Abstract
Objective
To evaluate the clinical usefulness of positron emission tomography (PET) using 18F-fluorodeoxyglucose (FDG) in patients with follow-up or suspected recurrent biliary cancer in a multicenter study.
Methods
We performed a retrospective review of 50 patients who underwent FDG-PET (either integrated PET/CT or manual fusion of dedicated PET and CT) scans for post-treatment surveillance of biliary cancer. Recurrence was suspected in 40 of these patients on the basis of tumor marker levels, and/or findings of conventional imaging (group A). Clinical findings in the remaining 10 patients showed them to be disease-free (group B). The diagnostic performance and clinical impact of PET were analyzed.
Results
Recurrence was confirmed in 28 out of the 40 patients in group A, and 1 of the 10 patients in group B. Patient-based analysis showed that the sensitivity, specificity, and accuracy of PET for detecting recurrence were 86% (25/29), 91% (19/21), and 88% (44/50), respectively. The one patient with recurrence in group B was correctly interpreted by PET. Positive test likelihood ratio and negative test likelihood ratio were increased from 1.69 to 9.05, and 0.08 to 0.32, respectively, after PET study. The findings of PET resulted in a change of management for 10 out of the 50 patients (20%) by initiating an unplanned treatment strategy (n = 7), by obviating the need for planned diagnostic procedures (n = 2), or by changing the treatment plan (n = 1).
Conclusion
FDG-PET/CT or PET with CT yielded helpful information in patients with suspected recurrent biliary cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite continuing advances in surgical and non-surgical therapeutic strategies, cancer recurrence and distant metastasis after initial treatment continue to be a major problem for patients with biliary cancer. Early and accurate detection of recurrence and distant metastasis has an important influence on therapy, and selection of appropriate treatment strategies can be expected to have a significant impact on overall survival. In devising therapeutic strategies taking into consideration patients’ quality of life, more accurate diagnosis acquired by recent imaging techniques is indispensable. Conventional morphological imaging modalities, including radiography, ultrasonography, computed tomography (CT), and magnetic resonance (MR) imaging are widely used to diagnose recurrent lesions. However, when used alone, these conventional imaging modalities are poor at visualizing small disseminated lesions, small lymph node metastases, and differentiating residual or recurrent tumor from scarring after therapy.
Positron emission tomography (PET) with 18F-fluoro-2-deoxy-d-glucose (FDG), which is based on the increased utilization, and thereby high uptake, of glucose by malignant cells, has opened a new field in clinical imaging. FDG-PET has been used successfully for the detection, staging, re-staging, and therapy monitoring of a large spectrum of various malignant tumors. Recently, integrated PET/CT, in which a full-ring-detector clinical PET scanner and multidetector row helical CT scanner are combined, makes it possible to acquire both metabolic and anatomic imaging data using a single device in a single diagnostic session and provides precise anatomic localization of suspicious areas of increased FDG uptake [1]. It is possible to diagnose cancer recurrence and distant metastasis by PET in the preclinical stage before it becomes evident by conventional imaging modalities in various malignancies [2–4]. Several studies describing the usefulness of FDG-PET for the differential diagnosis and initial diagnosis of biliary cancer have been published [5–10]. However, few studies have described the usefulness of FDG-PET for post-treatment evaluation [9–12].
The purpose of the present study was to assess the diagnostic accuracy of FDG-PET/CT or PET with CT in the diagnosis of recurrent biliary cancer and to assess its clinical impact in making decisions.
Materials and methods
Study population
We performed a multi-center (Dokkyo Medical University Hospital, Hokkaido University Hospital, Tohoku University Hospital, Hitachi General Hospital, National Cancer Center Hospital East, National Cancer Center Hospital, International Medical Center of Japan, and Kyoto University Hospital) retrospective observation study.Inclusion criteria were (1) post-operative state of biliary cancer, and (2) PET scans (either integrated PET/CT or manual fusion of dedicated PET and CT) were performed for surveillance of recurrent biliary cancer with or without suspicion of recurrence.If patients had repeated PET scans, we only analyzed the results of the first PET study after the initial surgery for biliary cancer.
Between June 2006 and December 2007, 50 consecutive patients (age range 47–82 years; median age 62 years) who had undergone surgery for histopathologically proven biliary cancer (gallbladder in 6 patients, cholangiocarcinoma in 12 patients, and bile duct cancer in 32 patients) entered into this study, which was approved by the respective institutional review boards. Informed consent was obtained from each patient after the nature of the procedures had been fully explained. Thirty patients had undergone only surgery, 19 had undergone chemotherapy after surgery, and 1 had undergone chemoradiotherapy after surgery (Table 1). The time interval between the initial surgery and PET examination ranged from 8 months to 7 years (median, 20 months), and the interval between the last treatment and PET examination ranged from 5 months to 6 years (median, 13 months).
Recurrence and distant metastasis of biliary cancer was suggested in 40 patients (group A) by elevated levels of tumor markers (CA 125 > 35 units/ml, or CA 19–9 > 35 units/ml) (n = 25 patients), abnormal findings in conventional morphological imaging modalities, such as ultrasonography, CT, or MR imaging studies (n = 10), and both elevated tumor marker levels and conventional imaging finding (n = 5). The remaining 10 patients were all clinically considered disease-free (group B). In this group, there were no abnormal findings in terms of tumor marker levels or conventional imaging findings. PET studies were performed at the request of physicians or the patients themselves.
FDG-PET or PET/CT study
Whole-body FDG-PET/CT (n = 30) or PET (n = 20) scans were performed after patients had fasted for at least 4 h. At 60 min after the intravenous administration of 250–370 MBq FDG, imaging of trajectory upper thigh to skull base was performed using a dedicated full ring BGO-based dedicated PET scanner (Advance, GE Healthcare), an LSO PET/CT scanner (Biograph Duo, CTI/Siemens), a BGO PET/CT scanner (Discovery LS/ST, GE Healthcare), and a GSO PET/CT scanner (Gemini, Philips Medical System). PET images were reconstructed with attenuation correction by an ordered-subsets expectation maximization algorithm, but specific parameters for image reconstruction depended on each institution’s method. All PET studies were conducted under the guidelines issued by the Japanese Society of Nuclear Medicine.
Data interpretation and image analysis
At least two experienced radiologists/nuclear medicine physicians interpreted the PET, CT, and fused images of integrated PET/CT examination visually by consensus. In dedicated PET studies, after making image fusion between PET and CT images manually on a workstation (AquariusNetStation, TeraRecon), PET, CT, and fused images were read by at least two experienced radiologists/nuclear medicine physicians. In interpreting these images, all readers had knowledge of the clinical findings, including tumor markers, and of the results of all the available imaging studies. Diagnostic ability was determined on a patient basis and also on a lesion location basis (local, various sites of metastasis, such as lymph nodes, bone, liver, and lung and peritoneal dissemination). A lesion on PET was considered suspicious if the metabolic activity was higher than background activity allowing for the normal biodistribution. Semiquantitative analysis using standardized uptake value (SUV) was not done in this study, and no cut-off values for SUV were set for differentiating lesions in interpreting PET images. Lymph nodes with increased FDG uptake were deemed positive for metastatic spread, even if they were smaller than 1 cm in short-axis diameter.Lung metastasis was mostly interpreted by CT and not PET, because small lung metastases show little or no FDG uptake. Peritoneal dissemination was considered positive when there was peritoneal, mesenteric, or intestinal thickness or mass formation with increased FDG uptake (Table 2).
The final diagnosis was obtained from the results of histopathological examination (n = 15), or clinical follow-up (n = 35) for periods longer than 6 months on the basis of tumor marker levels, CT or MR imaging findings, and PET/CT findings.
PET/CT or PET with CT was considered to be of value if it provided additional information that led to cancellation of previously planned diagnostic procedures or if it resulted in the initiation of previously unplanned treatment or changed the previously planned therapeutic approach.
Statistical analysis
We performed a patient-based analysis of the PET/CT or PET or with CT results based on the consensus verdict in general. Sensitivity, specificity, and accuracy were calculated using standard statistical formulas. The change of positive test likelihood ratio and negative test likelihood ratio after PET study were calculated. In addition, the clinical impact obtained by FDG-PET/CT or PET with CT for therapeutic strategy was also quantified.
Results
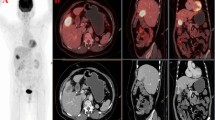
Recurrence was confirmed in 28 out of the 40 patients in group A, and 1 of the 10 patients in group B. In group A comprising 40 patients with suspected recurrence, PET/CT or PET with CT correctly diagnosed 24 out of 28 patients with recurrent disease and 10 out of 12 patients without recurrence. In group B comprising 10 patients suspected to be disease-free, PET/CT or PET with CT correctly diagnosed 1 of 1 patient with recurrent disease and 9 of 9 patients without recurrence. Positive test likelihood ratio and negative test likelihood ratio were increased from 1.69 to 9.05, and 0.08 to 0.32, respectively, after PET study. Patient-based analysis showed that the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of PET/CT or PET or with CT in groups A and B were 86.2, 90.5, 92.2, 82.6, and 88.0%, respectively, whereas those in group A were 85.7, 83.3, 92.3, 71.4, and 85.0%, respectively, and those in group B were 100, 100, 100, 100, and 100%, respectively. Some representative cases are shown in Figs. 1, 2. Four false negative PET results were, a missed tiny local recurrence, a missed lymph node metastasis, a missed tiny liver metastasis, and a missed tiny peritoneal dissemination. Two false positive PET results were, over-diagnosed cholangitis and post-surgical change.
A 76-year-old female postoperative cholangiocarcinoma patient with LN metastasis and peritoneal dissemination. Although axial contrast-enhanced CT taken one month ago (a) showed no abnormal findings, axial PET (b), CT (c), and fused-image (d) showed tiny LN metastasis in the hepatic portal region (arrow). Although axial contrast-enhanced CT taken one month ago (e) showed no abnormal findings, axial PET (f), CT (g), and fused-image (h) showed tiny peritoneal dissemination at the hepatic surface (arrow)
PET/CT or PET with CT resulted in a change of treatment management for 9 out of the 40 patients in group A by initiating an unplanned treatment strategy (n = 6), by obviating the need for planned diagnostic procedures (n = 2), or by changing the treatment plan (n = 1). PET/CT or PET with CT resulted in a change of treatment management for 1 out of the 10 patients in group B by initiating an unplanned treatment strategy.
Discussion
The diagnostic performance of PET with CT and PET/CT was reasonably high in our series, and was comparable to that in previously reported series. Four groups have investigated the usefulness of FDG-PET or PET/CT for postoperative surveillance of patients with biliary cancer and reported a sensitivity of 89–100% and specificity of 93–100% [9–12]. Two authors demonstrated that PET or PET/CT showed better diagnostic accuracy for recurrence than CT [11, 12]. Corvera et al. [10] performed FDG-PET scans in 33 postoperative patients with biliary cancer (gallbladder in 10 patients and cholangiocarcinoma in 23 patients) and found that the sensitivity and specificity of PET for recurrence were 89% and 100% and the findings of PET influenced management in 9% of 33 patients. Jadvar et al. [12] performed FDG-PET or PET/CT scans in 24 postoperative patients with biliary cancer and reported that the sensitivity and specificity of PET or PET/CT for recurrence were 94% and 100% while the respective figures for CT were 82% and 43%. Chikamoto et al. [11] performed FDG-PET scans in 18 postoperative patients with hilar bile duct cancer and reported that the sensitivity and specificity of PET for recurrence were 100% and 93% and the sensitivity of enhanced CT was 25%. Anderson et al. [9] performed FDG-PET examinations in 5 postoperative patients with biliary cancer and reported that the sensitivity and specificity of PET for recurrence were 100% and 100%. Our results are consistent with their data, and the number of patients in our series was relatively large.
In our series, the positive test likelihood ratio and negative test likelihood ratio were increased from 1.69 to 9.05, and 0.08 to 0.32, respectively, after PET/CT or PET study. This means that PET/CT or PET demonstrates a much greater incremental diagnostic value to the conventional and clinical diagnostic information if the PET is positive.
Several studies describing the usefulness of FDG-PET for the differential diagnosis and initial diagnosis of biliary cancer have demonstrated that the detection rate of FDG-PET in the nodular type of the biliary tumor was superior to that of the infiltrating type and PET/CT can be helpful to detect unsuspected distant metastasis, but may offer only modest accuracy for regional lymph node staging [5–10].
Although integrated FDG-PET/CT is an accurate complementary modality for providing good anatomic and functional localization, even PET/CT could not detect tiny lesions, tiny local recurrence, lymph node metastases, or peritoneal dissemination in our series. PET and PET/CT can only detect lymph nodes that have a certain volume of malignant cells sufficient to change the observed glucose metabolism, and neither of these modalities can detect micrometastasis. The spatial resolution of PET scans is insufficient for detection of microscopic metastases to lymph nodes [13]. With a given spatial resolution of 4–6 mm with currently available PET and PET/CT systems, the detection of microscopic lesions remains challenging. Improving the spatial resolution and sensitivity of PET and PET/CT scanners and developing new, more specific radioactive tracers may help to overcome this limitation in the future.
The liver represents one of the main targets of metastatic spread of biliary cancer, and PET or PET/CT is a useful modality to detect liver metastasis with a certain volume [14]. However PET/CT is unable to detect tiny liver metastasis. Recently contrast-enhanced MRI is widely accepted and Squillaci et al. demonstrated that the Gd-enhanced MRI had a better sensitivity to detect tiny liver metastasis than PET/contrast enhanced CT [15]. Moreover, a new contrast medium of MRI, gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) may replace ultrasound or enhanced CT and become the preferred contrast agent for detecting liver metastases [16]. When liver metastasis is highly suspected, contrast-enhanced MRI should be recommended.
This study had certain limitations. The gold standard for any analysis is histological confirmation of the findings. However, clinical follow-up is also a valid way to evaluate diagnostic accuracy and response to therapy, and it would have been unethical to investigate all PET/CT-detected lesions by invasive procedures. Positive findings are easy to confirm, but negative findings only mean that we were unable to acquire positive findings during the follow-up period, making it uncertain whether the findings were truly negative. Second, not all data were based on PET/CT, and 40% of them are from PET studies. If they were conducted by PET/CT, the sensitivity or accuracy might have been different. However, in this investigation, all PET images were manually fused on a workstation. Although misregistration can happen, it is expected that higher diagnostic accuracy would be obtained than PET only interpretation. Third, conventional morphological imaging modalities, such as radiography, ultrasonography, CT, and MR imaging, which were used to detect recurrent lesions were not performed in all patients and were variable in individual patients.
In conclusion, FDG-PET/CT yielded helpful information in patients with suspected recurrent biliary cancer, like in cases of various other cancers.
References
Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–79.
Kitajima K, Murakami K, Yamasaki E, Hagiwara S, Fukasawa I, Inaba N, et al. Performance of FDG-PET/CT in the diagnosis of recurrent endometrial cancer. Ann Nucl Med. 2008;22:103–9.
Kitajima K, Murakami K, Yamasaki E, Domeki Y, Kaji Y, Sugimura K. Performance of FDG-PET/CT for diagnosis of recurrent uterine cervical cancer. Eur Radiol. 2008;18:2040–7.
Nogami M, Nakamoto Y, Sakamoto S, Fukushima K, Okada T, Saga T, et al. Diagnostic performance of CT, PET, side-by-side, and fused image interpretations for restaging of non-Hodgkin lymphoma. Ann Nucl Med. 2007;21:189–96.
Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43–50.
Kim JY, Kim MH, Lee TY, Hwang CY, Kim JS, Yun SC, et al. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. Am J Gastroenterol. 2008;103:1145–51.
Moon CM, Bang S, Chung JB, Park SW, Song SY, Yun M, et al. Usefulness of 18F-fluorodeoxyglucose positron emission tomography in differential diagnosis and staging of cholangiocarcinomas. J Gastroenterol Hepatol. 2008;23:759–65.
Furukawa H, Ikuma H, Asakura-Yokoe K, Uesaka K. Preoperative staging of biliary carcinoma using 18F-fluorodeoxyglucose PET: prospective comparison with PET+CT, MDCT, and histopathology. Eur Radiol. 2008;18:2841–7
Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90–7.
Corvera CU, Blumgart LH, Akhurst T, Dematteo RP, D’Angelica M, Fong Y, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206:57–65.
Chikamoto A, Tsuji T, Takamori H, Kanemitsu K, Uozumi H, Yamashita Y, et al. The diagnostic efficacy of FDG-PET in the local recurrence of hilar bile duct cancer. J Hepatobiliary Pancreat Surg. 2006;13:403–8.
Jadvar H, Henderson RW, Conti PS. [F-18]fluorodeoxyglucose positron emission tomography and positron emission tomography: computed tomography in recurrent and metastatic cholangiocarcinoma. J Comput Assist Tomogr. 2007;31:223–8.
Kitajima K, Murakami K, Yamasaki E, Fukasawa I, Inaba N, Kaji Y, et al. Accuracy of FDG PET/CT in detecting pelvic and paraaortic lymph node metastasis in patients with endometrial cancer. AJR Am J Roentgenol. 2008;190:1652–8.
Bipat S, van Leeuwen MS, Comans EFI, Piji ME, Bossuyt PM, Zwinderman AH, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis. Meta-analysis. Radiology. 2005;237:123–31.
Squillaci E, Manenti G, Mancino S, Ciccio C, Calabria F, Donieli R, et al. Staging of colon cancer: whole-body MRI vs. whole body PET/CT—initial clinical experience. Abdom Imaging. 2008;33:676–88.
Hammerstingl R, Huppertz A, Breuer J, Balzer T, Blakeborough A, Carter R, et al. Diagnostic efficacy of gadoxetic acid (primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008;18:457–67.
Acknowledgments
This work was supported in part by the Grant-in-Aid for Cancer Research (17-12) from the Ministry of Health, Labour and Welfare, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kitajima, K., Murakami, K., Kanegae, K. et al. Clinical impact of whole body FDG-PET for recurrent biliary cancer: a multicenter study. Ann Nucl Med 23, 709–715 (2009). https://doi.org/10.1007/s12149-009-0297-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-009-0297-6