Abstract
Heterogeneously catalyzed transesterification reaction is known to be the most appropriate process for producing biodiesel from triglyceride-containing feedstock as it ensures catalyst reusability and easy product separation, lowers production costs, and makes biodiesel affordable. This work investigates the influence of calcination temperature (700–1000 °C) on the performance of CaO catalyst obtained from eggshells for biodiesel synthesis from waste frying oil (WFO). The prepared CaO catalyst was characterized using various techniques (TGA/DTA, N2 adsorption–desorption isotherm (BET), FTIR, CO2-TPD, XRF, XRD, and SEM). Taguchi optimization method with an orthogonal array was used to investigate the effect of process parameters (time, catalyst amount, methanol/oil ratio, and temperature). Reaction time was observed to be the most influential variable according to the design. Under the optimal transesterification conditions (i.e., at 50 °C for 3 h using WFO/methanol molar ratio of 1:10 with catalyst dosage of 0.75 wt.%), the biodiesel yield attained 90.81% when eggshell derived-catalyst calcined at 850 °C (CEG-850) was used. The remarkable performance of the CEG-850 could be attributed to its high basic strength (749 \(\upmu\)mol/g), improved surface area (15.4 m2/g), and dominance of basic sites on its surface. The blended fuel with 10% by volume biodiesel (B10) exhibited improved fuel properties compared to blended fuel with 50% by volume biodiesel (B50), which confirmed the suitability of a low-level biodiesel blend in the diesel engine. More than 54% biodiesel yield was achieved after the seventh cycle, depicting better stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Since energy demand keeps increasing and the search for appropriate methods to mitigate the greenhouse gas emission caused by fossil diesel utilization is ongoing, the subject of renewable energy and appropriate technology for its sustainability has dominated the global scene [1, 2]. Among the numerous alternative energy sources, biodiesel has been considered one of the eco-friendly substitutes [3]. However, its high cost of production and unavailability of raw material compared to petroleum-based diesel have been a major challenge for its commercialization, mostly in the undeveloped nations [1, 4].
In general, the economic feasibility of biodiesel production is heavily influenced by feedstock costs [5]. As previously stated, raw material costs account for more than 60% of total production costs [6]. Furthermore, processing technology is critical to the economics of biodiesel production [5]. Many studies have been investigated to reduce biodiesel production costs, but a large number of these studies focused more attention on new catalyst synthesis and yield increase [7,8,9,10]. However, the idea of using second-generation (non-edible) feedstock, such as waste frying/cooking oil, animal fat, Jatropha curcas oil, etc., to substitute edible oils should be the main consideration for the biodiesel production process. Biodiesel synthesis from waste frying oil (WFO) can lower prices and production cost as well as encourages reasonable biomass utilization [11].
According to Pukale et al. [12], the global production of WFO is estimated to be 29 million tonnes annually. WFO is one of the household wastes generated through food frying with vegetable oil (edible). The generated WFO is not appropriate for human consumption because of the harmful components present in it. Besides, its usage as food has been discouraged because of its damaging effect on body enzymes [13]. In addition to this, inappropriate management of WFO depicts a severe disposal problem, which represents economic and environmental challenges [14]. Therefore, conversion of WFO to biodiesel would ensure production sustainability, price stability, and a clean and healthy environment [15, 16]. In this regard, biodiesel has been synthesized from WFO in the presence of different catalysts [4, 4, 14, 17, 18]. For example, Shu et al. [19] produced biodiesel via methanolysis of WFO over a carbon-based solid acid catalyst. WFO-based biodiesel was synthesized using fly ash-supported ZnO catalyst in a batch reactor [16]. In another study reported by Olutoye et al. [9], transesterification of WFO with methanol over a barium-modified clay catalyst was carried out in order to synthesize fatty acid methyl esters (FAME). Furthermore, ZnO nanoparticle loaded on pumice was utilized as a solid catalyst for the FAME synthesis via methanolysis of WFO [20]. The application of optimized eggshell-derived CaO catalyst to catalyze biodiesel production from WFO is barely investigated.
Chicken eggshell is among the waste generated from households, eateries, and hotels due to egg consumption. Improper disposal of eggshells could pose environmental and health hazards. However, the valorization of eggshell wastes could serve as an avenue to mitigate the waste burden on the environment. The valorization strategies include converting waste to value-added material [1]. For example, since eggshell contains more than 90% CaCO3, it could be converted to CaO via calcination at elevated temperature and used as a catalyst for biodiesel production. Interestingly, bio-derived catalysts from eggshell, animal bone, and biomass have high potential and less harmful environmental impacts [5]. The heterogeneous catalysts prepared from eggshells have been used to catalyze transesterification reactions, and such studies are well reported [1, 3, 21,22,23,24,25,26,27,28].
The recent research is geared toward utilizing heterogeneous catalysts for biodiesel production due to the numerous challenges associated with homogeneous catalysts [3, 9, 29]. Although CaO catalysts have been synthesized from various bird eggshells for biodiesel synthesis [3, 24, 26, 28, 30,31,32], very few attempts have been made to investigate the calcination temperature effect on the catalytic performance of the resulting CaO catalyst. Only Abdul Patar et al. [33] optimized the eggshell calcination process between smaller temperature ranges of 900 °C to 950 °C and concluded that calcination temperature above 900 °C could guarantee better catalyst performance. Though the authors did not study the decomposition trend of the chicken eggshell by TGA/DTA analysis prior to calcination, they could not ascertain the consistent temperature for calcination. However, Sharma et al. [24] reported that decomposition of eggshell begins at 700 °C and complete decomposition occurs at 850 °C, whereby CaCO3 present in the eggshell might have degraded to CaO and CO2. Recently, Singh and Verma [34] reported that 800 °C was a suitable temperature for the formation of CaO from chicken and duck eggshells. Comparing the results reported by Abdul Patar et al. [33] and Sharma et al. [24], a significant correlation between the calcination temperature and eggshell-derived CaO catalyst performance is yet to be established. Moreover, many studies reported on CaO catalyst synthesis from eggshells failed to illustrate the influence of calcination temperature on its formation and activity [1, 3, 28, 35].
Thus, in this study, chicken eggshell was employed as a heterogeneous catalyst after calcination to catalyze WFO conversion to biodiesel. The influence of calcination temperature on CaO formation from eggshells was investigated. Additionally, the factors affecting the transesterification process, including catalyst loading, methanol/WFO ratio, time, and temperature, were optimized using the Taguchi approach. Furthermore, an attempt was made to improve fuel and physicochemical characteristics of biodiesel via blending with fossil diesel.
2 Materials and methods
2.1 Materials
Chicken eggshells and WFO were collected from ABUAD Guest House’s Kitchen, Ado-Ekiti, Nigeria, while methanol (CH3OH, 95%), n-hexane, and methyl heptadecanoate were procured from Topjay Scientific Limited, Ado-Ekiti, Nigeria. Diesel was obtained from a Total Petrol Station, Ibadan, Nigeria.
2.2 Synthesis of CaO catalyst
The catalyst preparation started by soaking the collected eggshells and washing them with clean water to remove dirt. The white membrane adhered to the shell was gently detached. Subsequently, the neatly rinsed eggshells were dried in an oven at 90 °C for 12 h to remove excess water. The dried eggshell was ground into a fine powder using 0.6 mm sieve mesh to obtain particles less than 0.6 mm in size. After that, a considerable portion of the sieved eggshell powder was calcined at different temperatures (700, 800, 850, 900, and 1000 °C) for 4 h in a muffle furnace. Henceforth, the obtained catalyst samples refer to CEG-T, where T indicates calcination temperature, e.g., CEG-700 depicts a sample of eggshell powder calcined at 700 °C.
2.3 Catalyst characterization
The thermal degradation trend of chicken eggshell-based catalyst was studied by using a thermogravimetry/differential analyzer (TGA/DTA) (DTG 60, Shimadzu, Japan) in the temperature range of 30–900 °C under N2 carrier flow rate of 100 mL/min at heating rate of 15 °C/min. The specific area and pore sizes of the as-synthesized catalyst samples were evaluated using N2 sorption isotherm analyzer (Micromeritics ASAP 2010) via Brunauer–Emmett–Teller (BET) and Barrett-Joyner-Halenda (BDJ) methods, respectively. Prior to analysis, all analyzed samples were degassed at 250 °C for 5 h to remove any adsorbed molecules from the pores and surfaces.
Surface functional group characterization of the raw eggshell (REG) and CEG-850 was conducted using a Spectrum TWO infrared (FTIR) spectrometer (Perkin Elmer, USA). The spectra of the samples were recorded in the wavenumber range from 4000 to 400 cm−1. Also, a scanning electron microscope with high resolution (JEOL-JSM 7600F) was used to evaluate the external morphologies of the REG and CEG-850 samples. The crystallographic structure and phases of the active CEG-850 catalyst were evaluated using an X-ray diffractometer (Rigaku D/Max-III, Tokyo, Japan) with Cu k \(\propto\) (\(\uplambda\) = 1.542 Å) irradiation at a scan rate of 0.02°/min.
The basic strength of the active CEG-850 catalyst was evaluated by temperature-programmed desorption of carbon dioxide (CO2-TPD) analysis using a Micromeritics Chemisorption analyzer (AutoChem II 2920). However, the chemical composition of the CEG-850 catalyst was determined by an X-ray fluorescence (XRF) analyzer (Pananalytical Minipal 4).
2.4 Process optimization by Taguchi method
In the current study, the Taguchi method was applied to evaluate the influence of operating variables of transesterification process on biodiesel yield. The main factors investigated were CEG-850 loading, methanol/WFO molar ratio, reaction temperature, and time, while the process response (output factor) was the biodiesel yield. A total of 9 experiments were carried out based on the L9 orthogonal array approach. For statistical calculations, signal/noise ratio (S/N) was utilized to analyze the experimental results.
S/N ratio is a dimensionless quantity that evaluates the difference between the process output variable and the desired value [36]. It also takes into account both the process mean and the variance. According to the Taguchi method, there are three specific goals in an experiment, including the smaller, the better (minimizing the response), the larger, the better (maximizing the response), and the target is best (achieving a given target value). However, the main goal herein is to maximize the yield of biodiesel. Thus, the S/N ratio for the larger, the better criterion (Eq. 1) is required to achieve the main objective.
where y is the biodiesel yield mean and n is the repetition number of experiments.
Table 1 presents the investigated process factors and their levels for biodiesel production via the transesterification process. It is worth mentioning that preliminary studies were carried out to determine the extreme value of the studied factors.
2.5 Biodiesel production process
Conversion of WFO to biodiesel via transesterification process was conducted using the synthesized CaO catalyst from eggshell in a two-neck round bottom flask coupled with a condenser, thermometer, and temperature-controlled magnetic stirrer. Before the commencement of the reaction, methanol and the needed quantity of CaO catalyst were fed into a flask and stirred at 60 °C for 20 min to liberate calcium methoxide. After that, the methanol-catalyst mixture was added to the preheated WFO contained in the reactor, and stirring immediately started after coupling of the reactor to prevent diffusion limitation. The catalytic reaction process was conducted with varying operational conditions through the Taguchi design approach (see Table 2). The WFO weight was fixed at 50 g while the stirring rate was also kept constant at 400 rpm.
Upon completion of the reaction, the reaction products were separated via centrifugation for 10 min at 7500 rpm. Thereafter, the centrifuged products, comprising reaction products (glycerol and biodiesel), were poured into a separating funnel, and the biodiesel, whose yield was determined as given in Eq. 2, was obtained as the upper product.
where Y is biodiesel yield in % while \({M}_{B}\) and \({M}_{\mathrm{WFO}}\) are masses of biodiesel and WFO, respectively.
2.6 Analysis of synthesized biodiesel
In an attempt to ascertain the quality of the prepared biodiesel, its physicochemical properties (see Table 3) were evaluated using the American Standard for Testing Material (ASTM) procedures. Furthermore, the functional groups contained in the produced biodiesel and WFO were determined using the same FTIR spectrometer mentioned in Sect. 2.3. Moreover, fatty acid methyl ester content in the produced biodiesel was evaluated with the aid of gas chromatography (Agilent GC, 7890A, USA) combined with flame ionization detector (FID) and capillary column (J & W DH-5HT, dimension: 15 m \(\times\) 0.32 mm \(\times\) 0.1 \(\mu\)m) as discussed in our previous study [4].
2.7 Blending of biodiesel with diesel
To improve the properties of the produced biodiesel and enhance its quality for better engine performance, the WFO biodiesel was blended with petroleum-based diesel in different quantities such as B10 (10%.v/v biodiesel and 90%.v/v diesel) and B50 (50%.v/v biodiesel and 50%.v/v diesel). The biodiesel-diesel mixture was blended in a 250 mL beaker placed on a magnetic stirrer at 25 \(\pm\) 2 °C (ambient temperature) and 300 rpm until a well-blended fuel was obtained. The pure biodiesel and pure diesel would henceforth refer to as B100 and B0, respectively. The physicochemical and fuel characteristics of B0, B100, B10, and B50 were determined in line with ASTM standard.
3 Results and discussion
3.1 As-synthesized catalyst characterization
To gain insight into the properties of the as-prepared CaO catalysts from chicken eggshell, TGA/DTA, N2 sorption isotherm, FTIR, SEM, CO2-TPD, XRD, and XRF analyses were carried out and discussed as follows.
3.1.1 TGA/DTA analysis
Figure 1 depicts the decomposition trend of raw eggshell during TGA/DTA analysis. As evident in the figure, between 100 °C and 700 °C, there was significant weight loss due to the removal of organic matters and moisture. As reported by Sharma et al. [24], this stage might signify the commencement of decomposition. However, the second degradation stage, which occurred between 700 and around 800 °C, indicated decomposition of CaCO3 present in the eggshell to CaO [3]. This observation agreed with result reported by Singh and Verma [34], who concluded that chicken eggshell could be transformed to CaO and CO2 at 800 °C. In addition, the degradation (TGA) curve was almost flat above 800 °C, which suggested the completion of the decomposition process.
3.1.2 N2 adsorption–desorption analysis
The N2 sorption analysis conducted on REG and CEG catalysts (see Table 4) showed some important textural characteristics of the samples studied. As shown in Table 4, it was observed that CEG-850 exhibited a high surface area and pore volume compared to its counterparts. Interestingly, the specific area and pore volume values obtained showed a consistent increase, with the increase of the calcination temperature from 700 up to 850 °C. However, the textural properties started to decline as the temperature exceeded 850 °C, probably due to the merging of small particles to form large agglomerates, which resulted in pore collapse (sintering) and reduced surface area [3, 10]. However, the higher BET surface area exhibited by the CEG-850 sample suggested that its surface was dominated by active sites, which could overcome the diffusion problem. Based on these results, eggshell calcined at 850 °C was selected as the best among the prepared CaO catalysts and analyzed further to know more about it.
3.1.3 FTIR analysis
Figure 2 depicts the detected functional groups on the surfaces of the REG and CEG-850 samples. As seen in the figure, the main peaks in the REG sample occurred at 3641–3487 cm−1 (O–H stretching vibration), 2480 cm−1 (C-N stretching), 1724 cm−1 (C = O stretching), 1416 cm−1 (C-O asymmetric stretching of carbonate), 1032 cm−1 (P-O-C band for aliphatic amines), 874 cm−1 (C-O out-of-plane bending of carbonate), and 712 cm−1 (C-O in-plane bending of carbonate). The appearance of fifth, seventh, and last peaks on the spectrum of REG confirmed the presence of calcium carbonate in the raw eggshell sample [3, 24]. However, after the calcination process, some of the peaks that were corresponded to \({\mathrm{CO}}_{3}^{2-}\) molecules disappeared or diminished, indicating a significant reduction in the mass of the functional group attached to the CaCO3 [24]. Meanwhile, the CEG-850 sample exhibited a sharp peak at 3639 cm−1 (O–H stretching vibration) formed as a result of atmospheric moisture adsorption onto the surface of the synthesized CaO, which led to the formation of Ca(OH)2, as also confirmed by XRD analysis (see Fig. 5). This result was in line with the result reported by Tan et al. [3], who prepared CaO catalysts from ostrich and chicken eggshells.
3.1.4 SEM analysis
Figure 3 shows the microstructural (SEM) images of REG and CEG-850 catalyst showing the CaO grains formation upon calcination (Fig. 3b) while the REG sample (Fig. 3a) possessed small and irregular particles. The structural changes observed after calcination suggested the decomposition of CaCO3 in the chicken eggshell into CaO and CO2 [34].
3.1.5 CO2-TPD analysis
The basic strength (total basic sites) of a heterogeneous catalyst, which acts as an active center during the catalytic reaction, is a prime trait of any solid catalyst in the biodiesel production process [37, 38]. The result of CO2-TPD analysis to determine total basic sites on synthesized CaO catalyst (CEG-850) from eggshell is depicted in Fig. 4. The CEG-850 catalyst sample exhibited two desorption peaks at 326 °C and 550 °C. The first corresponded to the basic sites of medium strength, similar to the data reported by Laskar et al. [37]. However, the second peak, which was located between 500 and 600 °C, confirmed the presence of stronger basic sites due to the CO2 desorption that occurred at elevated temperature [3]. Overall, the total basic amount of 749 \(\mu\)mol/g indicated that stronger basic sites at 326 °C and 550 °C were part of the active centers during the transesterification process.
3.1.6 XRD analysis
Figure 5 shows the X-ray diffractogram of CEG-850 catalyst, indicating typical characteristic peaks of CaO and Ca(OH)2 as major and minor phases, respectively. The peaks of CaO occurred at 2 \(\theta\) equal to 32.4°, 36.6°, 39.4°, 50.8°, 54.3°, and 71.6°, which agreed with the result reported by Tan et al. [3] while the peaks at 17.9°, 34.3°, and 62.9° were attributed to the Ca(OH)2. The detection of Ca(OH)2 was as a result of reaction between CaO and atmospheric moisture [24], which could be concluded that the synthesized CEG-850 catalyst was a mixture of CaO and Ca(OH)2.
3.1.7 XRF analysis
Table 5 presents the result of XRF analysis, and it was revealed that the calcined eggshell catalyst was largely dominated by CaO (97.39%) along with the traces of other metal oxides. The presence of CaO as a major phase in the CEG-850 sample played a significant role in the transesterification of WFO make biodiesel [33, 37], indicating that CaCO3 in eggshell could be transformed into CaO via calcination [39].
3.2 Impact of calcination temperature on yield of biodiesel
In furtherance of our desire to establish the optimum calcination temperature based on biodiesel yield, it was planned to calcine the raw eggshell at different temperatures ranging from 700 to 1000 °C. In this regard, the synthesized catalyst samples (CEG-700, CEG-800, CEG-850, CEG-900, and CEG-1000) were tested for transesterification of WFO using methanol/oil molar ratio of 10:1, catalyst loading of 0.5 wt.% and reaction time of 2 h at 60 °C. The average values of the WFO biodiesel yields obtained using the catalyst samples are displayed in Fig. 6. As evident in the result, the REG sample was inactive due to the presence of CaCO3, which could not drive the transesterification reaction to equilibrium. However, there was an increase in the FAME yield as calcination temperature increased from 700 °C to 850 °C but started to decrease as the temperature rose above 850 °C, attributing it to the occurrence of sintering (pore structure collapse) which reduced the surface area of the catalyst and consequently inhibited its activity [10]. These results suggested that CEG-850 was the most active catalyst among the synthesized catalysts. Its remarkable performance in the transesterification reaction resulted from CaO dominance on the catalyst surface, which enhanced the basic strength, thus improving the activity of the CEG-850 sample.
3.3 Process optimization by Taguchi method
The 3 level-4 factor L9 orthogonal matrixes, experimental responses obtained during the biodiesel production process, and values of S/N ratio estimated using Eq. 1 are presented in Table 2. Herein, we assumed that the large S/N ratio is desirable as it signifies the optimal transesterification process and suggests the best levels for each parameter [40].
The main effect plot in terms of the S/N ratio of biodiesel yield against transesterification process parameters (methanol/WFO ratio, time, catalyst amount, and temperature) is depicted in Fig. 7. The plot revealed that the optimum levels and influential variables could be easily identified. As seen in the figure, the optimum factors for maximum yield of biodiesel were found to be 10:1, 3 h, 0.75 wt.%, and 50 °C for methanol/oil molar ratio, time, catalyst loading, and temperature, respectively, which resulted in the predicted biodiesel yield of 88.47% (S/N ratio = 38.94). Upon identifying the optimal levels, further experimental tests were conducted in order to validate the predicted response, and the maximum experimental biodiesel yield and corresponding S/N ratio were 90.81% and 39.16, respectively.
Furthermore, it was evident that methanol/WFO molar ratio and catalyst loading had a maximum point at the middle level, which implied that excessive methanol and catalyst amount could inhibit the separation of the desired product (biodiesel) and glycerol phases, thereby resulting in low biodiesel yield [3, 41]. While the reaction time exhibited the highest point at the maximum region, the reaction temperature had the highest point at the low region. According to Yee and Lee [42], either high reaction temperature or high reaction time is needed to achieve high biodiesel yield. However, based on the optimization study, the maximum biodiesel yield was obtained at 50 °C and 3 h, thus indicating that the process adopted in this current work was energy efficient, guaranteeing sustainability and a friendly environment.
Table 6 shows the contribution degree of each of the parameters studied based on range statistics. The first rank, which signified the most contributing parameter for the biodiesel production process, indicated that reaction time significantly influenced the catalytic reaction. However, the methanol/WFO ratio, among the parameters studied, showed the least significant effect.
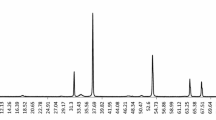
3.4 Analysis of FAME content in produced biodiesel
The results of GC-FID analysis conducted on biodiesel produced under optimum conditions are depicted in Fig. 8 and Table 7, which revealed that the methyl palmitate, methyl oleate, and methyl linoleate were the three dominant components in the synthesized biodiesel. The results further showed that the biodiesel produced from WFO contained a total of 19.63% saturated and 76.39% unsaturated FAME contents, thus suggesting that the produced biodiesel would have better oxidation stability and improved cold flow properties [41, 43].
3.5 Functional groups analysis
As evident in Fig. 9, there was a change in the spectrum of WFO upon its conversion to methyl esters (biodiesel, B100). The characteristic peaks at 2960–2945 cm−1, 1753 cm−1, and 1211 cm−1 corresponded to –CH3 asymmetric stretching vibration, -C = O stretching of ester, and –C-O stretching of ester, respectively [7, 41, 44], thus confirming the formation of methyl esters. Comparing the spectra of B0 (pure diesel) and B100 (pure biodiesel), the ester functional group was absent in the former, indicating that they were not derived from the same source. In spite of this, biodiesel could replace diesel as it exhibited almost the same functional groups as seen in Fig. 9.
Furthermore, as seen in Fig. 9, B10, B50, and B100 exhibited similar FTIR spectra pattern, where absorption bands at around 2960 cm−1 (-CH3 asymmetric stretching), 2930 cm−1 (-CH2 asymmetric stretching), 1750 cm−1 (-C = O stretching), 1470–1458 cm-1 (-CH2 scissors vibration), 1230–1000 cm−1 (-C-O stretching), and 700 cm−1 (rocky vibration of (CH2)n) were detected [45,46,47]. It appeared that the intensity of peak corresponded to the ester group reduced after blending of B100 with B0 in 1:9 mixing proportion to produce B10. Although the presence of oxygen-containing functional groups such as C = O (ester carbonyl group) and C-O (ester stretching vibration) could not be ruled out in blended fuel, the B10 appeared to have almost the same functional groups with B0 as also corroborated by fuel and physicochemical analysis results (Table 3). This observation was also affirmed by Zaharin et al. [48], who reported that a high biodiesel/fossil diesel blend ratio would result in blended fuel with poor fuel properties. According to Xue et al. [49], the presence of oxygen-containing functional groups in fuel enhances combustion and results in low CO emission, thus confirming the suitability of low-level blended fuel such as B10 to power compression ignition (CI) engine and vehicular diesel engine.
3.6 Fuel, physical, and chemical characteristics of biodiesel and blended fuels
In an attempt to enhance the fuel properties of the synthesized WFO biodiesel, it was blended with petrodiesel, and the properties of the resulting blended fuel were investigated, and the results are shown in Table 3. It was found that the properties of B100 (synthesized biodiesel) were in line with the ASTM D675 standard [50]. However, upon blending of B100 with B0 to make blended fuels (B10 and B50), the properties appeared to be improved and more comparable to pure diesel, especially B10, thereby suggesting the suitability of the diesel–biodiesel blends to power vehicular and CI engine without resulting in engine operational problems [3, 51].
Also, it could be observed that that flash point of B100 significantly reduced after blending from 178.4 °C to 49.0 °C for B10 and 58.0 °C for B50, which signified a remarkable improvement in the volatility of the synthesized biofuel. Since the flash points of B10 and B50 were greater than that of B0, as seen in Table 3, the blended fuels could be safely stored and transported [52]. Interestingly, the cloud point and pour point (cold flow properties) of the B100 were greatly improved by the blending, which suggested that the utilization of the blends in the engine could not cause blockage of the fuel filter. This observation agreed with findings reported by Verma and Sharma [53]. Notably, the B10 exhibited better fuel properties than B50, indicating that the former could be used in several diesel vehicles without modifying their engines [54].
3.7 Reusability of CEG-850 catalyst
After the catalytic reaction, the used catalyst was removed from product mixtures through centrifugation, thoroughly rinsed in solvent (hexane) to remove oil molecules, and then recalcined at 600 °C for 1 h in a furnace. The recovered CEG-850 catalyst was thereafter employed to catalyze WFO conversion for seven times under optimum transesterification process conditions (temperature = 50 °C, methanol/WFO molar ratio = 10:1, catalyst loading = 0.75 wt.%, and time = 3 h). As shown in Fig. 10, the yield of biodiesel gradually decreased from 89.44% to 54.22%. However, a considerable loss of more than 30% in catalytic activity was noticed after the last cycle, depicting a reduction in the number of active sites due to the catalyst surface poisoning by oil molecules and washing during the recovery process, which inhibited biodiesel yield [9].
4 Conclusions
Waste chicken eggshell-derived CaO catalyst with high basic strength was successfully synthesized and utilized to catalyze transesterification of WFO to biodiesel. XRD, FTIR, CO2-TPD, and BET analyses, among other characterization techniques, confirmed the dominance of CaO in the catalyst sample calcined at 850 °C. It was found that the CEG-850 catalyst exhibited better catalytic activity for WFO conversion due to the high basicity and surface area, which enhanced biodiesel yield. Under optimum operating conditions (i.e., at 50 °C for 3 h using a WFO/methanol ratio of 1:10 and 0.75 wt.% catalyst loading), the highest biodiesel yield of 90.81% was attained. Also, the produced biodiesel contained 76.39% unsaturated and 19.36% saturated esters, suggesting that the WFO-based biodiesel had better oxidation stability and improved cold flow properties. The physicochemical and fuel characteristics of the produced biodiesel significantly improved after blending with diesel. CEG-850 sample exhibited good activity and stability as a heterogeneous base and cheap catalyst for the conversion of low-grade feedstock to biodiesel.
Data availability
Not applicable.
References
Odetoye TE, Agu JO, Ajala EO (2021) Biodiesel production from poultry wastes: waste chicken fat and eggshell. J Environ Chem Eng 9(4):105654
Olutoye MA, Hameed BH (2013) A highly active clay-based catalyst for the synthesis of fatty acid methyl ester from waste cooking palm oil. Appl Catal A 450:57–62
Tan YH, Abdullah MO, Nolasco-Hipolito C, Taufiq-Yap YH (2015) Waste ostrich- and chicken-eggshells as heterogeneous base catalyst for biodiesel production from used cooking oil: catalyst characterization and biodiesel yield performance. Appl Energy 160:58–70
Yusuff AS, Bhonsle AK, Bangwal DP, Atray N (2021) Development of a barium-modified zeolite catalyst for biodiesel production from waste frying oil: process optimization by design of experiment. Renew Energy 177:1253–1264
Alagumalai A, Mahian O, Hollmann F, Zhang W (2021) Environmentally benign solid catalysts for sustainable biodiesel production: a critical review. Sci Total Environ 768:144856
Cho HJ, Kim JK, Cho HJ, Yeo YK (2013) Techno-economic study of a biodiesel production from palm fatty acid distillate. Ind Eng Chem Res. https://doi.org/10.1021/ie3016516
Taufiq-Yap YH, Abdullah NF, Basri M (2011) Biodiesel production via transesterification of palm oil using NaOH/Al2O3 catalysts. Sains Malaysiana 40:587–594
Yusuff AS, Adeniyi OD, Azeez MA, Olutoye MA, Akpan UG (2019) Synthesis and characterization of anthill-eggshell-Ni-Co mixed oxides composite catalyst for biodiesel production from waste frying oil. Biofuel Bioprod Biorefinery 13:37–47
Olutoye MA, Wong SW, Chin LH, Amani SW, Asif M, Hameed BH (2016) Synthesis of fatty acid methyl esters via the transesterification of waste cooking oil by methanol with a barium-modified montmorillonite K10 catalyst. Renew Energy 86:392–398
AlSharifi M, Znad H (2019) Development of a lithium based chicken bone (Li-Cb) composite as an efficient catalyst for biodiesel production. Renew Energy 136:856–864
Avinash A, Murugesan A (2019) Economic analysis of biodiesel production from waste cooking oil. Energ Source Part B Econ Plan Policy. https://doi.org/10.1080/15567249.2017.1319438
Pukale DD, Maddikeri GL, Gogate PR, Pandit AB, Pratap AP (2015) Ultrasound assisted transesterification of waste cooking oil using heterogeneous solid catalyst. Ultra SonoChem 22:278–286
Jin Y, Tian S, Guo J, Ren X, Li X, Gao S (2016) Synthesis, characterization and exploratory application of anionic surfactant fatty acid methyl ester sulfonate from waste cooking oil. J Surfactant. https://doi.org/10.1007/s11743-016-1813-z
Vinyes E, Oliver-sola J, Ugaya C, Rieradevail J, Gasol CM (2013) Application of LCSA to used cooking oil was management. Int J Life Cycle Assess 18:445–455
Moyo LB, Iyuke SE, Muvhiwa RF, Simate GS, Hlabangana N (2020) Application of response surface methodology for optimization of biodiesel production parameters from waste cooking oil using a membrane reactor. S Afr J Chem Eng 35:1–7
Yusuff AS, Bhonsle AK, Trivedi J, Bagwal DP, Singh LP, Atray N (2021) Synthesis and characterization of coal fly ash supported zinc oxide catalyst for biodiesel production using used cooking oil as feed. Renew Energy 170:302–314
Yusuff AS, Adeniyi OD, Olutoye MA, Akpan UG (2018) Kinetic study of transesterification of waste frying oil to biodiesel using anthill-eggshell-Ni-Co mixed oxide composite catalyst. Pet Coal 60:157–167
Santos S, Puna J, Gomes J (2020) A review on bio-based catalyst (immobilized enzymes) used for biodiesel production. Energies 13(11):3013
Shu Q, Nawaz Z, Gao J, Liao Y, Zhang Q, Wang D, Wang J (2010) Synthesis of biodiesel from a model waste oil feedstock using a carbon-based solid acid catalyst: reaction and separation. Biores Technol 101:5374–5384
Yusuff AS, Bello KA (2019) Synthesis of fatty acid methyl ester via transesterification of waste frying oil by a zinc-modified pumice catalyst: Taguchi approach to parametric optimization. React Kinet Mech Catal 128:739–761
Yusuff AS, Popoola LT (2019) Optimization of biodiesel production from waste frying oils over alumina supported chicken eggshell catalyst using experimental design tool. Acta Polytechnica 59:88–97
Zeng D, Zhang Q, Chen S, Liu S, Chen Y, Tian Y, Wang G (2015) Preparation and characterization of a strong solid base from waste eggshell for biodiesel production. J Environ Chem Eng 3:560e564
Aharon G, Piker A, Tabah B, Perkas N (2016) A green and low-cost room temperature biodiesel production from waste oil using eggshell as catalyst. Fuel 182:34–41
Sharma YC, Singh B, Korstad J (2010) Application of an efficient non-conventional heterogeneous catalyst for biodiesel synthesis form pongamia pinnata oil. Energy Fuel 24:3223–3231
Khenthrong F, Luadthong C, Nualpaeng W, Changsuwan P, Tongprem P, Viriya-empikul N, Faungnawakiji K (2012) Industrial waste as the heterogeneous catalysts for microwave-assisted biodiesel production. Catal Today 190:112–116
Jazie AA, Paramarik H, Sinha ASK (2013) Eggshell as eco-friendly catalyst for transesterification of rapeseed oil: optimization for biodiesel production. Int J Sustain Dev Green Econ 2:27–32
Chen GY, Shan R, Shi JF, Yan BB (2014) Ultrasonic-assisted production of biodiesel from transesterification of palm oil over ostrich eggshell-derived CaO catalysts. Biores Technol 171:420–432
Nijiu S, Meera S, begum KM, Anatharaman N, (2014) Modification of eggshell and its application in biodiesel production. J Saudi Chem Soc 186:702–706
Yusuff AS, Adeniyi OD, Olutoye MA, Akpan UG (2017) A review on application of heterogeneous catalysts in the production of biodiesel from vegetable oils. J Appl Sci Process Eng 4:142–157
Viriya-empikul N, Krasae P, Puttasawat B, Yoosuk B, Chollacoop N, Faugnawakij K (2010) Waste shells ofmollusk and egg as biodiesel production catalysts. Biores Technol 101:3765–3767
Wei Z, Xu C, Li B (2009) Application of waste eggshell as low-cost solid catalyst for biodiesel production. Biores Technol 100:2883–2885
Cho YB, Seo G (2010) High activity of acid-treated quail eggshell catalysts in the transesterification of palm oil with methanol. Biores Technol 101:8515–8519
Abdul Patar MA, Nasir NF, Osman SA, Isa NM (2020) Optimization of calcination temperature of eggshell catalyst and palm oil biodiesel production for blending of B10 petroleum diesel fuel. J Adv Res Fluid Mech Ther Sci 69:60–72
Singh TS, Verma TN (2020) Analysis of the effect of temperature on the morphology of eggshell calcium oxide catalyst: catalyst production for biodiesel production. Sci Iran Trans B Mech Eng 27:2915–2923
Pandit PR, Fulekar MH (2017) Eggshell waste as heterogeneous nanocatalyst for biodiesel production: optimized by response surface methodology. J Environ Manag 198:319–329
Yusuff AS, Kumar M, Obe BO, Atray N (2021) Calcium oxide supported on coal fly ash (CaO/CFA) as an efficient catalyst for biodiesel production from Jatropha curcas oil. Top Catal. https://doi.org/10.1007/s11244-021-01478
Laskar IB, Deshmukhya T, Bhanja P, Paul B, Gupta R, Chatterjee S (2020) Transesterification of soybean oil at room temperature using biowaste as catalyst: an experimental investigation on effect of co-solvent on biodiesel yield. Renew Energy 162:98–111
Syazwami ON, Teo SH, Islam A, Taufiq-Yap Y (2017) Transesterification activity and characterization of natural CaO derived from waste venus clam (Tapes belchari S.) material for enhancement of biodiesel production. Process Saf Environ Prot 105:303–315
Obadiah A, Swaroopa GA, Kumar SV, Jeganathan KR, Ramasubbu A (2012) Biodiesel production from palm oil using calcined waste animal bone as catalyst. Biores Technol 116:512–516
Yusuff AS, Ajayi OA, Popoola LT (2021) Application of Taguchi design approach to parametric optimization of adsorption of crystal violet dye by activated carbon from poultry litter. Sci Afr 13:e00850
Yusuff AS, Gbadamosi OA, Popoola LT (2021) Biodiesel production from transesterified waste cooking oil by zinc-modified anthill catalyst: parametric optimization and biodiesel properties improvement. J Environ Chem Eng 9:104955
Yee KF, Lee KT. (2008) Palm oil as feedstock for biodiesel production via heterogeneous transesterification: optimization study. Int Conf Environ (ICENV) 1–14
Serrano M, Oliveros R, Sanchez M, Moraschini A, Matinez M, Aracil J (2014) Influence of blending vegetable oil methyl esters on biodiesel fuel properties: Oxidative stability and cold flow properties. Energy 65:109–115
Betiku E, Okeleye AA, Ishola NB, Osunleke AS, Ojumu TV (2019) Development of a novel mesoporous biocatalyst derived from kola nut pod husk for conversion of Kariya seed oil to methyl esters: a case of synthesis, modeling and optimization studies. Catal Lett 149(7):1772–1789
Li H, Niu S, Lu C, Wang Y (2015) Comprehensive investigation of the thermal degradation characteristics of biodiesel and its feedstock oil through TGA-FTIR. Energy Fuels 29:5145-51–53
Faraguna F, Racar M, Jukic A (2019) Test method for determination of different biodiesel (fatty acid alkyl esters) content in diesel fuel using FTIR-ATR. Renew Energy 133:1231–1235
Li H, Liu F, Ma X, Cui P, Gao Y, Yu M, Guo M (2019) Effects biodiesel blends on the kinetic and thermodynamic parameters of fossil diesel during thermal degradation. Energy Convers Manag 198:111930
Zaharin MSM, Abdullah NR, Najafi G, Sharudin H, Yusaf T (2017) Effects of physicochemical properties of biodiesel fuel blends with alcohol on diesel engine performance and exhaust emissions: a review. Renew Sustain Energy Rev 79:475–493
Xue J (2013) Combustion characteristics, engine performances and emissions of waste edible oil biodiesel in diesel engine. Renew Sustain Energy Rev 23:350–365
Ramos M, Dias APS, Puna JF, Gomes J, Bordado JC (2019) Biodiesel production processes and sustainable raw materials. Energies 12(23):4408
Tan YH, Abdullah MD, Hipolito CN (2015) The potential of waste cooking oil-based biodiesel using heterogeneous catalyst derived from various calcined eggshells coupled with an emulsification technique: a review on the emission reduction and engine performance. Renew Sustain Energy Rev 47:589–603
Olutoye MA, Sulaiman B, Yusuff AS (2016) Bi-ZnO heterogeneous catalyst for transesterification of crude Jatropha oil to fatty acid methyl ester. Adv Res 7:1–8
Verma P, Sharma MP (2016) Review of process parameters for biodiesel production from different feedstocks. Renew Sustain Energy Rev 62:1063–1071
Alternative fuels Data Center, Energy efficiency & Renewable Energy, US Department of Energy
Acknowledgements
The authors appreciate Mr. Samuel Attah of University of Ibadan, Nigeria, Mr. Ewere Donatus of ABUAD, Ado-Ekiti, Nigeria, and Mukesh K Poddar of CSIR-Institute of Petroleum, Dehradun, India, for the kind assistance in the catalyst and fuel analyses.
Funding
The authors received no financial supports for this research or publication of the article.
Author information
Authors and Affiliations
Contributions
The original idea was conceived, designed, and executed by ASY. KAY wrote the first draft of the manuscript and managed the software validation. AII managed the literature survey while both ASY and KAY managed the analyses of the study performed and the spectroscopy analysis. All authors read and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yusuff, A.S., Thompson-Yusuff, K.A. & Igbafe, A.I. Synthesis of biodiesel via methanolysis of waste frying oil by biowaste-derived catalyst: process optimization and biodiesel blends characterization. Biomass Conv. Bioref. 14, 1781–1792 (2024). https://doi.org/10.1007/s13399-022-02389-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02389-1