Abstract
In the present study conversion of waste cooking oil to biodiesel has been carried out via simultaneous esterification and transesterification reaction over silica sulfuric acid as a solid acid catalyst. The process variables that influence the fatty acid methyl ester (FAME) conversion, such as reaction temperature, reaction time, catalyst concentration and methanol to oil molar ratio were investigated and optimized using Taguchi method. Highest FAME production obtained under the optimized condition was 98.66 %. Analysis of variance revealed that temperature was the most significant factor effecting the FAME production among four factors studied. From the kinetic study, the reaction was found to follow pseudo first-order kinetics and rate constant of the reaction under optimum condition was 0.00852 min−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Production of biodiesel as an alternative source of petroleum derived fuels has gained immense interest worldwide in the recent years as crude oil prices are near an all time high. Biodiesel is most commonly derived by catalytic transesterification of vegetable oils and animal fats [1]. Worldwide rising cost of oil feedstocks in the recent years has prompted greater biofuel investments, since 70–95 % of the total biodiesel production cost arises from the cost of raw materials [2]. In the current scenario, the high cost of biodiesel is the major obstacle for its commercialization. Hence, exploring ways to reduce the high cost of biodiesel is of much interest in recent biodiesel research, especially for those methods concentrating on minimizing the raw material cost. In this context, use of waste cooking oil (WCO) as a biodiesel feedstock has received considerable attention in the recent past due to cheaper cost and easy availability. Furthermore, production of biodiesel from WCO can also expel environmental hazards and waste disposal problems [3].
Although homogeneous catalysts have been advantageous in terms of faster reaction rates and high activity for transesterification reactions; salt formation and waste water generation during product purification are major issues limiting their applications [4]. In order to overcome the inherent drawbacks of homogeneous catalysts, various heterogeneous catalysts have been studied for the transesterification due to their recyclability and ease of operation [5]. Solid base catalysts have been quite successful for biodiesel production, but their sensitivity towards the presence of free fatty acids (FFAs) and water in oil feedstocks prefers solid acid catalysts [5, 6]. Solid acids are found to be more prominent for the transesterification of high FFA containing low-cost feedstocks due to their ability to simultaneously catalyze esterification and transesterification reaction [7, 8]. Furthermore, simple catalyst removal step, catalyst regeneration and reduction of corrosion-related problems promote them for the continuous mode operations [9]. Among various solid acid catalysts available, sulfated metal oxides [8–10], carbon-based solid acids [11, 12], sulfonic acid ion exchange resins [13], heteropolyacids [14], and zeolites [15] were tested effectively for the production of biodiesel using WCO as a feedstock. However, higher reaction temperatures, active site leaching, thermal stability, catalyst regeneration and low surface area are the major issues associated with the use of solid acid catalysts [16]. Furthermore, significant impact of various reaction parameters requires careful optimization to achieve high quality biodiesel [6]. Understanding of kinetics of the transesterification reaction is essential for the process design and to provide parameters that can be used to predict the extent of reaction at any time under particular conditions. Although kinetics of homogeneously catalyzed transesterification is well studied [17, 18], inconsiderable attempts are made for heterogeneously catalyzed reactions. Recently, Qing and coworkers [19] have investigated the kinetics of solid acid-catalyzed transesterification of waste oil and established that forward and reverse reaction followed pseudo second order kinetics. In another work, kinetics of soybean oil transesterification over solid base catalysts has been investigated and the order of reaction was found to be 1 or 3 depending upon the type of catalyst used [20].
Within the scope of this work, silica sulfuric acid (SSA) has been screened for the transesterification of WCO as a solid acid catalyst. Simple catalyst preparation, easy recovery and regeneration of SSA prompted us to take a step forward in the application of this catalyst for the biodiesel production from waste feedstock [21]. The chosen catalyst has been used in an optimization study for determining the best reaction conditions by means of statistical experimental design using Taguchi method. Most significant parameter effecting the FAME production was determined by analysis of variance (ANOVA). The reaction kinetics of WCO transesterification was evaluated under optimized reaction conditions and reaction order and rate constant were obtained by determining FAME production at regular time intervals.
Experimental
Materials and Methods
WCO with an acid value of 2.36 mg KOH/g oil was obtained from local catering house in Surat, India. The WCO sample used in present study was from fryer peanut oil since caterers use peanut oil. Table 1 summarizes various physico-chemical properties of WCO used in present study. All the properties of WCO given in Table 1 are determined according to ASTM and AOCS standard procedures (listed in Table 1). Fatty acid profile of WCO was determined by gas chromatograph (GC) as per ASTM D1585-96 guidelines. Silica gel (60–120 mesh) was collected from Merck and other reagents of analytical grade such as, chlorosulfonic acid, methanol, hexane, petroleum ether and ethyl acetate were purchased from Labort Fine Chem. Pvt. Ltd., India.
Catalyst Synthesis and Characterization
SSA was synthesized as described previously [22]. Typically, silica gel (60 g) was charged in 500 ml suction flask containing gas outlet tube and constant dropping funnel. Chlorosulfonic acid (0.2 mol) was added drop-wise for the period of 30 min. HCl gas evolved immediately was absorbed in NaOH solution. In order to complete removal of HCl gas, the resultant mixture was stirred at room temperature for 30 min. The solid product (SSA) was collected and stored in desiccator. Synthesized catalyst was characterized by powder X-ray diffraction (XRD), FTIR and acid–base titration method. XRD was performed using Miniflex X-ray diffractometer by Rigaku (Tokyo, Japan). The radiation source used was Cu Kα with X-ray generator tube operating at 30 kV and 15 mA. Samples were scanned in the Bragg angle (2θ) range of 0–60°. The solid-state IR spectrum of SSA was recorded using KBr disc technique on FTIR-8400S spectrophotometer by Shimadzu (Tokyo, Japan) in the range of 4,000–400 cm−1.
Design of Experiment
In order to study the effect of various reaction parameters on the FAME production and to determine the optimum reaction conditions for obtaining the maximum purity, a systematic approach developed by Taguchi [23, 24] was followed. The details of Taguchi method are provided in the supplementary data. The yield of FAME has been considered as the main response for present study. Experiments were designed for four parameters namely reaction temperature, oil/methanol ratio, catalyst dosage and reaction time at four levels. Physical values of these factors are provided in Table 2. The factors and their values were carefully selected after conducting preliminary experiments. Considering the fact that acid-catalyzed reactions are much slower and requires higher reaction temperature in order to obtain higher conversion [5–7], the reaction temperature was varied in the range of 65 to 150 °C. It was observed that, the effect of temperature was insignificant above 120 °C, giving almost constant FAME production (results are not shown). Hence, 120 °C was selected as a maximum temperature limit. The reaction was carried out for maximum period of 8 h, unless otherwise stated. Considering the rate of reaction to be directly proportional to number of active sites, catalyst concentration was varied over a range of 2 to 5 % in the present study. Stoichiometrically, transesterification of one mole of triglyceride (TG) requires 3 mol of methanol. Although high oil to methanol ratio is preferred to drive the reaction in forward direction [1, 2], preliminary studies revealed that at higher oil to methanol ratio (1:30) FAME production was reduced to some extent (results are not shown here). Hence, oil to methanol ratio was varied in the range of 1:5 to 1:20. Based on the Taguchi method, L16 orthogonal array was constructed and accordingly experiments were carried out. All the reactions were performed in duplicate in order to ensure reproducibility. Statistical analysis using ANOVA was performed using Minitab® software to study the influence of each parameter.
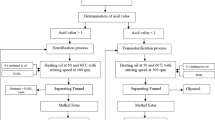
Transesterification of WCO
Transesterification reactions were carried out in a 700-ml laboratory-stirred autoclave (Amar Equipments, India) equipped with pressure gauge, temperature controller, internal cooling coil and sample outlet valve. WCO (100 g) was fed into the reactor and preheated to 60 °C followed by addition of catalyst–methanol mixture. The reaction mixture was then stirred at 600 rpm in order to avoid mass transfer limitations as stated elsewhere [2, 10]. Reaction mass was heated at desired temperatures and allowed to react further for different lengths of time. All the reactions were performed at autogeneous pressure. After completion of reaction, product was filtered, excess of methanol was evaporated in a rotary evaporator and the resulting mixture containing FAME (upper layer) and glycerol (lower layer) was allowed to settle for 24 h. FAME layer was collected, washed with water and dried over anhydrous sodium sulfate for complete removal of water.
FAME Characterization
The yield of FAME from WCO was calculated using 1H NMR analysis performed on Brucker-Avance II series NMR analyzer (500 MHz) (Germany) using CDCl3 [99.8 % D with 0.01(% v/v) TMS] solution and yields were calculated as described in the literature [25, 26]. The relevant signals chosen for the integration were those of methoxy group in FAME (3.66 ppm, singlet) and those of R-methylene protons present in all TG derivatives (2.3 ppm, triplet) [25, 26]. The yield of FAME was calculated by the equation proposed by Knothe [25], which is as given below:
Where, A ME is the integration value of the protons of methyl esters and \( {A}_{{\upalpha\ \mathrm{CH}}_2} \) is the integration value of the protons of the methylene protons.
Catalyst Reusability and Leaching Test
In order to study the reusability of catalyst, the catalyst was separated from the reaction mass by filtration and washed twice with acetone and hexane to remove polar and non-polar impurities. Catalyst was reused after drying overnight at 100 °C. Acidity measurements were carried out after each run in order to test leaching of acidic sites.
Results and Discussion
Catalyst Characterization
The textural properties of fresh and spent SSA were examined by XRD analysis and their patterns are presented in Fig. 1. The actual phases were mixture of hydrogen silicate hydrate, H2Si14O29.5·4H2O (cubic) and cubic silicon oxide (SiO2) [27]. No significant changes observed in XRD patterns of the fresh and spent catalyst, revealed retention of framework structure throughout the reaction. FTIR spectrum of catalyst (Fig. 2) confirmed the presence of sulfonic acid sites on silica support by showing major absorption peaks at 600–700 cm−1 (S–O stretching), 1,120–1,230 cm−1 (O═S═O stretching modes). Symmetric and anti symmetric Si–O–Si stretching bands were found near 851 cm−1 and 1,000–1,200 cm−1, respectively. Acidity of SSA was estimated by acid–base titration method and found to be 3.6 mmol H+/g SiO2 suggesting strong acidic nature.
Optimization of Reaction Parameters
The effect of reaction parameters on the transesterification of WCO using SSA as a solid acid catalyst was investigated and FAME production for each parameter was optimized. Table 3 summarizes the experimental matrix for the L16 array with corresponding yield of FAME. Even though each experiment is carried out identically, results of the process for FAME production could be different due to correlation and interaction of each parameter. Using the results obtained, the S/N ratio was found considering “higher is better” case using the following equation (Eq. 2) [23, 28]:
Where, n is the number of repetition done for the given experiment and y i is the yield of ith experiment. Ross [23] has identified three types of S/N ratios, such as larger-the-better, smaller-the-better and nominal-the-best. Based on the process parameters and aim of the present study, the S/N ratio for “larger-the-better” case was selected to maximize the FAME production. All experiments were performed in duplicate to ensure the reproducibility and S/N ratio was calculated accordingly. The values of S/N ratio are reported in Table 3. Using an individual S/N ratio, the total S/N ratio for each factor at each level was calculated. For an example, total S/N ratio for factor A at level 1 (66.62) can be found by sum of the individual S/N ratio for experiment no. 1–4, i.e. (6.28, 16.55, 20.56, and 23.16). The values of total S/N ratios are presented in Table 4. Figure 3 depicts the graphical representation of the factor effects at each level. Figure 3 shows gradual increase in FAME production with increase in temperature and reaching the maximum value at 120 °C. This may be attributed to the accelerated activation of carboxylic group of TGs at high temperature, making it available for the nucleophilic attack by hydroxyl group of alcohol (CH3OH). The activation of TG is difficult due to possible interference of long alkyl chains of TG molecule with the activation of its carbonyl group. In order to favor the methanol nucleophilic attack on the triglyceride, a comparatively high reaction temperature is needed to activate this carbonyl group [5, 6]. Despite their insensitivity to FFAs and water present in the feedstock, acid-catalyzed transesterification reactions are largely ignored due to their relatively slower reaction rates [5]. According to Lotero et al. [29] at higher temperatures, the extent of phase separation decreases and rate constants increases resulting in shorter reaction time. Freedman et al. [17] also suggested that acid-catalyzed reactions commonly require temperatures above 100 °C. Results observed in present study are in accordance with existing literatures, wherein FAME production increased significantly at high temperatures [11–14, 16] especially, when solid acids were involved. In order to determine the effect of time on FAME production, the reactions were carried out at different times. S/N ratio for the reaction time was found to be increasing from lower to higher level signifying the effect of time length. As Fig. 3 depicts, the FAME production increased gradually with increase in time and reached a maximum of >97 % at 8 h. Catalyst loading and oil to methanol ratio are the another important parameters that need to be optimized to obtain high ester yields. Figure 3 also indicates gradual increment in FAME production when catalyst concentration was increased from 2 to 4 %. This enhanced activity is accredited to increasing number of active sites available for the reaction, which is a common feature of solid acid catalysts. However, at higher concentration little decrease in FAME production was observed, which may be ascribed to the limitation imposed by the equilibrium of the reaction [30]. Moreover, in a three phase system (oil/methanol/catalyst), a higher dosage of catalyst tends to lead to higher viscosity resulting in mass transfer limitations for the reactants to reach the catalytic sites [31]. Although acid-catalyzed transesterification achieves greater and faster conversion at higher alcohol concentrations, increasing alcohol ratio requires recovery resulting in higher production costs [5]. In the present study, oil to methanol ratio was optimized in the range of 1:5 to 1:20 and increase in FAME yield was observed with increasing alcohol concentration. Canakci and Van Gerpen [32] investigated various alcohol ratios to find optimum ratio for transesterification of TG and proposed that 30:1 is the optimum ratio for transesterification of oil when reaction is catalyzed by sulfuric acid. Recently, Petchmala et al. [33] also demonstrated that 1:25 oil to methanol ratio was required to achieve 90 % methyl ester conversion when sulfated zirconia as a solid acid catalyst for transesterification of purified palm oil. However, ratio of alcohol used in present study is still lower than those reported in related literatures [17, 31–34].
It should be noted that, Fig. 3 shows the effect of controllable factors on S/N ratio measured independently, i.e. without considering the interactions between these factors. Sometimes, these interactions may affect the results significantly. The present work was aimed to study the effect of individual parameters on FAME production. Hence, effects of interaction between the reaction parameters are not considered in present case. To study the interaction of reaction parameters, different experimental design may be required. This may be regarded as limitation of present experimental design.
In order to validate the Taguchi method, it is further essential to perform confirmation experiment. The level corresponding to maximum S/N ratio among all four levels was selected for the optimal set of parameters. From Table 4 and Fig. 3, it can be observed that the optimal parameters identified are A4B4C4D4 viz. reaction temperature 120 °C, reaction time 8 h, catalyst loading 5 % and oil to methanol ratio 1:20. The confirmation experiment was performed in duplicate using the optimum conditions obtained by Taguchi method and the results are reported in Table 5. It clearly demonstrates that the value of FAME production and S/N ratio under the optimized conditions are higher than maximum FAME production and highest S/N ratio obtained in the L16 array (Table 3—entry 15). Thus, the results obtained here validated an application of the Taguchi method for biodiesel production from WCO using SSA as solid acid catalyst.
In order to analyze the influence of each parameter studied on reaction as well as FAME production, ANOVA is essential. In the present case, ANOVA was performed to check the individual influence of each reaction parameter on purity of FAME converted (Table 6), where significance of each factor was determined by comparison of factor variance. The F value for temperature was found highest among all parameters, which reveals that temperature is affecting the process to a greater extent followed by oil to methanol ratio. Furthermore, p value for each factor suggests that null hypothesis may be rejected. However, F test provides only qualitative information for the determination of most important factor [35]. For the quantitative analysis, percentage contribution for each factor was obtained. Table 6 depicts that contribution for the temperature was found to be the highest within all parameters. The percentage contribution of temperature was 84.30 %, whereas, other factors contributed in the range of 4.41–6.28 %. Based on ANOVA, the order of factors according to their levels of influence can be given as follows: Temperature (A) > Oil to methanol ratio (D) > Time (B) > Catalyst dosage (C). The present study further suggests that a minor variation in temperature may lead to a dramatic change in the response.
1H NMR Characterization of FAME
The use of 1H NMR analysis is convenient and fast in monitoring a reaction, because a small aliquot can be extracted from the batch reaction at any given time and the 1H NMR spectrum analysis provides quantitative information pertaining to the chemical species present in the reaction. The extent of transesterification of WCO was determined by 1H NMR spectroscopy. 1H NMR spectra of WCO and FAME are represented in Figs. 4 and 5, respectively. Integration of the areas under these signals in the mentioned equation gives the FAME production and the conversion calculated accordingly was 98.66 %. Purity of FAME is further confirmed by the absence of peaks corresponding to triglycerides (4.22–4.26 ppm), di- or mono glycerol (small peaks at 3.4–4.1 ppm) in the 1H NMR spectra (Fig. 5).
Reusability of Catalyst
In order to reduce the cost of biodiesel production, catalyst reusability is important. In the present case, catalyst can be reused several times after simple regeneration, for transesterification of WCO under optimized conditions. Results indicated that the catalyst can be reused for three times without significant loss of activity (Fig. 6). However, little decrement in FAME production was observed in subsequent runs. The observed catalyst deactivation could be due to modification of the catalyst structure or leaching of active sites at high reaction temperature. In order to assess the effect of reaction on catalyst structure, catalyst collected after third reuse and washed with acetone and hexane. No change is observed in XRD pattern of this spent catalyst (Fig. 1) confirming retention of structure during the reaction. This clearly demonstrates that catalyst deactivation is not related to the modification of catalyst structure. The contribution of leaching on catalytic activity reduction was investigated by measuring the acid strength of catalyst after three runs. The acidity of SSA is found to be decreased to 2.78 mmol H+/g SiO2, which accounted for ∼76 % of the initial value (3.6 mmol H+/g SiO2). This may be ascribed to leaching of active sites during the reaction. However, it should be noted that SSA can be easily regenerated for further use by re-acidulation as described in the “Experimental” section. This can be further evidenced from XRD patterns (Fig. 1), which shows retention of silica structure throughout the reaction.
Kinetics of the Reaction
To correlate experimental data and quantify the effects of reaction time and temperature, a detailed study on kinetic behavior was carried out for conversion of WCO to FAME over SSA under optimized reaction conditions. The transesterification reaction involves three reversible steps, where TGs reacts with methanol to give diglycerides (DGs) in first step. DGs on further reaction with methanol gives monoglycerides (MGs) in second step, which on further reaction with methanol provides FAME and glycerol as a final products. The SSA catalyzed transesterification reaction is initialed by protonation of carbonyl oxygen of TG molecule (Figure 1S—supplementary data). This increases the electrophilicity of the adjoining carbon atom, making it more susceptible to nucleophilic attack by methanol molecule. This results in the formation of unstable tetrahedral intermediate. Finally, the proton transfer from hydroxyl group to glyceridic oxygen atom results in formation of methyl ester molecule. This sequence is repeated twice to produce three FAME molecules and glycerol as the final product. According to Kusdiana and Saka [36], percent yield of methyl ester can be used as the only parameter to monitor the rate of reaction and three step conversions from TG-DG, DG-MG, and MG-FAME can be simplified in terms of conversion of TG to FAME. Since, FAME is the common product in all reaction steps involved, the overall reaction can be stoichiometrically expressed as a one-step reaction in the following manner, ignoring intermediate products (DGs and MGs).
This reaction is further repeated two times to give two more FAME molecule and glycerol as final product. Since the transesterification is a reversible reaction, high methanol to oil ratio is commonly preferred to drive the reaction in forward direction. In the present study, kinetics of transesterification has been studied with respect to FAME production (%) as a function of time. Since the methanol was used in large quantity (1:20), the reaction is assumed to proceed as a pseudo first-order reaction as a function of FAME [34]. In the present work, transesterification kinetics of WCO has studied with respect to % FAME as a function of time. Therefore, the first-order rate constant of the reaction can be expressed by Eq. (3) as follows:
Where, (FAME) = % of FAME, t = time, k = rate constant. The extent of FAME production can be calculated from 1H NMR of the product withdrawn at regular time intervals. Determination of reaction kinetics using aliquot sampling and 1H NMR analysis has been recently demonstrated by Morgenstern et al. [37]. The results obtained using 1H NMR analyses were found in agreement with the results reported from the traditional GC analysis. For this, FAME yield was determined at regular time intervals of 1 h using 1H NMR under previously optimized reaction conditions viz. reaction temperature 120 °C, catalyst amount 4 % w/w (w.r.t. oil) and oil to methanol ratio 1:20. From the conversion data obtained (X a), plots of −ln (1 − X a) versus reaction time were obtained to determine the rate of reaction (Fig. 7). From Fig. 7, it can be concluded that the reaction followed pseudo first-order kinetics and the rate constant (k) of the reaction calculated was 0.0085 min−1. These results are in accordance with the previously reported studies [19, 32, 38], where various homogeneous and heterogeneous natures are employed for biodiesel production. Earlier, Canakci and Van Gerpen [32] demonstrated that, the rate of the transesterification reaction follows pseudo first-order kinetics when high methanol to oil ratio was involved. However, compared to homogeneously catalyzed reactions, heterogeneous catalytic reaction process involves complex steps such as, adsorption of reactants onto the catalyst surface, reaction on the catalyst surface and desorption of products [19]. Hence, in order to find the exact rate constant of the reaction, interaction of reactants with catalyst surface and formation of intermediate products is required to be considered. As the present study provides simplified kinetics of transesterification of WCO in terms FAME production, the order of the reaction and rate constant obtained here represents overall nature of reaction.
Comparison with Other Catalysts
Table 7 compares the results of present work with some reported solid acid catalysts for similar class of reactions. Although reaction conditions employed in the present work are comparatively moderate, the FAME production obtained was comparable to other reported results for similar class of catalysts, where relatively harsh reaction conditions (high reaction temperatures) are employed [10, 11, 40].
Fuel Properties of Biodiesel from WCO
The physico-chemical characteristics of biodiesel derived from WCO under study are summarized in Table 8 and compared with ASTM standards. The fuel properties of the biodiesel were determined according to ASTM D6751 standard, and it was found that, the biodiesel properties were very close to diesel fuel specifications and in accordance with the ASTM specifications. The specific gravity of biodiesel (0.8806 g/cm2) was found slightly higher than that of diesel (0.85 g/cm2). Similarly, kinematic viscosity was also found on the higher side (5.6 mm2/s) compared to other reported values [4, 42]. This may be attributed to correlation of viscosity with unreacted triglycerides. The flow properties (cloud point) of biodiesel were found at lower end and in accordance with conventional diesel revealing its suitability at cold conditions.
Conclusion
Silica sulfuric acid has been investigated as an efficient solid acid catalyst for the biodiesel production from WCO (obtained from groundnut oil) for the first time. Based on the Taguchi method, the optimum reaction conditions within the selected parameters were found to be, reaction temperature 120 °C, reaction time 8 h, catalyst loading 4 % w/w and oil to methanol ratio 1:20, obtaining maximum FAME production (98.66 %). ANOVA conceded that the temperature was the most statistically significant parameter affecting the FAME yield. The Taguchi method provided a systematic approach to optimize the process of biodiesel production from WCO, using few well-defined experimental sets for optimization of the designed parameters. Kinetic study of the reaction under optimal conditions revealed that the reaction followed pseudo first-order kinetics and rate of the reaction was 0.00852 min−1. The catalyst can be reused up to three runs without loss of activity under the optimized conditions.
Abbreviations
- WCO:
-
Waste cooking oil
- SSA:
-
Silica sulfuric acid
- FAME:
-
Fatty acid methyl ester
- FFAs:
-
Free fatty acids
- ANOVA:
-
Analysis of variance
- XRD:
-
X-ray diffraction
- TG:
-
Triglyceride
- FTIR:
-
Fourier transform infrared
- 1H NMR:
-
1H nuclear magnetic resonance
- O/M :
-
Oil to methanol molar ratio
- S/N :
-
Signal to noise
- S :
-
Sum of squares of S/N ratio
- DF:
-
Degree of freedom
- V :
-
Variance
- F :
-
F value
- t :
-
Time
- k :
-
Rate constant
- E a :
-
Activation energy
References
Ma F, Hanna MA (1999) Biodiesel production: a review. Bioresour Technol 70:1–15. doi:10.1016/S0960-8524(99)00025-5
Zheng Y, Dube MA, McLean DD, Kates M (2003) Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour Technol 89:1–16. doi:10.1016/S0960-8524(03)00040-3
Ultu Z, Kocak MS (2008) The effect of biodiesel fuel obtained from waste cooking oil on direct injection diesel engine performance and exhaust emissions. Renew Energy 33:1936–1941. doi:10.1016/j.renene.2007.10.006
Phan AN, Phan TM (2008) Biodiesel production from waste cooking oils. Fuel 87:3490–3496. doi:10.1016/j.fuel.2008.07.008
Kiss AA, Diamin AC, Rothenberg G (2006) Solid acid catalysts for biodiesel production—towards sustainable energy. Adv Synth Catal 348:75–81. doi:10.1002/adsc.200505160
Sharma YC, Singh B, Korstad J (2010) Advancements in solid acid catalysts for ecofriendly and economically viable synthesis of biodiesel. Biofuels Bioprod Bioref 5:69–92. doi:10.1002/bbb.253
Helwani Z, Othman MR, Aziz N, Kim J (2009) Solid heterogeneous catalysts for transesterification of triglycerides with methanol: a review. Appl Catal A 363:1–10. doi:10.1016/j.apcata.2009.05.021
Lam MK, Lee KT, Mohamed AR (2010) Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: a review. Biotechnol Adv 28:500–518. doi:10.1016/j.biotechadv.2010.03.002
Park YM, Lee DW, Kim DK, Lee JS, Lee KY (2008) The heterogeneous catalyst system for the continuous conversion of free fatty acids in used vegetable oils for the production of biodiesel. Catal Today 131:238–243. doi:10.1016/j.cattod.2007.10.052
Peng BX, Shu Q, Wang JF, Wang GR, Wang DZ, Han MH (2008) Biodiesel production from waste oil feedstocks by solid acid catalysis. Process Saf Environ Prot 86:441–447. doi:10.1016/j.psep.2008.05.003
Shu Q, Gao J, Nawaz Z, Liao Y, Wang D, Wang J (2010) Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon based solid acid catalyst. Appl Energy 87:2589–2596. doi:10.1016/j.apenergy.2010.03.024
Lou WY, Zong MH, Duan ZQ (2008) Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour Technol 99:8752–8758. doi:10.1016/j.biortech.2008.04.038
Ozbay N, Oktar N, Tapan NA (2008) Esterification of free fatty acids in waste cooking oils (WCO): role of ion exchange resins. Fuel 87:1789–1798. doi:10.1016/j.fuel.2007.12.010
Kulkarni MG, Gopinath R, Meher LC, Dalai AK (2006) Solid acid catalyzed biodiesel production by simultaneous esterification and transesterification. Green Chem 8:1056–1062. doi:10.1039/B605713F
Brito A, Borges ME, Otero N (2007) Zeolite Y as a heterogeneous catalyst in biodiesel fuel production from used vegetable oil. Energy Fuel 21:2380–3283. doi:10.1021/ef700455r
Lopez DE, Goodwin JG Jr, Bruce DA, Furuta S (2008) Esterification and transesterification using modified-zirconia catalyst. Appl Catal A 339:76–83. doi:10.1016/j.apcata.2008.01.009
Freedman B, Pryde EH, Mounts TL (1984) Variables affecting the yields of fatty esters from transesterified vegetable oils. J Am Oil Chem Soc 61:1638–1643. doi:10.1007/BF02541649
Jain S, Sharma MP, Rajvanshi S (2011) Acid base catalyzed transesterification kinetics of waste cooking oil. Fuel Proc Technol 92:32–38. doi:10.1016/j.fuproc.2010.08.017
Qing S, Jixian G, Yuhui L, Jinfu W (2011) Reaction kinetics of biodiesel synthesis from waste oil using a carbon-based solid acid catalyst. Chin J Chem Eng 19(1):163–168. doi:10.1016/S1004-9541(09)60193-2
Singh AK, Fernando SD (2007) Reaction kinetics of soybean oil transesterification using heterogeneous metal oxide catalysts. Chem Eng Technol 30(12):1716–1720. doi:10.1002/ceat.200700274
Shah KA, Maheria KC, Parikh JK (2011) Effect of reaction parameters on the catalytic transesterification of cottonseed oil using silica sulfuric acid. Ener. Source Part A. Manuscript accepted. Manuscript ID - UESO-2011-0997.R2
Zolfigol MA (2001) Silica sulfuric acid/NaNO2 as a novel heterogeneous system for production of thionitriles and disulfides under mild conditions. Tetrahedron 57:9509–9511. doi:10.1016/S0040-4020(01)00960-7
Ross PJ (1996) Taguchi techniques for quality engineering, 2nd edn. McGraw–Hill, New York
Taguchi G, Chowdhury S, Wu Y (2005) Taguchi's quality engineering handbook. Wiley, New Jersey
Knothe G (2006) Analyzing biodiesel: standards and other methods. J Am Oil Chem Soc 83:823–833. doi:10.1007/s11746-006-5033-y
Samios D, Pedrotti F, Nicolau A, Reiznautt QB, Martini DD, Dalcin FM (2009) A transesterification double step process—TDSP for biodiesel preparation from fatty acids triglycerides. Fuel Proc Tech 90:599–605. doi:10.1016/j.fuproc.2008.12.011
Shaterian HR, Ghashang M, Feyzi M (2008) Silica sulfuric acid as an efficient catalyst for the preparation of 2H-indazolo [2,1-b] phthalazine-triones. Appl Catal A 345:128–133. doi:10.1016/j.apcata.2008.04.032
Prajapati DP, Desai MA, Parikh JK (2011) Fractional factorial design for optimization of extraction of essential oil from Cymbopogon winterianus by hydrodistillation. Res J Chem Environ 15:903–908
Lotero E, Liu Y, Lopez DE, Suwannakarn K, Bruce DA, Goodwin JG (2005) Synthesis of biodiesel via acid catalysis. Ind Eng Chem Res 44:5353–5363. doi:10.1021/ie049157g
Thitsartam W, Kawi S (2011) Transesterification of oil by sulfated Zr-supported mesoporous silica. Ind Eng Chem Res 50:7857–7865. doi:10.1021/ie1022817
Kim HJ, Kang BS, Kim MJ, Park YM, Kim DK, Lee JS, Lee KY (2004) Transesterification of vegetable oil to biodiesel using heterogeneous base catalyst. Catal Today 93–95:315–320. doi:10.1016/j.cattod.2004.06.007
Canakci M, Van Gerpen J (1999) Biodiesel production via acid catalysis. Trans ASAE 42:1203–1210
Petchmala A, Laosiripojana N, Jongsomjit B et al (2010) Transesterification of palm oil and esterification of palm fatty acid near- and super-critical methanol with SO4–ZrO2 catalysts. Fuel 89:2387–2392. doi:10.1016/j.fuel.2010.04.010
Melero JA, Bautista LF, Morales G, Iglesias J, Briones D (2009) Biodiesel production with heterogeneous sulfonic acid-functionalized mesostructured catalysts. Energy Fuel 23:539–547. doi:10.1021/ef8005756
Yildiz YS (2008) Optimization of bomplax red CR-L dye removal from aqueous solution by electrocoagulation using aluminium electrodes. J Hazard Mater 153(1–2):194–200. doi:10.1016/j.jhazmat.2007.08.034
Kusdiana D, Saka S (2001) Kinetics of transesterification of rapeseed oil to biodiesel fuel as treated in supercritical methanol. Fuel 80(5):693–698. doi:10.1016/S0016-2361(00)00140-X
Diasakov M, Loulodi A, Papyannakos N (1998) Kinetics of non-catalytic transesterification of soybean oil. Fuel 77:1297–1302. doi:10.1016/S0016-2361(98)00025-8
Vyas AP, Subrahmanyam N, Patel PA (2009) Production of biodiesel through transesterification of Jatropha oil using KNO3/Al2O3 solid catalyst. Fuel 88:625–628. doi:10.1016/j.fuel.2008.10.033
Wang Y, Ou S, Liu P, Xue F, Tang S (2006) Comparison of two different processes to synthesize biodiesel by waste cooking oil. J Mol Catal A 252:107–112. doi:10.1016/j.molcata.2006.02.047
Ramachandran K, Sivakumar P, Suganya T, Renganathan S (2011) Production of biodiesel from mixed waste vegetable oil using and aluminium hydrogen sulphate as a heterogeneous catalyst. Biores Technol 102:7289–7293. doi:10.1016/j.biortech.2011.04.100
Lam MK, Lee KT, Mohamed AR (2009) Sulfated tin oxide as solid superacid catalyst for transesterification of waste cooking oil: an optimization study. App Catal B 93:134–139. doi:10.1016/j.apcatb.2009.09.022
Lin YF, Wu YG, Chang CT (2007) Combustion characteristics of waste-oil produced biodiesel/diesel fuel blends. Fuel 86:1772–1780. doi:10.1016/j.fuel.2007.01.012
Singh SP, Singh D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew Sust Energ Rev 14:200–216. doi:10.1016/j.rser.2009.07.017
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 74 kb)
Rights and permissions
About this article
Cite this article
Shah, K.A., Parikh, J.K. & Maheria, K.C. Optimization Studies and Chemical Kinetics of Silica Sulfuric Acid-Catalyzed Biodiesel Synthesis from Waste Cooking Oil. Bioenerg. Res. 7, 206–216 (2014). https://doi.org/10.1007/s12155-013-9363-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-013-9363-y