Abstract
Objectives
The purpose of this study was to evaluate the adverse effects of chronic marked hyperglycemia on clinical diagnostic performance of positron emission tomography (PET) using 18F-fuorodeoxyglucose (FDG).
Methods
Fifty-seven scans of 54 patients, who received FDG-PET for the diagnosis of various cancer(s), and who showed high plasma glucose level of more than 200 mg/dl at the time of administration of FDG in spite of at least 4-h fasting, were retrospectively analyzed. In the clinical follow-up, this high plasma glucose was confirmed as chronic hyperglycemia derived from uncontrolled diabetes (n = 32) and untreated diabetes (n = 25). Based on the final diagnosis of malignancy obtained by histopathology or clinical follow-up for at least 6 months, the diagnostic performance of visual PET analysis was evaluated.
Results
Excluding nine scans of nine patients without sufficient follow-up, final diagnosis was obtained in 48 scans of 45 patients. In 36 scans of 36 patients, at least one malignant lesion was finally confirmed, and true-positive and false-negative results were obtained in 30 and six cases, respectively. Six cases showed false-negative results due to low FDG-avid pathological characteristics (hepatocellular carcinoma, etc.), chemotherapeutic effect or small tumor size. Overall, the patient-based sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy were 83, 83, 94, 63 and 83%, respectively. In lesion-based diagnosis, 56 of 75 lesions (74%) were depicted by PET, while 19 lesions were negative on PET, also due to low FDG-avid characteristics or small size (less than 15 mm).
Conclusions
At the time of chronic hyperglycemia (not acute hyperglycemia), the adverse effect caused by high plasma glucose level was minimum. The FDG uptake of the tumor maintained a sufficiently high level for visual clinical diagnosis in most cases, except in the cases of low FDG-avid tumors or small lesions (15 mm in size).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Positron emission tomography (PET) using 18F-fluoro-2-deoxy-d-glucose (FDG) has been widely used as a functional imaging tool in oncology [1]. It is known that the accumulation of FDG can be influenced by plasma glucose level on injection of FDG, because FDG is a radiolabeled glucose analog. There have been many reports mentioning the effects of hyperglycemia on FDG uptake. In vitro studies indicated that accumulation of FDG in cancer cells declined with the increase of glucose level in the medium [2]. Torizuka et al., however, reported in their in vitro study that adenocarcinoma cells did not significantly change FDG uptake in the case of chronic hyperglycemia, while acute hyperglycemia markedly reduced FDG uptake [3]. In addition, Zhao et al. reported in their animal model that insulin loading decreased the FDG uptake into tumors, while glucose loading did not [4]. In a human study, hyperglycemia may considerably decrease FDG uptake in tumors, impair image quality and may also adversely affect the diagnostic accuracy [5, 6]. It is reported that negative PET results should be interpreted with caution in patients with hyperglycemia, who had been suspected of having pancreatic cancer [7]. It is also recommended to reschedule PET scan when the plasma glucose level is greater than 150–200 mg/dl on injection of FDG [8]. However, most of these reports with human cases focus on acute hyperglycemia and not on chronic hyperglycemia.
Acute hyperglycemia caused by meal intake can be avoided, while we have to consider the clinical application of FDG-PET scan for patients with chronic hyperglycemia, despite their prior fasting, due to diabetes. In such cases, it is not always easy to reschedule a PET scan in clinical practice. Especially, patients with pancreatic cancer have accompanying uncontrollable diabetes, and it may be preferable to perform a PET scan in spite of their hyperglycemia, rather than wait until their hyperglycemia is diminished, resulting in possible delay of treatment. For this reason, it is important to assess how much and how often chronic hyperglycemia, with a plasma glucose level of greater than 200 mg/dl, can affect the actual diagnostic accuracy in a clinical oncologic situation.
The purpose of this study was to investigate the influence of chronic hyperglycemia caused by diabetes in the diagnostic performance of FDG-PET in a clinical setting.
Patients and methods
Patients and plasma glucose evaluation
Between January 2001 and December 2005, 3459 patients underwent 4655 FDG-PET scans in our institute for suspected or known malignant disease. In our institute, patients were instructed to fast for more than 4 h before FDG injection, until the end of PET scanning. Patients who had just had a meal and patients with acute hyperglycemia caused by insufficient fasting were excluded from this investigation. Among the fasting patients, there were 71 scans of 68 patients, who showed high plasma glucose level greater than 200 mg/dl at the time of administration of FDG. Among them, 14 patients with hyperglycemia caused by unknown reasons were excluded because confirmation of diabetes was not sufficient in clinical follow-up. Finally, there were 57 scans of 54 patients (1.2%) (male:female = 38:16, mean age: 64.8 ± 9.0, ranging from 42 to 79 years old) who showed chronic hyperglycemia (greater than 200 mg/dl) at the time of administration of FDG (mean glucose level:255.5 ± 69.3, ranging from 201 to 666 mg/dl) in spite of prior fast for at least 4 h. The reasons for chronic hyperglycemia were confirmed in the clinical follow-up as follows: poorly controlled diabetes (n = 32) and untreated diabetes (n = 25). At the time of administration of FDG, 2 ml of the patient’s blood was drawn via the cubitus vein and the drawn blood was collected in a plastic tube containing sodium fluoride, heparin and EDTA (ethylenediaminetetraacetic acid)-Na2, immediately. The sample was centrifuged for 3 min at the speed of 3,000/min. The plasma sample was analyzed by GOD (glucose oxidase)-electro-enzymatic assay using automated analyzer, GA1160 (ARKRAY, Kyoto, Japan).
PET scanning
Fluorine-18 was synthesized by the nucleophilic substitution method [9] with an 18F-FDG-synthesizing instrument (F-100, Sumitomo Heavy Industries, Tokyo, Japan) and a cyclotron (CYPRIS-325R, Sumitomo Heavy Industries, Tokyo, Japan). After 4-h of fasting, patients received an intravenous injection of approximately 370 MBq (10 mCi) of FDG, and PET was performed using a whole-body PET scanner (GE advance, GE healthcare, Milwaukee, WI). The scanner had 18 rings, which provided 35 tomographic sections at 4.25-mm intervals. Image reconstruction with segmented attenuation correction was performed by ordered subsets expectation maximization algorithm. The intrinsic transaxial resolution of the PET machine was measured as 3.8 m full width at half maximum (FWHM) at the center of cross section, and 7.3 mm FWHM at a radius of 20 cm (radial) from the center of cross section. The effective resolution after reconstruction was measured as 4.0 mm FWHM at the center of cross section, and 6.6 mm at a radius of 20 cm from the center of cross section.
Interpretations and analyses
PET images were interpreted visually by at least two experienced nuclear medicine physicians, using corresponding CT and MRI images (performed within 1 week before or after the date of FDG-PET study), which permitted accurate identification of the tumor relative to anatomic landmarks. CT examinations were performed with the use of Hi-Speed Advantage (GE Medical Systems; Milwaukee, WI) or CT-W2000 or CT-W3000 (Hitachi Medico; Tokyo, Japan) scanners. Pre-contrast, early and delayed post-contrast images were obtained in all patients with abdominal lesions, and pre-contrast and post-contrast images were obtained in all patients with neck lesions, while contrast images were not obtained for the patients with chest lesions. MR imaging using T1 and T2-weighted fast spin-echo (SE) imaging was performed on a 1.5-T superconducting unit; Magnetom Symphony (Siemens, Erlangen, Germany) or Sigma Horizon (General Electric Medical System, Milwaukee, WI) with a body phased array coil for the patients with abdominal lesions, and with a neck phased array coil for the patients with neck lesions. However, MR imaging was not performed in patients with chest lesions. The patient-based diagnostic accuracy was calculated, according to final diagnoses determined by histopathology (n = 20) or clinical follow-up for at least 6 months (n = 28). Also, the frequency of false-negative findings was evaluated. In addition, FDG accumulation in malignant lesions, brain (cerebellum), liver (right lobe) and muscle (gluteal muscle) was also analyzed semi-quantitatively by calculating the standardized uptake value (SUV) in the regions of interest (ROIs) with the size of 5 × 5 mm2 placed over the areas.
The ROI was placed in tumor areas that showed the highest FDG activity, and the maximum SUV (SUVmax) in the ROIs of the lesion was defined as the SUV of the tumor.
If patients had more than six malignant lesions, only five lesions were analyzed. Physiological FDG uptake in the brain, liver and muscle (hip muscle was used as reference) was also examined in each case by calculating the averaged SUV of the five ROIs in five different slices of each organ. For the comparison of normal FDG uptake in brain, liver and muscle, randomly chosen 50 normoglycemic patients who received FDG-PET study for the evaluation of cancer in the body, not brain or liver tumor, between January 2001 and December 2005 were analyzed (mean glucose level: 88.5 ± 12.4).
Results
Nine scans of nine patients without sufficient oncologic follow-up were excluded from the analysis, because of unconfirmed final cancer status. Since three patients had a PET scan twice, we analyzed 48 scans in 45 patients. In these three patients, two scans were performed with more than 1-year interval between two scans. Therefore, we defined these two scans as two different cases. At least one malignant site was finally diagnosed in 36 of 48 cases (Table 1), while the final diagnoses were negative (no malignant lesion) in 12 of 48 cases (Table 2).
In 36 cases with malignant lesion, 30 cases showed true-positive primary lesion by PET diagnosis even under hyperglycemia, while six cases showed false negative. In 30 cases with true-positive results, 19 cases showed multiple foci. Among these 19 cases, two cases had numerous lung metastases and one had multiple bone metastases, but many lesions were clearly depicted by PET (Fig. 1). Among the 12 cases who had no malignant lesion, 10 cases were accurately diagnosed as true-negative and two cases were interpreted as positive with the final diagnosis of false positive (Table 2). These false-positive findings were considered to be due to the physiological uptake in the colon and biloma. Six false-negative cases for primary lesion were small-sized bile duct cancer (n = 1), hepatocellular carcinoma (well-differentiated: n = 2, poorly differentiated with small size: n = 1; n = 3), lung cancer (adenocarcinoma, post-radiation, n = 1), and pancreatic cancer (scirrhous type, n = 1) (Fig. 2) (Table 3). In 11 cases of lung cancer in this study, tumor detectability was 10/11 cases (91%) with only one false-negative case. In 11 cases of pancreatic cancer, tumor detectability was 10/11 cases (91%) with only one false-negative case (Fig. 2). On the other hand, there was only one case of bile duct cancer and four cases of hepatocellular carcinoma (tumor detectability was 0 and 25%, respectively). Overall, the patient-based sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy were 83, 83, 94, 63 and 83%, respectively.
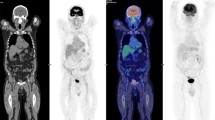
A 64-year-old diabetic male with multiple lung metastases (patient#7). The plasma glucose level on administration of FDG was 291 mg/dl. CT (a) shows multiple pulmonary nodules in bilateral lung fields (a), and moderate to intense uptake is seen on PET (b MIP image, c axial image), demonstrating multiple lung metastases. Most of the nodules (up to 5 lesions) in the lung fields (white arrows) were demonstrated on PET in the corresponding area (black arrows), while a small nodule in the right middle lobe was not demonstrated on PET (white and black circles). However, it is unknown whether this small lesion was clearly depicted under normoglycemia or not, because the size of the lesion was too small
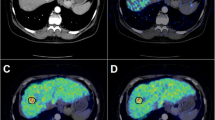
A 69-year-old diabetic male with pancreatic cancer (patient#30). The plasma glucose level on administration of FDG was 212 mg/dl. CT revealed a mass in the pancreatic head portion (a white arrow); however, only faint uptake was just observed in the corresponding area with SUVmax of 2.4 (b MIP image, black circle). Pancreatic cancer of 35 mm in size was confirmed by surgery. Pathological examination revealed that the tumor was tubular adenocarcinoma of the scirrhous type
In lesion-based diagnosis, 56 of 75 lesions (74%) were depicted by PET, while 19 lesions were negative on PET mainly due to small size (less than 15 mm) (Table 3). Thirteen false-negative lesions for additional lesions were three bone metastases (two osteoblastic lesions, one post-radiotherapeutic lesion), one lung lesion with ground-glass opacity (well-differentiated adenocarcinoma) and 9 small lesions (less than 15 mm in size).
In the quantitative analysis of FDG uptake in cancer tissue, there was no apparent decrease in the SUVmax of tumor, despite hyperglycemia (Fig. 3a). SUVmax of tumor did not show a tendency to continuous decrease with the increase of plasma glucose level (n.s.). Even in the false-negative cases, FDG uptake was kept relatively high (more than 2.0) for several lesions (Table 3). With regard to the physiological uptake, FDG uptake in the brain showed a significant constant decrease with the increase in plasma glucose level (R 2 0.3888) and tended to be lower in the present study (4.5 ± 1.0), as compared to that (11.8 ± 2.6) in the randomly chosen 50 patients with normoglycemia (mean glucose level:88.5 ± 12.4) (Fig. 3b). However, uptake in the liver (3.3 ± 0.5) and muscle (1.4 ± 0.4) was not influenced significantly by chronic hyperglycemia, as compared to those (2.9 ± 0.8, 1.3 ± 0.3) in the randomly chosen 50 patients with normoglycemia (Fig. 3c, d). These results suggest that FDG uptake in cancer tissue under hyperglycemia can be kept relatively high, despite high plasma glucose level and the decrease in brain FDG uptake.
Scatter gram for SUV and plasma glucose level in tumor and each organ. The relationship between FDG uptake and plasma glucose level was shown in tumor (a), brain (b), liver (c) and muscle (d). (a) Regarding tumor FDG uptake, SUV ranged widely from 1.8 to 12.8. Even under high plasma glucose level, most of the tumors showed FDG uptake higher than 3.0 and there was no significant correlation between SUV and plasma glucose level (R 2 0.0219). Seven perpendicular bars were also shown on the left side of the scattered dots, which represented the average ± SD of SUV calculated in each cancer under normal plasma glucose level in our institute (see “Discussion”). On the other hand, the brain showed negative relationship with plasma glucose levels. Brain FDG uptake (SUVmax = 4.5 ± 1.0) ranged from 1.6 to 6.6, which was significantly lower than those in the normoglycemic patients (SUV = 11.8 ± 2.6). There was a significant inverse correlation between brain SUV and plasma glucose level (R 2 0.3888). FDG uptake of liver and muscle was quite stable in the hyperglycemic condition. There was no significant correlation between liver and muscle FDG uptake and plasma glucose level (R 2; liver: 0.0132, muscle: 0.0019)
Discussion
Our results suggested that tumor FDG uptake maintains a sufficiently high level for visual clinical diagnosis in a majority of cases (except for low FDG-avid tumors or small lesions) with chronic hyperglycemia (more than 200 mg/dl). Thirty of 36 patients showed positive results in the main tumor. Overall, the patient-based diagnosis was with a high level of accuracy, as sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy were 83, 83, 94, 63, and 83%, respectively. In both lung cancers and pancreatic cancers, tumor detectability was 91% with only one false-negative case, which was a satisfactory level for clinical oncologic diagnosis even under normoglycemia. Lesion-based diagnosis also showed high sensitivity (74%). This result is compatible with those of previous studies performed with lung cancers and uterine cervix cancers [10, 11]. It means that in a majority of clinical situations, FDG-PET study could be performed despite the high plasma glucose level, if the high glucose level was introduced due to chronic hyperglycemia.
In this investigation, it must be noted that about one-third of FDG-negative lesions (6/19 lesions) consisted of low FDG-avid lesions and that about two-thirds (11/19 lesions) consisted of small lesions (less than 15 mm) (Table 3). Pancreatic cancer sometimes shows relatively low FDG accumulation, mainly because of its desmoplastic pathological character and the resultant low tumor cell cellularity [12]. Well-differentiated hepatocellular carcinoma is known for its relatively low detection rate, because well-differentiated cancers tend to have lower FDG accumulation than those of poorly differentiated cancers [13, 14]. Osteoblastic bone metastases frequently had low FDG uptake [15]. Well-differentiated adenocarcinoma of lung with ground-glass opacity is known to have low FDG avidity [16]. Thus, these low FDG-avid pathological characters may be one of the main causes of false-negative results in this study. Regarding small lesions (smaller than 15 mm in size), it is not the problem of the plasma glucose level, but the problem of the resolution of the PET device. In these false negative cases, recent advance of PET/CT or PET scan using breath-hold technique, etc., may hammer out a solution, regardless of plasma glucose level.
Let us check the six false-negative primary lesions in detail (Table 3). First of all, a case of pancreatic cancer showed lower FDG uptake. According to the previously published data of our institute, the averages of SUVmax under normoglycemia in pancreatic cancer were 7.2 ± 3.5 [13]. Therefore, the SUV = 2.4 was relatively low for a common invasive ductal adenocarcinoma of the pancreas (Fig. 3a). However, not only hyperglycemia, but also the scirrhous pathological character may affect FDG uptake, because of the low tumor cell cellularity. Secondly, three false-negative cases with hepatocellular carcinomas should be discussed (patient#11–13). According to the previously published data of our institute, the averages of SUVmax under normoglycemia in well, moderate and poorly differentiated hepatocellular carcinoma were 3.4 ± 1.4, 4.5 ± 1.8 and 10.1 ± 6.5, respectively [13]. The average SUV of normal liver was also shown to be 2.9 ± 0.8; therefore, well-differentiated hepatocellular carcinoma shows almost the same FDG uptake as that of the normal liver tissue (Fig. 3a, c). The three cases in this study showed the pathology, SUV and tumor size as follows: patient#11, well, SUV = 2.9 and 25 mm; patient#13, well, SUV = 3.4 and 22 mm; patient#12, poor, SUV = 1.8 and 14 mm, respectively. We suspected that the former two cases were false negative in PET scan due to the well-differentiated pathological character of the tumor. We also suspected that the latter case with poorly differentiated type showed negative PET finding mainly due to the small size. Thirdly, the false-negative result of patient#18 with lung cancer was due to chemotherapeutic effect. The pathology of patient#18 was not obtained, because this patient was treated with systemic chemotherapy, but the cytology by pretreatment bronchoscopy revealed adenocarcinoma, which is known to be a relatively lower FDG-avid tumor. According to the previously published data of our institute, the averages of SUVmax under normoglycemia in adenocarcinoma (AC) and squamous cell carcinoma (SCC) of lung were 7.3 ± 5.8, 15.2 ± 4.7, respectively [17]. The SUV = 2.1 in this case was relatively low for a common adenocarcinoma of the lung (Fig. 3a). However, this patient had received chemotherapy 2 weeks before the PET scan and that was why PET showed false-negative result. Bile duct cancer (patient#2; hilar bile duct cancer) also is discussed. According to previously published and in-house data of our institute, the averages of SUVmax of intrahepatic and hilar/extrahepatic bile duct cancer under normoglycemia were 8.5 ± 3.5 and 4.1 ± 1.6, respectively [17]. The SUV of 3.8 in this case was not as low, compared to the average of hilar/extrahepatic bile duct cancers (Fig. 3a). Hilar and extrahepatic cholangiocarcinomas diagnosed by obstructive jaundice tend to be small in size; therefore, their FDG uptake was usually lower than that of the intrahepatic one. The tumor size of patient#2 was also small and thin, and the accurate size was not available in the pathological report. The result of false-negative PET scan in this case may be due to the small size of the tumor.
In the additional 13 lesions with false-negative result (Table 3), the first 4 lesions with larger tumor size were suspected to have low FDG avidity, due to osteoblastic character (bone metastasis), post-radiation (bone metastasis) and well-differentiated character (GGO). The following two lesions of small size were hepatocellular carcinoma with well-differentiated character (patient#13). The other negative lesions were less than 11 mm in size.
Therefore, we could conclude that FDG accumulation in the FDG-avid tumor of a certain size (larger than 15 mm) remained sufficiently high for visual diagnosis in the majority of cases even under chronic hyperglycemia. On the other hands, FDG diagnosis at the time of chronic hyperglycemia must be performed carefully in cases of low FDG-avid tumors, such as pancreatic cancers, hepatocellular carcinomas and well-differentiated lung adenocarcinomas. In addition, clinicians should pay attention to the possibility of the presence of false-negative small-sized lesions at the time of chronic hyperglycemia.
Diabetes mellitus, especially type 2, is now found in almost every population. A variety of evidence suggests that without effective prevention and control program, the prevalence will continue to increase globally [18]. Patients with diabetes are known to be at risk of several cancers. There is increasing evidence that diabetes and/or hyperglycemia are independent risk factors and/or predictors at least in respect of cancer of the colon, pancreas, female breast and endometrium and, in men, of the liver and bladder [19]. In the present study, we encountered only 1% of chronic hyperglycemic patients in 5 years of clinical experience, but much more cancer patients with hyperglycemia or uncontrolled diabetes may be expected in the near future.
In general, FDG-PET has not been performed in known diabetic patients having cancers, because of the evidences from several papers, which suggest poor diagnostic results due to hyperglycemia. However, most of previous papers did not pay attention to the type of hyperglycemia, acute or chronic. When a nuclear physician encounters a patient with unexpected hyperglycemia in clinical FDG-PET study, he needs to find out the cause of hyperglycemia: acute hyperglycemia caused by insufficient fasting or chronic hyperglycemia under fasting condition caused by diabetes. For patients with insufficient fasting, it is recommended to reschedule and perform the PET scan under appropriate fasting condition at a later date. If patients had hyperglycemia caused by severe or poorly controlled diabetes, PET scan may not be canceled and can be performed after obtaining permission of the referring physician. Also, the consent of the patient, informing the possibility of false-negative results, has to be obtained. PET scan is performed without delay because (1) re-examination at a later date after treatment for diabetes would cause a possible delay in the treatment, especially in patients with an aggressive cancer, such as pancreatic cancer; (2) even under hyperglycemia, FDG-PET has a satisfactory diagnostic performance and may detect unexpected distant metastasis or may show other useful information.
Contrary to the results of tumors, there was an inverse correlation between brain FDG uptake and blood glucose level (Fig. 3). Although glucose is the only energy source of brain tissue, chronic hyperglycemia is known to have deleterious effects on brain tissue because excessive influx of glucose results in increased intracellular protein glycation and generation of free radicals [20, 21]. A variety of natural protective mechanisms against hyperglycemia have been investigated and many researches using animal model are focusing on glucose transporters’ expression on blood–brain barrier (BBB) [22, 23]. In brain tissue, vascular endothelial and vascular smooth muscle cells express the ubiquitous GLUT-1 predominantly, and these cells autoregulate their rate of glucose transport in response to changes in ambient glucose levels. This results in the decrease of transport up to 50%, by reduction of GLUT-1 expression on exposure to high glucose levels [24]. Our results are supposed to be compatible with these animal studies.
In the present study, the muscle and liver uptakes were kept stable in the hyperglycemic condition (>200 mg/dl) (Fig. 3). Chronic hyperglycemia is known to have deleterious effects on muscle cells [25]. Muscle cells may have a natural protective mechanism against hyperglycemia, but there has been little information. The predominant type of glucose transporters for muscle is GLUT-4 and it is known to be insulin dependent [26]. Further evaluation is needed. Muscle FDG uptake may be a good indicator for the differentiation between chronic hyperglycemia due to diabetes and acute hyperglycemia (or normoglycemia) after oral ingestion, because oral ingestion usually effects the FDG accumulation of muscles. Therefore, in case of acute hyperglycemia, muscle FDG uptake would rise according to plasma glucose level and this high muscle uptake might affect the results of oncologic diagnosis.
Limitations of the present study must be mentioned. In the present study, the presence or absence of malignant lesion was confirmed in about half of the cases by other imaging modalities, with clinical follow-ups for at least 6 months. Regarding the mainly targeted tumors (primary lesions), our confirmation was definite in the present study, while additional lesions, such as small lymph nodes or small metastatic lesions, might not be accurately confirmed due to limited information or limitation in the resolution of other imaging modalities. Especially in a case of advanced cancer under chemotherapy, there is no way to confirm such kind of small lesions in the follow-up observation. It cannot be denied that there is a possibility of the presence of more false-negative (probably small) lesions from the pathological point of view. In addition, our PET machine in this study was not a PET/CT. For the diagnostic performance with PET/CT, the results of sensitivity or detectability might be better, especially at the level of SUV 2.0–3.0 (patient#2, 18, 30). Secondly, it was impossible to compare tumor FDG uptake under hyperglycemia to that under normal glucose level in the same patient immediately after treatment for diabetes. Comparison of tumor FDG accumulation between two scans in the same patient under high and normal glucose level would be interesting. There was, however, no patient in the present study who received FDG-PET scans again under normal glucose level after treatment for diabetes without receiving cancer treatment. In our study, there were only three patients who received re-examination of FDG-PET within 3 months after treatment for diabetes and cancer (Table 4). In all cases, the effects of the change in the plasma glucose level after treatment of diabetes were not apparent.
Conclusions
FDG accumulation in the FDG-avid tumor of a certain size (larger than 15 mm) remained high enough for visual diagnosis in the majority of the cases, even under chronic hyperglycemia (more than 200 mg/dl). Under fasting condition with chronic hyperglycemia caused by severe diabetes in this study, diagnostic results showed its sensitivity, specificity and diagnostic accuracy as 83, 83 and 83%, respectively, which is supposed to be almost satisfactory for clinical oncologic diagnosis. Therefore, PET scan can be performed in uncontrolled or untreated diabetic patients under fasting condition, rather than re-examination with FDG-PET at a later date, because re-examination after treatment of diabetes may cause possible delay in treatment, especially in patients with an aggressive cancer, such as pancreatic cancer.
References
Bomanji JB, Costa DC, Ell PJ. Clinical role of positron emission tomography in oncology. Lancet Oncol. 2001;3:157–64.
Wahl RL, Henry CA, Ethier SP. Serum glucose: effects on tumor and normal tissue accumulation of 2-[F-18]-fluoro-2-deoxy-d-glucose in rodents with mammary carcinoma. Radiology. 1992;183:643–7.
Torizuka T, Clavo AC, Wahl RL. Effect of hyperglycemia on in vitro tumor uptake of tritiated FDG, thymidine, l-methionine and l-leucine. J Nucl Med. 1997;38:382–6.
Zhao S, Kuge Y, Tsukamoto E, Mochizuki T, Kato T, Hikosaka K, et al. Effects of insulin and glucose loading on FDG uptake in experimental malignant tumours and inflammatory lesions. Eur J Nucl Med. 2001;28:730–5.
Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer—a PET study. J Nucl Med. 1993;34:1–6.
Diederichs CG, Staib L, Glatting G, Beger HG, Reske SN. FDG-PET: elevated plasma glucose reduces both uptake and detection rate of pancreatic malignancies. J Nucl Med. 1998;39:1030–3.
Zimny M, Bares R, Fass J, Adam G, Cremerius U, Dohmen B, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography in the differential diagnosis of pancreatic carcinoma: a report of 106 cases. Eur J Nucl Med. 1997;24:678–82.
Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885–95.
Kitano H, Magata Y, Tanaka A, Mukai T, Kuge Y, Nagatsu K, et al. Performance assessment of O-18 water purifier. Ann Nucl Med. 2001;15:75–8.
Gorenberg M, Hallett WA, O’Doherty MJ. Does diabetes affect [(18)F]FDG standardised uptake values in lung cancer? Eur J Nucl Med Mol Imaging. 2002;29:1324–7.
Chang YC, Yen TC, Ng KK, See LC, Lai CH, Chang TC, et al. Does diabetes mellitus influence the efficacy of FDG-PET in the diagnosis of cervical cancer? Eur J Nucl Med Mol Imaging. 2005;32:647–52.
Hatano E, Ikai I, Higashi T, Teramukai S, Torizuka T, Saga T, et al. Preoperative positron emission tomography with fluorine-18-fluorodeoxyglucose is predictive of prognosis in patients with hepatocellular carcinoma after resection. World J Surgery. 2006;30:1736–41.
Higashi T, Saga T, Nakamoto Y, Ishimori T, Fujimoto K, Doi R, et al. Diagnosis of pancreatic cancer using fluorine-18 fluorodeoxyglucose positron emission tomography (FDG PET)—usefulness and limitation in “clinical reality”. Ann Nucl Med. 2003;17:261–79.
Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13:427–33.
Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16:3375–9.
Mamede M, Higashi T, Kitaichi M, Ishizu K, Ishimori T, Nakamoto Y, et al. [18F]FDG uptake and PCNA, Glut-1, and Hexokinase-II expressions in cancers and inflammatory lesions of the lung. Neoplasia. 2005;7:369–79.
Seo S, Hatano E, Higashi T, Nakajima A, Nakamoto Y, Tada M, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts lymph node metastasis, P-glycoprotein expression, and recurrence after resection in mass-forming intrahepatic cholangiocarcinoma. Surgery. 2008;143:769–77.
Namageyo-Funa A, Nanavati P. The challenges of addressing primary prevention of diabetes: a response to recommendations from the chronic disease directors’ project. J Public Health Manag Pract. 2008;14:26–8.
Schiel R, Beltschikow W, Steiner T, Stein G. Diabetes, insulin, and risk of cancer. Methods Find Exp Clin Pharmacol. 2006;28:169–75.
Nishikawa T, Edelstein D, Du XL. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature. 2000;404:787–90.
Guyot LL, Diaz FG, O’Regan MH, Song D, Phillis JW. The effect of topical insulin on the release of excitotoxic and other amino acids from the rat cerebral cortex during streptozotocin-induced hyperglycemic ischemia. Brain Res. 2000;872:29–36.
Duelli R, Maurer MH, Staudt R, Heiland S, Duembgen L, Kuschinsky W. Increased cerebral glucose utilization and decreased glucose transporter Glut-1 during chronic hyperglycemia in rat brain. Brain Res. 2000;858:338–47.
Alpert E, Gruzman A, Totary H, Kaiser N, Reich R, Sasson S. A natural protective mechanism against hyperglycemia in vascular endothelial and smooth-muscle cells: role of glucose and 12-hydroxyeicosatetraenoic acid. Biochem J. 2002;362:413–22.
Nagamatsu S, Sawa H, Wakizaka A, Hoshino T. Expression of facilitative glucose transporter isoforms in human brain tumors. J Neurochem. 1993;61:2048–53.
Peiró C, Lafuente N, Matesanz N, Cercas E, Llergo JL, Vallejo S, et al. High glucose induces cell death of cultured human aortic smooth muscle cells through the formation of hydrogen peroxide. Br J Pharmacol. 2001;133:967–74.
Ishiki M, Klip A. Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology. 2005;146:5071–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hara, T., Higashi, T., Nakamoto, Y. et al. Significance of chronic marked hyperglycemia on FDG-PET: is it really problematic for clinical oncologic imaging?. Ann Nucl Med 23, 657–669 (2009). https://doi.org/10.1007/s12149-009-0288-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-009-0288-7