Abstract
Douglas-fir (Pseudotsuga menziesii) forest residues were physically fractionated through sieving. The bark and wood were separated for large-sized fractions (>12.7 mm), and their contents were determined. The chemical compositions of the large fractions were calculated based on the contents and chemical compositions of the bark and wood. The chemical compositions of the fine fractions were analyzed. The bark and wood content in the fine fractions was calculated based on the measured glucan and lignin contents in each fraction. It was found that fractionation by particle/chip size can effectively fractionate bark and wood and therefore lignin from carbohydrates. The large-sized fractions (>12.7 mm) represent approximately 60 % of the collected forest residues but only contain approximately 37 % of the total bark and 35 % of the total ash, or a selectivity over bark and ash of 1.6 and 1.7, respectively. Pretreatment of forest residues by sulfite pretreatment to overcome recalcitrance of lignocelluloses and subsequent enzymatic hydrolysis revealed the presence of 14.3 % bark can reduce substrate enzymatic digestibilities (SED) 16 % compared with that from a bark-free sample. The SED of a bark is 41 % compared with 73 % for wood when pretreated under the same conditions. Separating pretreatment of bark from wood is beneficial for producing a more enzymatically digestible substrate. The results from the present study could have significant implications for harvesting forest residues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forest residues from commercial plantation harvesting and forest thinning operations are a substantial biomass resource that can be utilized for bioenergy and biochemical production [1]. In the USA, recovering 70 % of harvest residues or approximately 35 million tons is equivalent to 17.6 million tons of carbon from fossil fuel [2]. As a result, using forest residues as an energy source can have a positive effect on reducing carbon air emissions through carbon dioxide sequestration by photosynthesis [3, 4]. A significant amount of work has been conducted on harvesting forest residues [5–7]. Currently, harvested wood chips from forest residues are used primarily as hog fuel for bioenergy productions in boilers or gasifiers [7]. With the development of the carbohydrate platform for conversion of biomass to bioenergy [4, 8], it is of interest to evaluate the potential of forest residues for fermentable sugar production.
Although a significant amount of research has been conducted on woody biomass bioconversion through chemical and physical pretreatment [8], forest residues present unique properties that can be detrimental to bioconversion using the carbohydrate platform. First of all, forest residues contain a significant amount of bark, the layer external to the cambium, that accounts for about 15–25 % of the whole tree mass [9]. Bark is normally considered unsuitable for fermentable sugar production due to its high content of extractives, inorganic compounds (ash), and lignin, and its low content of carbohydrates [10, 11]. Although bark-free wood is attractive for bioconversion, debarking forest residues is not economically feasible. Secondly, the forest residues are often harvested in the form of wood chips through mechanical preprocessing [5]. The resultant wood chips or particles have a range of sizes and shapes. It is intuitive that large wood chips are most likely from large branches with relatively low bark and ash contents, while small wood particles are from twigs, including even needles and leaves, with high bark and ash contents. As a result, a size fractionation through sieving would be expected to fractionate the forest residues chemically. Elucidating the variation of chemical compositions of forest residues with particle size has practical importance for developing economically and environmentally beneficial harvesting guidelines. For example, it is possible to leave the fractions with high bark content on the ground as nutrients and soil conditioner [12] and collect only the fractions with high carbohydrate content for conversion to fermentable sugars.
The objectives of this study are (1) to demonstrate size fractionation of forest residues can increase feedstock quality in terms of carbohydrates by effectively separating bark and ash from wood, and (2) to study the effects of bark content as well as separate processing of bark from wood in pretreatment and enzymatic hydrolysis on feedstock saccharification efficiency. Specifically, a forest residues sample harvested from a Douglas-fir (Pseudotsuga menziesii) resulting from a regeneration harvest was used. The sample was first sieved to different size fractions. The mass distribution and chemical composition, along with ash content of the different fractions, were determined. As a softwood, Douglas-fir has high lignin content, and few pretreatments are effective to remove its recalcitrance for enzymatic saccharification [13]. The sulfite pretreatment to overcome recalcitrance of lignocelluloses (SPORL) was selected in the present study because of its effectiveness for producing fermentable sugars from softwoods [14–17]. Sugar productions from a bark-free, a bark-only, and a wood and bark composite sample were separately determined and compared to understand the effects of bark content and different processing strategies on sugar production. The overall goal of this research is to develop effective strategies for collecting forest residues and converting to fermentable sugars.
Materials and Methods
Enzymes and Chemicals
CTec-2, a commercial cellulase enzyme, was provided by Novozymes of North America (Franklinton, NC, USA). The average activity of the enzyme was 150 filter paper unit (FPU)/mL determined using a literature method [18].
Forest Residues
Forest residues consist of tops, branches, broken, or defective tree parts, and trees not meeting grade specifications. Depending on the harvest method, forest residues accumulate at roadside as a byproduct of forest operations or the residues are brought to roadside in a separate operation. The economics of forest residue collection heavily favor the residues as a byproduct (former) practice [19]. The timing of harvest and timing of residue removal can affect the percentage of bark and fines. Logs harvested in the spring have less bark, particularly those subject to mechanical processing [20]. Forest residues left to dry in the forest have fewer needles [21]. The Douglas-fir forest residues tested here came from a roadside pile resulting from a regeneration harvest in a mixed conifer stand at Berry Creek, Douglas County 43.052101 N, 123.621085 W (Datum WGS-84) owned by Roseburg Resources (Roseburg, OR, USA). The forest residues were still green and had needles and twigs attached. The sample was from an area that had been harvested between July and August 2011 and chipped on September 20, 2011. The residues was chipped using a two-knife 450-hp mobile horizontal chipper (Bruks Group, Sweden) mounted on a forwarder base. About 16 kg (wet weight) of chipped residues were shipped to the USDA Forest Service, Forest Products Laboratory, Madison, WI, USA.
Fractionation and Particle Size Distribution
The Douglas-fir forest residues sample as received with a moisture content of 39.5 % was fractionated through sieving. A total of 9 kg (wet weight) was processed through a nested stack of 11 sieves. The sieve sizes were 3.2, 4.8, 6.4, 9.5, 12.7, 15.9, 19.05, 22.2, 25.4, 28.6, and 31.8 mm. The set of sieves was placed on the horizontal sieve shaker (USPN 7905, Williams Standard, Williams Apparatus Company, Watertown, NY, USA). These sieves were selected based on the estimated range of particle sizes in the sample. After 10 min of sieving, the oven dry (od) mass retained on each sieve was determined using the wet weights and the moisture contents of each fraction measured gravimetrically by oven drying an aliquot sample overnight. The particle size mass distributions of the od and wet wood chips were then calculated.
Bark and Wood Contents and Chemical Compositions
It is nearly impossible to physically separate bark from wood for fine fractions with very small particle sizes, i.e., fractions I to IV (Table 1). Therefore, bark and wood contents in these small-particle fractions could not be measured. However, a very uniform and representative aliquot sample can be easily obtained from these fractions for chemical composition analysis. The measured lignin and glucan contents (the two major components in the sample) can be used to calculate the wood and bark contents in each of these fine fractions by simultaneously solving the following equations:
where G and L are glucan and lignin content, respectively. WDf and BKf are respective wood and bark contents to be determined for fraction f. Subscripts f, wd, and bk represent fraction f, wood, and bark, respectively. A sensitivity analysis was conducted to examine errors in the calculated bark and wood content for each fraction based on the measurement standard deviations of glucan and lignin in chemical composition analysis. It was found that the calculated bark and wood content are most sensitive to bark lignin content L bk. The results listed in Table 2 were calculated using a standard deviation of 2 % in L bk, while the deviations of other measured parameters in Eqs. 1a and 1b were assumed zero. The maximal experimental standard deviation of chemical composition was less than 0.4 % (Table 2).
Bark and wood can be easily separated for larger-sized fractions, i.e., fractions V to XII, and the bark and wood content, WD f and BK f , can be determined. However, it is very difficult to get a small but representative sample from each of these large-sized fractions for chemical composition analysis. Therefore, the chemical composition of these samples was calculated from the measured bark and wood content and the chemical composition of the wood and bark,
where C stands for component, e.g., lignin, glucan, and mannan; therefore, C f is the component content of fraction f. C wd and C bk are the respective component contents of wood and bark, measured via separating the bark from wood for randomly selected pieces of the forest residues, as listed in Table 2. The chemical composition of wood and bark was assumed not to change with particle size. The measured standard deviations of bark and wood content were used to calculate the errors in the chemical composition of each calculated fraction (Table 1).
The bark and ash contents and the chemical composition of the “whole” collected Douglas-fir residues were calculated based on the mass distribution by particle size and the bark and ash contents and the compositions of each size fraction according to the following equation:
where f i is the mass fraction of fraction i.
Fractionation Selectivity
Fractionation selectivity over bark or ash can be defined as the fraction of the accept forest residues as of the total residues divided by the fraction of bark or ash in the accept sample as of the total bark or ash, respectively, as follows:
where \( {f_i}, {f_i}^{\text{bk}}, {f_i}^{\text{ash}} \) are the oven dry mass fractions of the forest residues, bark, and ash of fraction i, respectively. Equations 4a and 4b indicate that selectivity is also the bark or ash content in the initial unfractionated sample over the bark or ash content in the accept sample.
Composite Samples for SPORL Pretreatment
Selected fractions of the fractionated forest residues were used to make composite samples for pretreatment and enzymatic saccharification studies according to the procedure described below. Based on the bark content (Table 1), the composite samples were made by combining fractions VI to XI with size between 12.70 and 28.58 mm as these fractions have very similar low levels of bark and ash content. Fraction V is a transition fraction in terms of bark content and is not used in the composite sample. Fraction XII was not used because the particle sizes were too large for our pretreatment equipment to process. However, the composite sample consisting of fractions VI to XI can represent fraction XII based on bark content and chemical compositions. Therefore, the composite sample represents approximately 60 % of the collected forest residues (Fig. 2a) but only contains approximately 37 % of the bark (Fig. 2b) and 35 % of the ash (Fig. 2c), as will be discussed in the “Results” section later. Manual debarking was applied to each fraction from VI to XI before combining. Composite samples of bark-only (U-bark, U stands for untreated), debarked wood-only (U-wood), and a mixture of wood and bark (U-mix) were each separately prepared by combining fractions VI to XI. The percentages of fractions VI to XI in the composite samples were based on the mass distribution of the fractions VI to XI in the combined samples and the wood and bark contents of each fraction (Table 2).
SPORL Pretreatment and Substrate Production
The composite samples of debarked wood (U-wood), bark (U-bark), and the mixture of wood and bark (U-mix) were subjected to SPORL pretreatment [14, 16]. SPORL pretreatments were conducted using three 1-L reactors mounted inside a rotating wood pulping digester in an autoclave configuration as described elsewhere [14, 16]. The three reactors were heated externally by steam. The digester is rotated at 2 rpm for mixing of biomass samples with chemicals during pretreatment. SPORL pretreatments were conducted at 180°C with a liquid to solids ratio (L/S) of 3. The sulfuric acid and sodium bisulfite charges based on od weight biomass solids were 2.21 and 8 (w/w) %, respectively. The pretreatment duration was 20 min, which is slightly shorter compared to our previous studies using softwood to show differences among different samples [14, 15]. Following pretreatment, each 1-L reactor was cooled using tap water while sealed. The residual solids remained intact which allowed for easy separation from the pretreatment hydrolysate (spent liquor) using a screen. The pretreatment spent liquor, mainly containing hemicellulosic sugars, was recovered and stored at 4°C until used for composition analysis. Each pretreated solid fraction was separately disk milled using an 8-in. manual-driven disk refiner (Andritz Sprout–Bauer Pressurized Refiner, Springfield, OH, USA) with a disk plate gap of 1 mm as described elsewhere [22]. Water was added to facilitate disk milling. The material collected was directly dewatered through pressing using a canvas bag to a solids content of about 30 %. The yield of solid (substrate) in the form of fibers or fiber bundles was then determined from the weight and moisture content of the collected substrate. The resultant solid substrates received no additional washing and were stored for chemical composition analysis and enzymatic hydrolysis.
Chemical Composition Analysis
The solid biomass substrates, both raw and pretreated, were ground using a Wiley mill (model #2, Arthur Thomas Co., Philadelphia, PA, USA) to pass a 40-mesh (~1 mm) screen. The resulting materials were hydrolyzed using sulfuric acid in two stages as described previously [16]. The hydrolysate was then analyzed for carbohydrates using an HPLC (Dionex IC-3000, Dionex Corporation, Sunnyvale, CA, USA) with pulsed amperometric detection (HPAEC-PAD) by the Analytical and Microscopy Lab of the Forest Products Laboratory. A Dionex Carbo Pac PA-1 Analytical column (4 × 250 mm) with a 4 × 20-mm PA-1 guard column was used. The Klason lignin content was measured gravimetrically after washing and drying the solid residues from the acid hydrolysis. The pretreatment spent liquor was also analyzed for fermentation inhibitors such as furan using the same HPLC with UV detection previously described [16]. For rapid analysis, glucose in the enzymatic hydrolysate was measured using a commercial glucose analyzer (YSI 2700S, YSI Inc., Yellow Springs, OH, USA). The reported data are the average of duplicate measurements.
Enzymatic Hydrolysis
All hydrolysis experiments were conducted using CTec-2 (Novozyme, Franklinton, NC, USA) at 2 % substrate solids (w/v) and pH approximately 5.2 in 50 mL of sodium acetate buffer (50 mM) on a shaker (Thermo Fisher Scientific, Model 4450, Waltham, MA, USA) at 50°C and 200 rpm. The enzyme loading was 20 FPU/g glucan. Triplicate hydrolysis runs were conducted. Hydrolysate samples were taken periodically for glucose determination. The average results from triplicate runs were reported. The standard deviations were used as error bars in plots. The substrate enzymatic digestibility (SED), defined as the percent glucan in the substrate saccharified enzymatically to glucose, was determined.
Results
Particle Size Distribution of the Douglas-fir Residues
The Douglas-fir forest residues fractions have considerable differences in color, shape, and bark and ash contents. As shown in Fig. 1, the small-sized fractions were needle-shaped with a greater aspect ratio than large-sized fractions. The small-sized fractions also tend to have a darker color than the large-sized fractions. The small-sized fractions I to IV also have much higher bark and ash contents than the large-sized fractions VI to XII (Table 1). The bark and ash content decreased with increasing particle size. The bark and wood content attained constant values of approximately 15 % and 85 %, respectively, for the large-sized fractions (VI to XII) when the particle size was greater than 12.7 mm (fraction VI). Fraction V is the transition fraction which has slightly higher bark and ash contents than those of the large-sized fractions VI to XII.
The results presented above suggest that physical fractionation based on particle size can effectively separate bark and ash from wood. To support this argument, we plotted the particle mass (Fig. 2a) along with bark mass (Fig. 2b) and ash mass (Fig. 2c) distributions by particle size fractions. Summing the results indicates that the small-sized fractions of I to IV has 25 % of the total mass (Fig. 2a) while containing approximately 50 % of the bark (Fig. 2b) and 55 % of the ash (Fig. 2c). Furthermore, the smallest fraction, I, with less than 5 % of the mass, contains more than 15 % of the bark and approximately 28 % of the ash. It is also notable that the small-sized fractions have slightly higher moisture content than the large-sized fractions (Fig. 2a) due to large surface area for moisture intake. Although these results were obtained from only one sample, it should be generally valid for most forest residues because the major sources of ash are from contaminants such as dirt, with small particle sizes, and bark. Bark is more friable than wood and can be easily broken down to small particles during harvesting and preprocessing.
Chemical Compositions of Residues Fractions
The small-sized fractions I to IV also have higher lignin content and lower carbohydrate content than the large-sized fractions VI to XII (Table 1). This is due to the small-sized fractions having higher bark content while bark inherently has higher lignin and lower carbohydrate contents than wood. As with bark content, the lignin content decreased as particle size increased for the small-sized fractions I to V and approached a constant value of approximately 32.5 % for large-sized fractions VI to XII. It is apparent that the physical fractionation by particle size also effectively fractionates the forest residues by chemical composition. We calculated the mass ratio between lignin and the sum of the three major polysaccharides in Douglas-fir, i.e., glucan, xylan, and mannan, for each particle size fraction and plotted the distribution in Fig. 3. The results clearly indicate that the ratios in the small-sized fractions I to VI are greater than the large-sized fractions VI to XII with ratios of approximately 0.6. The ratio for the smallest fraction I is over 1.2, more than double that of the large-sized fractions VI to XII.
Furthermore, the small-sized fractions also have relatively high moisture content (Fig. 2a). Therefore, physical fractionation by size can also help to separate water from wood. This may be beneficial when only the large fractions were collected and shipped in wet compared with collection and shipping the whole residues.
The bark and ash contents and the chemical compositions of the “whole” Douglas-fir residues were calculated according to Eq. 3 and listed in Table 1. The results indicate that collected residues have an ash content of only 1.5 % but a relatively high bark content of 22.6 %, which resulted in a high lignin content of 33.5 %. The residues have a cellulose (glucan) content of 37.3 %.
The Bark and Ash Contents and Chemical Compositions of the Composite Samples
The chemical compositions of composite samples U-bark and U-wood are assumed the same as those of bark and debarked wood, respectively, and are listed in Table 2. The chemical composition of the composite sample U-mix was calculated based on the fraction mix ratios and is also listed in Table 2. U-bark has high lignin and ash contents of 44.7 % and 3 %, respectively, and a low hollocellulose content with a glucan content of only approximately over 23 %. U-wood has a lignin content of 30.6 % and a glucan content of 41.8 %. The composite mixture of bark and wood (U-mix), made from large-sized fractions VI to XI, has 14.3 % bark, 0.85 % ash, and 32.6 % lignin. These values are all lower than those of the “whole” forest residues (Table 1), respectively. As a result, glucan content was enriched to 39.1 % in comparison with 37.3 % for the “whole” residue.
Effect of Bark on SPORL Pretreated Substrate Composition and Sugar Degradation
SPORL pretreatment produces two product streams. The liquid fraction (pretreatment hydrolysate or spent liquor) contains solubilized sugars largely derived from the hemicelluloses, sulfonated lignin, and various other chemicals. The solid substrate contains largely cellulose and residual lignin. Chemical compositions of pretreated composite samples of bark (T-bark, T stands for pretreated) from U-bark, wood (T-wood) from U-wood, and a mixture of bark and wood (T-mix) from U-mix are listed in Table 2. The percentage removals of lignin and carbohydrate from T-wood and T-mix by SPORL pretreatment are similar. T-mix has slightly higher lignin and lower glucan content than T-wood, which is expected because the composite wood sample U-wood had higher glucan and lower lignin contents. SPORL pretreatment removed significantly less lignin from T-wood or T-mix than from T-bark. Lignin removal was approximately 40 % for T-bark compared with less than 30 % from T-wood or T-mix. Xylan removal was only 77 % for T-bark compared with approximately 90 % from T-wood or T-mix (Table 2). SPORL pretreatments enriched glucan content more than lignin for all composite samples. Glucan enrichments were approximately 150 % for T-wood or T-mix and 166 % for T-bark, while lignin enrichments were approximately 120 % for all samples.
The extracted carbohydrates mainly hemicelluloses by SPORL pretreatment were hydrolyzed to sugars and further degraded to furan and organic acids. The sugar profiles of the pretreatment hydrolysate TL-wood and TL-mix, corresponding to T-wood and T-mix, respectively, are very similar (Table 3). TL-wood has slightly higher concentrations of various sugars than TL-mix except for arabinose because bark has higher arabinan content than wood. The hydrolysate TL-bark, corresponding to T-bark, has significantly lower sugar concentrations due to the low carbohydrate content of the U-bark sample. The total sugar concentration in TL-wood was 44.2 g/L, compared with 25.1 g/L in TL-bark, or 43 % lower. Specifically, glucose, xylose, and mannose concentrations in the TL-bark are 34 %, 71 %, and 44 % lower than that in TL-wood, respectively. The low carbohydrate content in U-bark not only resulted in lower sugar concentrations in its hydrolysate TL-bark but also lower amounts of HMF and furfural compared with U-wood (Table 3).
Effect of Bark on Substrate Enzymatic Saccharification
The SED of the pretreated substrate T-bark, T-wood, and T-mix were compared to evaluate the effect of bark on enzymatic saccharification of the forest residues after SPORL pretreatment. The results clearly indicate that bark negatively affects cellulose enzymatic saccharification (Fig. 4) The final SED of the pretreated composite bark sample, T-bark (lignin content 52.9 %, Table 2), was only 41 %, compared with 73 % for the debarked wood composite sample, T-wood (lignin content 37.4 %). The SED of the mixture of wood and bark composite sample, T-mix (lignin content of 41.8 %), was approximately 57 %. The difference in SED between T-wood and T-mix is primarily caused by the differences in the lignin content of the substrates and the amounts of lignin removal by SPORL pretreatment (the contents as well as the amounts of removal of hemicelluloses are almost the same, Table 2).
Effects of Separate Processing of Bark from Wood
Lignin in the bark affects enzymatic saccharification of substrate cellulose through two mechanisms: (1) bark is much more recalcitrant to cellulase enzymes than wood because it has more lignin, which blocks enzymatic access to cellulose; (2) lignin can adsorb cellulase to produce the so-called non-specific binding. The first mechanism is associated with pretreatment, while the second one is related to enzymatic hydrolysis. We analyzed the following scenarios to understand whether or not separate pretreatment or hydrolysis is technically beneficial for enzymatic saccharification:
-
1
Control: Combined pretreatment and hydrolysis of bark and wood. This is the pretreatment run using U-mix and enzymatic hydrolysis of the resultant pretreated substrate T-mix.
-
2
Separate pretreatments of bark (U-bark) from wood (U-wood), but combine pretreated bark (T-bark) with pretreated wood (T-wood) in enzymatic hydrolysis. For comparison purposes, the fraction of T-bark \( {f_{{{\text{T - bark}}}}} = 0.129 \) in the mixture (T-EH-mix) of T-bark and T-wood so that their corresponding amounts of U-bark and U-wood are identical to those in U-mix, i.e., bark content of 14.3 %.
-
3
Separate pretreatment of bark (U-bark) from wood (U-wood) and separate enzymatic hydrolysis of the resultant substrate T-bark from T-wood. For comparison purposes, the amounts of U-bark and U-wood used in separate pretreatment are identical to those in U-mix. The SED of this scenario can be determined by adding the glucose from separate enzymatic hydrolysis of T-bark and T-wood and divided by the total glucan in these two substrates, i.e.,
$$ {\text{SE}}{{\text{D}}_{{{\text{all}}\,{\text{separate}}}}} = 90\frac{{{\text{gl}}{{\text{s}}_{{{\text{T - bark}}}}}\, \cdot \,({f_{{{\text{T}}\,{\text{ - bark}}}}}) + {\text{gl}}{{\text{s}}_{{{\text{T - wood}}}}} \cdot (1 - {f_{{{\text{T - bark}}}}})}}{{{\text{Gl}}{{\text{n}}_{{{\text{T - bark}}}}} \cdot ({f_{{{\text{T - bark}}}}}) + {\text{Gl}}{{\text{n}}_{{{\text{T - wood}}}}} \cdot (1 - {f_{{{\text{T - bark}}}}})}} $$(5)where gls is the amount of glucose produced by enzymatic hydrolysis, and Gln is the pretreated substrate glucan content. The fraction of the pretreated bark, \( {f_{\text{T}}}_{{{\text{ - bark}}}} = 0.{129} \), same as that in Scenario (2), is determined based on the bark content of 14.3 % in U-mix and the yields of T-bark and T-wood (Table 2).
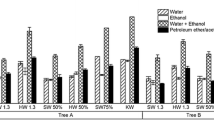
The results indicate that separate hydrolysis did not affect SED as shown in Fig. 5. The SED of the mixture T-EH-mix of T-bark and T-wood are almost identical to those calculated using Eq. 5. However, separate pretreatment of wood from bark is favorable to improve SED. This is because the presence of a small amount of bark, 14.3 %, marginally affects the available glucan for glucose production as bark has a low glucan content of only 23.2 %, but it can have a significant impact on removing wood recalcitrance in combined pretreatment as shown clearly in Fig. 4, i.e., bark and wood are interdependent in pretreatment. It is possible that a more severe pretreatment is required when the feedstock contains bark. This is supported by the relatively low degradation of sugars in the pretreatment hydrolysate as will be discussed in the next paragraph.
We compared the monomeric sugar and fermentation inhibitor profiles of pretreatment hydrolysate among TL-mix, TL-wood, TL-bark, and TL-hyp (a hypothetical sample made of TL-wood and TL-bark for separate pretreatment bark from wood with their amounts same as those in U-mix). The results in Table 3 suggest that combined pretreatment of bark and wood produced slightly more monomeric sugars and less inhibitors than individual bark and wood pretreatments (comparing TL-mix with TL-hyp). This again confirms that bark and wood are interdependent when they are pretreated together. Furthermore, synergy exists for hemicellulosic sugar recovery for the pretreatment conditions tested. This disagrees with literature findings that 0–30 % bark content did not influence pretreatment severity using SO2 catalyzed steam explosion [23].
Discussion
The findings discussed in the “Results” section could have significant implications to the practice of harvesting forest residues. This can be clearly seen from the cumulative distributions of residues oven dry mass, bark, and ash by particle size as shown in Fig. 6. The y coordinate, \( {\text{100 - Cumulative distribution }}\left( \% \right){ } = \frac{{\sum\nolimits_i^{\text{XII}} {{f_i}} }}{{\sum\nolimits_{\text{I}}^{\text{XII}} {{f_i}} }} \), represents the accumulation of the large-sized fractions (accept) from fraction i to the largest fraction XII. The fractions larger than 12.7 mm contain approximately 60 % of the collected forest residues, but only contain approximately 37 % of the bark and 35 % of the ash. We can use the selectivity defined by Eqs. 4a and 4b to further quantitatively demonstrate the effectiveness of fractionation for separating bark and ash from wood. The results indicate that using a mesh 2 screen (12.7 mm) can provide optimal separation bark and ash from wood as the selectivity over bark and ash are both maximized and achieved asymptotic values (Fig. 6). The fine fractions (reject) of approximately 40 % of the mass that contain most of the bark can be left uncollected. The large fractions (accept) also have slightly lower moisture content (Fig. 2a), beneficial to improve shipping economics. Literature indicated that Douglas-fir bark has significantly higher nutrient contents than wood [12, 24]. The nitrogen and potassium contents in Douglas-fir bark are approximately four to five and eight to ten times higher than those found in wood, respectively. Bark has high ion-exchange capacity and is a soil conditioner for good organic soil [24]. Because a fraction of forest residues needs to be left in the forest to supply nutrients and conditioning soil, fractionating small particles and leaving them in the forest is technically beneficial.
Leaving the fine fractions in the forest will result in a net loss of harvested residues, e.g., 40 % for all fractions below 12.7 mm for the present residues sample. It is of interest to know the net loss in glucose production. The results in Fig. 4 indicate that the reduction in SED at 96 h is 16 % due to the presence of 14.3 % bark in the composite mix sample and an additional reduction in SED of 16 % compared with that of the pure bark sample. Since pretreatment and hydrolysis of the “whole” residues was not conducted, using interpolation assuming ash is simply dead load and does not affect enzymatic hydrolysis, the SED of the “whole” residue with bark content of 22.6 % (Table 1) at 96 h was estimated to be 53 % based on the results shown in Fig. 4. Therefore, rejecting 40 % of the residues resulted in a glucose production loss of 30 % based on the estimated SED of 55 % and the glucan content of the whole residues (Table 1). This rejection loss varies with residues and the amount of desired residues to be left uncollected, and needs to be taken into account in harvesting.
The ability to capture the potential benefits of leaving fines in the forest depends on the collection method and downstream supply chain costs and benefits. Currently, collection methods favor utilization of roadside materials that have already been removed from the forest. Points of fractionation need to be evaluated to determine the best location. Tradeoffs involve cost of collection, effect on productivity of the comminution process, and efficiency of transport.
Conclusions
Physical fractionation of Douglas-fir forest residues through sieving can effectively fractionate bark and ash and therefore lignin from carbohydrates. For the forest residues studied, fractionation selectivity of wood over bark and ash using a mesh 2 screen is 1.6 and 1.7, respectively. Bark affects pretreatment to produce a less digestible substrate for subsequent enzymatic cellulose saccharification. With the presence of bark of 14.3 % in a mix sample, the reduction in SED is 16 % compared with that from a wood sample. The SED of a bark is 41 % compared with 73 % for wood when pretreated under the same conditions, indicating separate pretreatment optimization is required to improve SED forest residues containing bark. However, combined pretreatment of bark with wood has a slight synergistic effect that improves hemicellulosic sugar recovery and reduces the formation of fermentation inhibitors under pretreatment condition tested in this study. Separate enzymatic hydrolysis appears to have no effect on substrate. Together with the benefits of leaving bark in forests for soil improvement, the results obtained in this study suggest it is desirable and effective to fractionate forest residues by particle size, though the economics of where fractionation should take place yet needs to be determined. This has significant implications to the practice of harvesting, processing, and transport of forest residues.
References
Kirschbaum MUF (2003) To sink or burn? A discussion of the potential contributions of forests to greenhouse gas balances through storing carbon or providing biofuels. Biomass Bioenerg 24(4–5):297–310
Gan J, Smith CT (2006) Availability of logging residues and potential for electricity production and carbon displacement in the USA. Biomass Bioenergy 30(12):1011–1020
McKendry P (2002) Energy production from biomass (part 1): overview of biomass. Bioresour Technol 83:37–46
Zhu JY, Zhuang XS (2012) Conceptual net energy output for biofuel production from lignocellulosic biomass through biorefining. Prog Energy Combust Sci 38(4). doi:10.1016/j.pecs.2012.03.007
Koch P (1980) Harvesting energy chips from forest residues-some concepts for the southern pine region. General Technical Report SO-33. USDA Forest Service, Pineville, LA
Whittaker C, Mortimer N, Murphy R, Matthews R (2011) Energy and greenhouse gas balance of the use of forest residues for bioenergy production in the UK. Biomass Bioenergy 35(11):4581–4594
Eisenbies MH, Vance ED, Aust WM, Seiler JR (2009) Intensive utilization of harvest residues in southern pine plantations: quantities available and implications for nutrient budgets and sustainable site productivity. BioEnerg Res 2:90–98
Zhu JY, Pan XJ, Zalesny RS Jr (2010) Pretreatment of woody biomass for biofuel production: energy efficiency, technologies and recalcitrance. Appl Microbiol Biotechnol 87:847–857
David C, Atarhouch T (1987) Utilization of waste cellulose. VIII. Enzymatic hydrolysis of spruce bark by cellulases of Trichoderma viride. Appl Biochem Biotechnol 16:51–59
Kim KH, Tucker M, Nguyen Q (2005) Conversion of bark-rich biomass mixture into fermentable sugar by two-stage dilute acid-catalyzed hydrolysis. Bioresour Technol 96(11):1249–1255
Torget R, Himmel ME, Grohmann K (1991) Dilute sulfuric-acid pretreatment of hardwood bark. Bioresour Technol 35(3):239–246
Harkin JM, Rowe JW (1971) Bark and its possible use. Research Note FPL-091. USDA Forest Service, Madison, WI. pp 1–55
Pan XJ, Zhang X, Gregg DJ, Saddler JN (2004) Enhanced enzymatic hydrolysis of steam-exploded Douglas fir wood by alkali-oxygen post-treatment. Appl Biochem Biotechnol 113–16:1103–1114
Zhu JY, Pan XJ, Wang GS, Gleisner R (2009) Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour Technol 100(8):2411–2418
Zhu JY, Zhu W, OBryan P, Dien BS, Tian S, Gleisner R et al (2010) Ethanol production from SPORL-pretreated lodgepole pine: preliminary evaluation of mass balance and process energy efficiency. Appl Microbiol Biotechnol 86(5):1355–1365
Luo X, Gleisner R, Tian S, Negron J, Horn E, Pan XJ et al (2010) Evaluation of mountain beetle infested lodgepole pine for cellulosic ethanol production by SPORL pretreatment. Ind Eng Chem Res 49(17):8258–8266
Tian S, Luo XL, Yang XS, Zhu JY (2010) Robust cellulosic ethanol production from SPORL-pretreated lodgepolep pine using an adapted strain S. cerevisiae without detoxification. Bioresour Technol 101:8678–8685
Wood TM, Bhat M (1988) Methods for measuring cellulase activities. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol 160, Biomass (Part A, cellulose and hemicellulose). Academic, New York, pp 87–112
Perlack RD, Stokes BJ (2011) DOE. 2011. U.S. billion-ton update: biomass supply for a bioenergy and bioproducts industry. Oakridge National Laboratory, Oak Ridge
Murphy GE, Pilkerton SJ (2011) Seasonal impacts on bark loss for Douglas-fir and Ponderosa pine harvested on the Pacific Northwest Coast of the USA. Int J For Eng 22(1):35–41
Hakkila P (1989) Utilization of residual forest biomass. Springer, Berlin
Wang GS, Pan XJ, Zhu JY, Gleisner R (2009) Sulfite pretreatment to overcome recalcitrance of lignocellulose (SPORL) for robust enzymatic saccharification of hardwoods. Biotechnol Prog 25(4):1086–1093
Robinson J, Keating JD, Boussaid A, Mansfield SD, Saddler JN (2002) The influence of bark on the fermentation of Douglas-fir whitewood pre-hydrolysates. Appl Microbiol Biotechnol 59(4–5):443–448
Schowalter TD, Morrell JJ (2002) Nutritional quality of Douglas-fir wood: effect of vertical and horizontal position on nutrient levels. Wood Fiber Sci 34(1):158–164
Acknowledgements
This work, as part of the Northwest Advanced Renewables Alliance (NARA), was funded by the Agriculture and Food Research Initiative Competitive Grant no. 2011-68005-30416 from the USDA National Institute of Food and Agriculture (NIFA). We would also like to acknowledge Novozymes North America for their constant support by complementary providing cellulase enzymes. We would also like to thank Fred Matt of USDA Forest Products Laboratory for conducting detailed chemical composition analysis. The financial support from NIFA and the Chinese Scholarship Council made the visiting appointment of Zhang at the USDA Forest Products Laboratory possible.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was conducted while Zhang was a visiting student at the USDA Forest Products Laboratory and on official government time of Zhu and Gleisner.
Rights and permissions
About this article
Cite this article
Zhang, C., Zhu, J.Y., Gleisner, R. et al. Fractionation of Forest Residues of Douglas-fir for Fermentable Sugar Production by SPORL Pretreatment. Bioenerg. Res. 5, 978–988 (2012). https://doi.org/10.1007/s12155-012-9213-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-012-9213-3