefficient of variation (CV) for the IC50 values of the 13 OPs were 74.6 ng mL−1 and 33.9 %, respectively. The results demonstrated that this immunoassay was appropriate for class-specific determination of OPs due to its uniform sensitivity and broad specificity.
Furthermore, the cross-reactivity of MAb 3A7 in this study was quite different from other anti-OPs broad-specificity MAbs [15, 23]. The comparison of the sensitivity and broad specificity of the MAbs produced in this study and in the literature is shown in ESM Table S3. These data indicated that a different spacer arm structure for the haptens could result in MAbs with various sensitivities and broad specificity for OPs. The MAb produced in this study achieved the lowest average IC50 value and a CV value of IC50 for the 13 OPs. In addition, the ciELISA studied in this work revealed a high CR for more O,O-dimethyl OPs compared to early studies [15, 23]. MAb 3A7 showed the highest sensitivity for fenthion (O,O-dimethyl OP), but low CR for coumaphos (O,O-diethyl OP), which was different from the MAb developed by Xu et al. [23]. The result may be caused by the different position of carboxy group on the benzene ring of the generic haptens. The only difference between the two immune haptens used in the two studies is the position of the carboxy group on the benzene ring transferred from the para to the meta position. When haptens are covalently attached to the lysine of BSA, the hapten with the carboxyl in the para position of the benzene ring may form two aromatic ring structures, which is highly similar to the structure of coumaphos; but the hapten with carboxyl at the meta position of the benzene ring cannot be. Additionally, MAb 3A7 showed high sensitivity for both parathion and diazinon, but low CR for chlorpyrifos, bromophos-ethyl, and pirimiphos-ethyl, which was quite different from the MAbs developed by Piao et al. [15], demonstrating that the generic hapten, which possesses a carboxyl in the para position and a substituent in the meta position on the benzene ring, might result in a higher sensitivity to OPs with complex or bulky aromatic substituents such as diazinon chlorpyrifos, bromophos-ethyl, and pirimiphos-ethyl. However, the additional substituent in the benzene ring is not conductive to the promotion of a broad spectrum and uniform specificity of antibodies due to the enhanced specificity of the hapten. It is also worth noting that, in this study, the hapten with the carboxyl in the meta position and no additional substituent on the benzene ring led to higher sensitivity for fenthion and fenitrothion, both of which have substituents in the meta position of the benzene ring. This result demonstrated that the specific structures of the OPs rather than the general structures play an important role in the responses of MAbs.
Pretreatment of vegetable samples
The matrix effects of the prepared food samples are always the major bottleneck for the application of immunoassays to pesticide analysis [38]. A significant dilution of samples used to be performed to reduce matrix effects, but this dilution resulted in a significant reduction of the sensitivity of the assay [15, 24]. In this study, vegetable and fruit samples were pretreated by the QuEChERS approach based on d-SPE to decrease the matrix effects. Different combinations of sorbents shown in ESM Table S2 were tested for d-SPE, and the appropriate combination of sorbents was determined (see ESM Fig. S6). The results showed that the amount of PSA used for d-SPE influenced the ELISA significantly and that 100 mg of PSA was sufficient to purify 1 mL of the extraction matrix. GCB and C18 play a less important role in decreasing the matrix effects. Finally, the combination of 100 mg PSA + 150 mg anhydrous MgSO4 was selected for d-SPE pretreatment of 1 mL of the MeCN extract (ESM Fig. S6). The matrix effects of cucumber on noncompetitive ELISA with or without pretreatment by the QuEChERS approach were compared (Fig. 3a). The results showed that with the pretreatment of d-SPE, only a fourfold dilution could eliminate the matrix effects. By comparison, a dilution of more than 32 times was needed to eliminate the matrix effects for the samples without pretreatment. The d-SPE tests of cabbage, tomato, lettuce, apple, and peach extracts showed that cabbage, lettuce, and tomato also need a fourfold dilution to eliminate the matrix effects, but apple and peach need only a twofold dilution. Moreover, the matrix effects of cucumber on ciELISA due to unpurified and purified cucumber extracts were determined with parathion as the analyte (Fig. 3b, c). The results showed that after pretreatment with d-SPE, only a fourfold dilution could eliminate the matrix effects, while dilution 40 times was necessary to eliminate the matrix effects of the sample without pretreatment with d-SPE.
Analysis of the spiked samples
For the recovery study, in view of the sensitivity of the MAb to the matrix, all of the vegetable and fruit samples were pretreated with d-SPE and were diluted fourfold to eliminate the matrix effects before analysis by the optimum ciELISA. Standard ciELISA inhibition curves for the six OPs are presented in Fig. 4. As presented in Table 2, the recoveries for individual OPs ranged from 89.4 to 131.5 % and the CV ranged from 3.5 to 15.7 %. As shown in Table S4 (see ESM), the spiked OP mixtures could obviously be detected with an inhibition of 39.7, 63.9, and 51.6 % for the three spiked mixtures. The inhibitions of the spiked OP mixtures in the vegetable samples were compared with the pesticide mixtures spiked in PBS, and the recoveries were 93.4, 113.7 and 106.6 %, respectively. The results demonstrated that the ciELISA that was developed based on MAb 3A7 and hapten H-OVA was available to monitor OPs residues in vegetable and fruit sample pretreatment with d-SPE.
Comparison of ciELISA and GC–MS/MS analysis
The developed ciELISA was confirmed by GC–MS/MS. Cucumber samples without OPs were homogenized and separated into ten groups and were then spiked with parathion and parathion-methyl at 60, 120, 180, 240, and 480 ng g−1. Each group was separated into two parts. One of the two parts was detected by the optimum ciELISA, and the other part was detected by GC–MS/MS. The linear regression equations between the ciELISA and GC–MS/MS were determined as y = 23.238 + 0.899x, R 2 = 0.981 for parathion and y = 16.062 + 0.946x, R 2 = 0.992 for parathion-methyl. The correlation between data obtained from ELISA and GC–MS/MS is shown in Fig. 5 and good correlation of the two methods was observed. The result indicated that the developed ciELISA is suitable for the detection of OPs in cucumber samples.
Screening of real samples by ciELISA and GC–MS/MS
Forty vegetable and fruit samples (including 30 cucumber samples, 5 tomato samples, 3 apple samples, and 2 peach samples) were randomly collected from the markets of Taian. All of the samples were analyzed by ciELISA, followed by confirmation with GC–MS/MS. The broad-specificity ciELISA that had been developed was used as a semi-quantitative screening method determined according to the percent inhibition (percent inhibition was calculated by the equation (1 − A / A 0) × 100, where A is the absorbance of samples and A 0 is the absorbance of the PBS control). The results showed that the percent inhibition of ciELISA ranged from −5.6 to 16.6 %. The subsequent GC–MS/MS analysis showed that 5 of the 40 samples contain OPs, and all of the positive samples are cucumber samples. Cucumber sample 4 contains 8.2 ng g−1 phenthoate and 14.4 ng g−1 triazophos with a ciELISA percent inhibition of 10.6 %; cucumber sample 9 contains 6.2 ng g−1 chlorpyrifos-methyl and 6.4 ng g−1 triazophos, with a ciELISA percent inhibition of 4.6 %; cucumber sample 10 contains 3.2 ng g−1 diazinon, with a ciELISA percent inhibition of −1.6 %; cucumber sample 20 contains 20.2 ng g−1 diazinon and 18.6 ng g−1 pirimiphos-methyl, with a ciELISA percent inhibition of 16.6 %; cucumber sample 29 contains 28.7 ng g−1 diazinon, with ciELISA percent inhibition of 12.4 %. These results indicate that the ciELISA was suited for screening food samples containing OPs at a low concentration.
Conclusions
In summary, eight generic haptens were synthesized for OP immunological detection. Among these haptens, hapten B, a generic hapten O,O-diethyl O-(3-carboxyphenyl) phosphorothioate with a carboxy group in the meta position of the benzene ring, was covalently attached to BSA for use as an immunogen, and a broad-specificity MAb 3A7 was produced. Moreover, hapten H-OVA, possessing an O,O-dimethyl generic structure linked through a linear spacer arm, was selected as the coating antigen in the immunosorbent assay. The MAb showed high sensitivity to seven O,O-diethyl OPs and six O,O-dimethyl OPs and exhibited uniform specificity for the 13 OPs with the lowest known coefficient of variation (CV) of 50 % inhibition values (IC50). The average IC50 and CV of the IC50 values for the 13 OPs were 74.6 ng mL−1 and 33.9 %, respectively. For the recovery study, the vegetable and fruit samples were pretreated by a QuEChERS approach based on d-SPE to decrease the matrix effects, and the results demonstrated that the pretreatment was very effective. The accuracy of the ciELISA was compared with GC–MS/MS, and a good correlation of the two methods was observed. The ciELISA that we developed was also applied to real samples followed by confirmation with GC–MS/MS. The results demonstrated that the ciELISA is suitable for monitoring OP contamination in vegetable and fruit samples. In sum, the combination of an immunoassay and the QuEChERS approach shed light on the monitoring of OPs in food samples.
References
Lu CS, Barr DB, Pearson MA, Waller LA (2008) Environ Health Perspect 116:537–542
Liu D, Chen W, Wei J, Li X, Wang Z, Jiang X (2012) Anal Chem 84:4185–4191
Zheng Z, Zhou Y, Li X, Liu S, Tang Z (2011) Biosens Bioelectron 26:3081–3085
Long Q, Li H, Zhang Y, Yao S (2015) Biosens Bioelectron 68:168–174
Yi Y, Zhu G, Liu C, Huang Y, Zhang Y, Li H, Zhang J, Yao S (2013) Anal Chem 85:11464–11470
Palma P, Kuster M, Alvarenga P, Palma VL, Fernandes RM, Soares AMVM, de Alda MJ L, Barceló D, Barbosa IR (2009) Environ Int 35:545–551
Zhu P, Miao H, Du J, Zou J, Zhang G, Zhao Y, Wu Y (2014) J Agric Food Chem 62:7092–7100
Wu L, Song Y, Hu M, Zhang H, Yu A, Yu C, Ma Q, Wang Z (2015) Food Chem 176:197–204
Fernández-Alba AR, García-Reyes JF (2008) TrAC Trend Anal Chem 27:973–990
Seebunrueng K, Santaladchaiyakit Y, Srijaranai S (2014) Chemosphere 103:51–58
Zhang L, Wang Z, Wen Y, Shi J, Wang J (2015) Anal Methods 7:205–210
Hua X, Liu X, Shi H, Wang Y, Kim HJ, Gee SJ, Wang M, Liu F, Hammock BD (2014) RSC Adv 4:42445–42453
Lee WY, Lee EK, Kim YJ, Park WC, Chung T, Lee YT (2006) Anal Chim Acta 557:169–178
Gui W, Liu Y, Wang C, Liang X, Zhu G (2009) Anal Biochem 393:88–94
Piao YZ, Kim YJ, Kim YA, Lee HS, Hammock BD, Lee YT (2009) J Agric Food Chem 57:10004–10013
Li Y, Zhao F, Zhao L, Yang Z (2015) Food Anal Methods 8:420–427
Zikos C, Evangelou A, Karachaliou CE, Gourma G, Blouchos P, Moschopoulou G, Yialouris C, Griffiths J, Johnson G, Petrou P, Kakabakos S, Kintzios S, Livaniou E (2015) Chemosphere 119:S16–S20
Liu Y, Guo Y, Zhu G, Tang F (2014) J Food Prot 7:1052–1240
Liu Y, Lou Y, Xu D, Qian G, Zhang Q, Wu R, Hu B, Liu F (2009) Microchem J 93:36–42
Yan X, Tang X, Li H, Sheng E, Yang D, Wang M (2014) Food Anal Methods 7:1186–1194
Xu Z, Shen Y, Zheng W, Beier RC, Xie G, Dong J, Yang J, Wang H, Lei H, She Z, Sun Y (2010) Anal Chem 82:9314–9321
Li X, Zhang H, Ji Y, Zheng Z, Bian Q, Zhu G (2015) Food Agric Immunol 26:109–119
Xu Z, Wang H, Shen Y, Nichkova M, Lei H, Beier RC, Zheng W, Yang J, She Z, Sun Y (2011) Analyst 136:2512–2520
Mak SK, Shan G, Lee HJ, Watanabe T, Stoutamire DW, Gee SJ, Hammock BD (2005) Anal Chim Acta 534:109–120
Xu Z, Deng H, Deng X, Yang J, Jiang Y, Zeng D, Huang F, Shen Y, Lei H, Wang H, Sun Y (2012) Food Chem 131:1569–1576
Xu Z, Dong J, Wang H, Li Z, Beier RC, Jiang Y, Lei H, Shen Y, Yang J, Sun Y (2012) J Agric Food Chem 60:5076–5083
Wilkowska A, Biziuk M (2011) Food Chem 125:803–812
Sinha SN, Vasudev K, Rao MVV (2012) Food Chem 132:1574–1584
He Z, Chen S, Wang L, Peng Y, Luo M, Wang W, Liu X (2015) Anal Bioanal Chem 407:2637–2643
Omar N, Bakar J, Muhammad K (2013) Food Control 34:318–322
Bruzzoniti MC, Checchini L, De Carlo RM, Orlandini S, Rivoira L, Del Bubba M (2014) Anal Bioanal Chem 406:4089–4116
Kolberg DI, Prestes OD, Adaime MB, Zanella R (2011) Food Chem 125:1436–1442
McAdam DP, Hill AS, Beasley HL, Skerritt JH (1992) J Agric Food Chem 40:1466–1470
Wang J, Yu G, Sheng W, Shi M, Guo B, Wang S (2011) J Agric Food Chem 59:2997–3003
Xu Z, Zeng D, Yang J, Shen Y, Beier RC, Lei HT, Wang H, Sun Y (2011) J Environ Monit 13:3040–3048
Spinks CA (2000) Trends Food Sci Technol 11:210–217
Jang MS, Lee SJ, Xue X, Kwon HM, Ra CS, Lee YT, Chung T, Kor B (2002) Chem Soc 23:1116–1120
Nunes GS, Toscano IA, Barceló D (1998) TrAC Trend Anal Chem 17:79–87
Acknowledgments
This study was supported by National Natural Science Foundation of China (grant numbers 30972050, 31271873) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 4952 kb)
Rights and permissions
About this article
Cite this article
Zhao, F., Hu, C., Wang, H. et al. Development of a MAb-based immunoassay for the simultaneous determination of O,O-diethyl and O,O-dimethyl organophosphorus pesticides in vegetable and fruit samples pretreated with QuEChERS. Anal Bioanal Chem 407, 8959–8970 (2015). https://doi.org/10.1007/s00216-015-9055-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9055-x