Abstract
In oncology positron emission tomography/computed tomography (PET/CT) is a standard imaging procedure for cancer staging, restaging, treatment monitoring, and radiation therapy planning. Despite the availability of many radiotracers, 18F-fluoro-2-deoxy-2-D-glucose ([18F]FDG) is still the most used for most cancers staging.
Hyperglycemic conditions, antidiabetic oral medications, or insulin can have an impact on [18F]FDG PET/CT scan accuracy. A correct knowledge of how these conditions influence [18F]FDG distribution is fundamental for patients management before [18F]FDG PET/CT. International PET/CT guidelines propose protocols for patients’ adequate preparation and therapy management before [18F]FDG administration, in particular with regard to diabetic patients.
Adapted reprint with permission from: Finessi M, Bisi G, Deandreis D. Hyperglycemia and 18F-FDG PET/CT, issues and problem solving: a literature review. Acta Diabetol 2020; 57(3):253–262 https://doi.org/10.1007/s00592-019-01385-8
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Clinical Importance of 18F FDG PET/CT in Cancer Staging
In oncology positron emission tomography/computed tomography (PET/CT) is a standard procedure, commonly used in several cancers for staging, restaging, treatment monitoring, and radiation therapy planning. Despite many radiotracers being available, according to different applications, 18F-fluoro-2-deoxy-2-D-glucose ([18F]FDG) is the most widespread. Focusing on cancer TNM staging, local tumor (T) extension is frequently a prerogative of morphological imaging such as CT scan or MRI to evaluate local extension, but [18F]FDG PET can help in a better tumoral mass characterization, especially identifying necrotic or fibrotic areas inside the tumoral mass to guide biopsy or locoregional treatment. Furthermore [18F]FDG is a very important tool for lymph-node (N) and distant metastases (M) detection: baseline [18F]FDG PET/CT is fundamental to assess tumor burden, which not only is a prognostic factor but also is fundamental to plan the optimal therapeutic strategy (Gallamini et al. 2014).
Indications in Lymphomas
[18F]FDG PET/CT is recommended in disease staging in several tumors, in particular both in Hodgkin lymphoma (HL) and in [18F]FDG-avid non-Hodgkin lymphoma (NHL) subtypes. [18F]FDG PET/CT improves the accuracy of staging both for nodal and extranodal sites, allowing a staging change (sometimes upstaging) in 10–30% of patients, in particular for a better definition of splenic involvement and for its high sensitivity in the case of bone marrow involvement: in the case of bone marrow [18F]FDG PET/CT uptake in HL, bone marrow biopsy is in fact no longer indicated (Cheson et al. 2014). In the case of LH or [18F]FDG-avid NHL, [18F]FDG PET/CT is fundamental also in response to therapy assessment to define complete or partial response, stable or progressive disease using a 5-point scale (Deauville score), based on [18F]FDG lesion uptake compared to background (Cheson et al. 2014).
Carcinoma and Adenocarcinoma
[18F]FDG PET/CT has demonstrated a pivotal role also in several [18F]FDG-avid solid tumors. Despite T extension being often provided by conventional imaging (CT, MRI), PET/CT scan could provide additional information: for example solitary pulmonary solid nodules greater or equal to 8 mm can safely be considered benign if the PET-CT scan is negative (Madsen et al. 2016). On the other hand, in the case of advanced NSCLC the main advantage of [18F]FDG PET/CT is the assessment of tumor spread to the pleura (Gallamini et al. 2014). In the case of breast cancer, despite [18F]FDG PET/CT not being recommended for staging localized disease, evidences support its use in locally advanced breast cancer based on improved regional and distant staging, and also for its prognostic role, as baseline tumor glycolytic activity is associated with biological behavior and prognosis (Caresia Aroztegui et al. 2017).
The fundamental role of [18F]FDG PET/CT in solid tumors staging has been widely demonstrated in N and M assessment. For example in the case of NSCLC, [18F]FDG PET/CT is superior to contrast-enhanced CT (CeCT), by adding metabolic information able to disclose morphologically undetectable nodal dissemination: despite this superiority the sensitivity of PET-CT is in general insufficient to rule out mediastinal lymph node metastasis (Madsen et al. 2016) and surgical staging remains the standard (Gallamini et al. 2014). In the case of advanced breast cancer, the imaging-guided sentinel lymph node biopsy with 99mTc-colloid remains the gold standard, but the high positive predictive value of [18F]FDG PET/CT (≥90%) could guide an axillary lymph node dissection in the case of axillary node uptake (Gallamini et al. 2014).
In the case of melanoma staging, [18F]FDG PET/CT could add clinical information for patients with palpable or macroscopic locoregional nodal metastasis: approximately 60% of patients with locoregional nodal metastasis will develop distant metastases (Perng et al. 2015). In M detection, there’s no doubt on [18F]FDG PET/CT leading role, and it is widely supported by international guidelines. For example, in NSCLC no curative-intent treatment should be planned until a PET-CT scan has excluded occult distant metastases (Madsen et al. 2016) or, in the case of melanoma, numerous studies have shown that FDG PET/CT can detect M parameter with high sensitivity (86%) and specificity (91%) (Perng et al. 2015).
Tumor Uptake and Hyperglycemia
Several conditions can impact [18F]FDG PET/CT accuracy, first of all peculiar tumor [18F]FDG avidity related to tumor aggressiveness, histology, and metabolism. Hyperglycemia is the second most important condition that can impact [18F]FDG PET/CT scan results, and an accurate knowledge of [18F]FDG uptake mechanism and management of patient glycemic status before performing the exam are pivotal to obtain the maximum quality of the procedure.
Mechanism of [18F]FDG Uptake
The chemical structure of [18F]FDG is similar to natural glucose, differing in a carbon-2 atom labelled with [18F] (Ido et al. 1978), a radioisotope with a half-life of 109.8 min and a positron emission decay. Cancer tissue presents accelerated glucose metabolism even in the presence of oxygen, with an associated increase in lactate production (Potter et al. 2016). This phenomenon was firstly described by Warburg in 1920 and named “Warburg effect” (Warburg and Negelein 1924). On the basis of the increased lactate production even in the presence of oxygen, he hypothesized a mitochondrial dysfunction that was not supported by further research. Aerobic glycolysis, in fact, is a favorable metabolic pathway for tumoral cell because, despite generally leading to a lower production of ATP molecules compared to mitochondrial oxidative phosphorylation, on the other hand it is much faster and in the same amount of time, produces a higher number of ATP molecules (Chen et al. 2017).
Intracellular Pathways of Glucose and Labelled Glucose
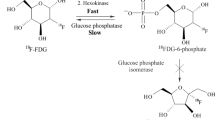
As normal glucose, [18F]FDG is transported by membrane specific glucose transporters (GLUT) into the cell cytosol, and phosphorylated by hexokinase to [18F]FDG 6-phosphate that, differently to glucose 6-phosphate, is not a good substrate for further enzyme action through the glycolytic chain, and it is trapped into the cell and not metabolized to carbon dioxide and water (Larson 2006).
Five different glucose transporters (GLUT) are responsible for [18F]FDG transport across cell membrane: GLUT-1 is ubiquitously expressed in the cell membrane of various tissues and it is up-regulated by several growth factors (Pauwels et al. 2000); GLUT-2 expression is regulated by glucose concentration and it is mainly expressed by intestine, kidney, liver, pancreatic islets, and brain (Theorens et al. 1988); GLUT-3 is expressed in neurons and ensures a glucose supply in the brain even in hypoglycemic conditions (Maher 1995); GLUT-4 is stimulated by insulin and it is expressed in skeletal and cardiac muscle, and in brown and white adipose tissue (Rea and James 1997); GLUT-5 is expressed in the small intestine and is responsible for fructose transport (Kayano et al. 1990).
In euglycemic status, several organs present a physiologic biodistribution of [18F]FDG that reflects GLUTs expression (Fig. 47.1). It is very intense in brain, moderate in liver, and weak in skeletal muscle; furthermore it is variable in the cardiac muscle because myocardial cells primarily use the beta oxidation pathway of fatty acids, but following a glucose load may present a metabolic shift toward glucose metabolism; moreover, myocardial cells protect themselves from hypoxic state by means of the “glucose-fatty acid cycle” (Randle et al. 1963).
Physiologic biodistribution of [18F]FDG. In fasting and euglycemic conditions [18F]FDG uptake reflects GLUTs expression: [18F]FDG uptake is intense in brain, because of GLUT-3 expression that ensures correct glucose levels even in hypoglycemic conditions, weak in skeletal muscle for GLUT-4 expression stimulated by insulin, variable in cardiac muscle that primarily uses free fatty acids, but may present a metabolic shift to glucose metabolism after glucose load; intense in the urinary system because of [18F]FDG physiological urinary excretion
Finally, urinary system presents high [18F]FDG concentration due to it physiological urinary tracer excretion. Despite the absence of glycosuria in physiological and euglycemic status, renal tubular cells, in addition to GLUTs, also present a sodium-dependent glucose transporter (SGLT) that is responsible for re-uptake of glucose from the filtrate in the proximal tubules, promoting glucose transport against its concentration gradient (Szabo et al. 2006). SGLT presents a lower affinity for [18F]-FDG compared to glucose, due to the replacement of a hydroxy group in D-glucose with a [18F] atom (Moran et al. 1999), and for this reason [18F]-FDG cannot be reabsorbed in the proximal tubules of the kidney and it is accumulated in the urine (Qiao et al. 2007).
Cancer Cell Pathways
Cancer cells [18F]FDG uptake can vary substantially depending on glycolysis levels at one site and it is associated with both increased GLUT and intracellular hexokinase expression (Brown and Wahl 1993); moreover, in cancer cells, glucose 6-phosphatase is markedly downregulated, so its levels are insufficient to break down [18F]FDG 6-phosphate (Larson 2006).
Generally, aggressive and proliferating tumors present a high expression of GLUT and hexokinases, so in these case [18F]FDG uptake is generally very high, but FDG avidity can differ among different histological subtype: Hodgkin Lymphoma, Diffuse Large B Cell Non-Hodgkin Lymphoma (DLBCL) or Follicular Lymphoma presents high [18F]FDG uptake, while other hematologic malignancies, such as Marginal Zone Lymphoma (ML), for example, demonstrate low [18F]FDG uptake (Fig. 47.2). Also solid tumors may have different [18F]FDG avidity. In the case of lung cancer, in fact, Non-Small Cell Lung Carcinoma (NSCLC) presents high [18F]FDG uptake, while tumors with neuroendocrine differentiation (typical carcinoid) present low/absent [18F]FDG uptake (Fig. 47.3). Other well-differentiated tumors that use different metabolic pathway, for example, membrane phospholipids turnover in cell membrane instead of glycolysis, such as Prostatic Carcinoma or Hepatocellular Carcinoma (HCC), present a very low [18F]FDG avidity and should be studied specifically with other PET tracers.
[18F]FDG PET/CT scan in two patients with recent diagnosis of pulmonary nodule prior to histological diagnosis: left apical node of patient A presents very high [18F]FDG uptake compared to medium lobe node of patient B. Subsequent histological diagnosis revealed in case A a NSCLC instead in case B a typical lung carcinoid
[18F]FDG uptake may be influenced not only by tumoral histological characteristics, but also by intra-tumoral heterogeneity in the case of intra-tumoral necrosis, that is responsible for low and heterogeneous uptake (Fig. 47.4) and cell dedifferentiation (undifferentiated neuroendocrine tumors, for example, may present high [18F]FDG uptake). Also different therapies effects may modify [18F]FDG uptake: cytotoxic therapies such as chemotherapy or external radiotherapy may reduce [18F]FDG uptake, but may also cause flogistic reaction with increased [18F]FDG uptake at that site. For these reason an interval of at least three weeks from the last cycle of chemotherapy and three months from the end of radiotherapy is often required, especially in hematologic malignancies, before performing [18F]FDG PET/CT scan to avoid false positive findings.
Effects of Hyperglycemia on [18F]FDG Distribution and PET/CT Scan Interpretation
Several clinical conditions may affect [18F]FDG uptake. Several published studies described the effects of hyperglycemia and hyperinsulinemia on [18F]FDG biodistribution. Hyperglycemia is the leading cause of altered biodistribution because, both in normal and in cancer cells, it leads to a direct competition between plasmatic glucose and [18F]FDG uptake (Di et al. 2018), reducing the binding site of [18F]FDG (Wahl et al. 1992). Reactive hyperinsulinemia to hyperglycemia results in a higher skeletal and myocardial muscle [18F]FDG uptake (Diederichs et al. 1998) for GLUT-4 up-regulation with lower uptake in cancer cells compared to physiological tissue leading to the risk of false negative findings. Also the standardized uptake value (SUV), a semiquantitative parameter expressing FDG concentration in tissues (Juweid and Cheson 2006), may be affected by blood glucose levels. SUV is the a-dimensional semiquantitative expression of the tracer uptake in a region of interest (ROI), for example, tumoral lesion, normalized by total amount of activity administered and body weight (Body weighted SUV—SUVbw) or body surface area (SUVbsa); it is used as SUVmax or mean or peak to compare [18F]FDG uptake on pre and post-therapy scan and to define response to therapy (Wahl et al. 2009). Furthermore, SUVs of healthy tissues, such as liver and mediastinal blood pool, are used as references in international criteria to define disease uptake and to assess response to therapy in lymphoproliferative disease (Barrington and Kluge 2017).
In 2013 Büsing et al. (2013) enrolled 90 patients with Blood Glucose Levels (BGL) ranging from 50 to 372 mg/dl to assess the impact of chronically elevated BGL on [18F]FDG tumor uptake and biodistribution in healthy organs. The authors found a significant association between BGL increase and both cerebral uptake reduction (p < 0.001) and muscle uptake increase (p < 0.001) and weak associations between BGL and liver uptake (p = 0.06), tumoral (p = 0.133), fat, lung, and spleen uptake expressed as SUVmax (p = 0.136–0.157).
Sprinz et al. in 2018 investigated the effects of glycemia on [18F]FDG uptake in healthy liver, brain, and lungs in 5623 patients that underwent PET/CT, stratified into four groups by serum glucose levels. All organs showed significant differences in mean SUVmax according to different groups (p < 0.001) in univariate analysis, while multivariate analysis adjusted for sex, age, and BMI, confirmed significant differences only for brain and liver and not for lung uptake.
The effect of BGL on SUVmax and SUV mean of [18F]FDG uptake was assessed in 8380 patients collected in a recent meta-analysis published in 2019 (Eskian et al. 2019). Patients were divided into 5 groups by BGL, and SUVmax and SUVmean values of tumor, brain, muscle, liver, and blood pool were recorded. Significant inverse correlations (p < 0.001) were found between BGL and SUVmax and SUVmean both in brain and in muscle, while positive correlations were found between BGL and SUVmax and SUVmean in liver (p = 0.001, p = 0004) and blood pool (p = 0.008, p < 0.001). No significant correlation was found between BGL and SUVmax or SUVmean in tumors.
All hyperglycemic groups compared to the euglycemic group presented significantly lower brain and muscle SUVs (p < 0.001 for both), while higher SUVmax and SUVmean in liver (p = 0.001, p = 0004) and blood pool (p = 0.008, p < 0.001) were reported. On the contrary, tumoral tissue presented significantly lower SUVmax only in the case of BGL >200 mg/dl. The explanation could be that in tumoral tissue GLUT could be not saturated even in the case of high BGL because tumoral cells overexpress GLUT in order to respond to the hypoxic condition subsequent to induced angiogenesis (Eskian et al. 2019; Yang et al. 2017) demonstrated in various cancer types (Macheda et al. 2005; Carvalho et al. 2011).
Diagnostic Strategies in Normal and Disglycemic Patients
In order to avoid hyperglycemic status guidelines to manage correct patient preparation for [18F]FDG PET/CT scan have been proposed by international societies such as the European Association of Nuclear Medicine (EANM) (Boellaard et al. 2014), the Society of Nuclear Medicine and Molecular Imaging (SNMMI) (Delbeke et al. 2006), the American College of Radiology (ACR) (American College of Radiology 2007), and the National Cancer Institute (NCI) (Shankar et al. 2006).
Non-diabetic patients should fast for at least 4–6 h and parenteral nutrition and intravenous fluids containing glucose should be discontinued for the same time before [18F]FDG injection to prevent high insulinemia. BGL must be measured by a glucometer prior [18F]FDG administration (Boellaard et al. 2014; Delbeke et al. 2006; American College of Radiology 2007; Shankar et al. 2006); for clinical routine the accepted upper BGL threshold is 200 mg/dL, while for research trials ranges between 126 and 150 mg/dL are recommended (Boellaard et al. 2014). In the case of hyperglycemia, it is possible to decrease BGL in patients by hydration until an acceptable level is achieved (Boellaard et al. 2014). The management of diabetic patient is more challenging providing specific recommendations in this setting (Boellaard et al. 2014) for diabetic patients treated by oral medication or insulin.
For diabetic patients treated with oral medication such as metformin, fasting for at least 4–6 h before [18F]-FDG injection in association with adequate hydration is recommended, without oral medication withdrawal to allow for a controlled blood sugar level. PET/CT scan should preferably be performed in the late morning. Metformin decreases BGL by lowering gluconeogenesis, increasing insulin sensitivity and enhancing glucose consumption by enterocytes (Rena et al. 2017). Its known action on the bowel assumes a critical role in [18F]FDG physiological distribution: metformin significantly increases [18F]FDG accumulation in the bowel, in particular in the colon (Martin and Saleem 2014) (Fig. 47.5a and b).
(a) [18F]FDG PET/CT scan performed for characterization of a hepatic lesion in a 41 year old diabetic patient treated with metformin. High intestinal uptake is visible in particular in descending colon. (b) [18F]FDG PET/CT scan performed for suspected occult lesion in a 51 year old diabetic patient treated with metformin and hepatic lesions suspected for metastases. High intestinal uptake is visible in particular in transverse and sigmoid colon
Massolo et al. (2013) in 2013 verified this phenomenon in fifty-three mice that performed dynamic acquisitions for [18F]FDG kinetic evaluation under fasting conditions over a 4-month study period. Mice were divided into 4 groups: untreated mice (group 1), mice exposed to metformin treatment for 48 h before each PET scan (group 2), mice treated for the whole study period (group 3), and mice in which treatment was interrupted 48 h before PET scan (group 4). They found that prolonged drug administration significantly increased bowel [18F]FDG uptake after a relatively long period of treatment and persisted after drug washout.
This increased [18F]FDG uptake has been also demonstrated in clinical practice.
A prospective study published by Gontier at al. (2008) demonstrated an intense, diffuse, and continuous pattern distribution of [18F]FDG along the bowel, strongly predominant in the colon, in patients treated with metformin. They enrolled fifty-five patients under oral medication for diabetes mellitus divided in two groups on the basis of anti-diabetic treatment (group 1a treated with metformin and group 1b in anti-diabetic treatment excluding metformin) and compared to control group (group 2, patients without diabetes mellitus). Patients treated with oral medication presented significantly increased [18F]FDG bowel uptake compared to controls (p < 0.001); [18F]FDG bowel uptake was significantly higher in group 1a compared to group 1b (p < 0.01).
The effect of metformin on [18F]FDG bowel uptake must kept in mind in the case of PET/CT scan performed for abdominal evaluation, in particular in the case of suspected peritoneal carcinomatosis, colo-rectal or gynecological neoplasm in which increased [18F]FDG bowel uptake could hide pathological uptake and induce false negative results (Gontier et al. 2008). No guideline consensus is available on the oral anti-diabetic treatment management in the case of abdominal evaluation, but several published studies on the timing of metformin discontinuation are available.
A retrospective study published in 2016 (Lee et al. 2016) aimed to assess the impact of metformin discontinuation up to 72 h in [18F]FDG bowel uptake. Two hundred and forty diabetic patients were divided into four groups on the basis of metformin discontinuation: <24 h (group A), 24–48 h (group B), 48–72 h (group C), and no metformin at all (control group). Compared with the control group, [18F]FDG uptake increased significantly from the ileum to the rectosigmoid colon in group A (p < 0.001), from the transverse to the rectosigmoid colon in group B (p < 0.001) and from the descending colon to the rectosigmoid colon in group C (p < 0.001), highlighting a suboptimal metformin discontinuation <72 h for images interpretation, in particular for the distal colon segments
Conversely, a prospective study published in 2010 (Oh et al. 2010) concluded that discontinuation of metformin for 2 days is feasible to reduce high [18F]FDG bowel uptake. One hundred thirty-eight diabetic patients were divided into two groups: group A treated with metformin and group B in which the regimen did not include metformin and they were compared to patients without diabetes mellitus (control group). Group A was divided into two subgroups on the basis of metformin discontinuation (group A1 continued metformin; group A2 stopped metformin treatment 2 days before PET/CT scan). Ten diabetic patients underwent two consecutive PET/CT scans before and after the discontinuation of metformin. Group A1 compared to group A2 and group B presented a significantly higher (p < 0.001) [18F]FDG bowel uptake. In 10 patients who underwent serial PET/CT scans, [18F]FDG bowel uptake decreased by 64% and hidden colorectal malignancies were revealed in two patients after the discontinuation of the drug.
Despite no consensus being available on the timing metformin discontinuation, in the case of [18F]FDG PET/CT scan for abdominal malignancies, a careful evaluation of patient’s drug treatment must be conducted, to ensure the optimal patient preparation in order to avoid false negative result preventing the rise of BGL.
In the case of treatment with insulin, European guidelines (Boellaard et al. 2014) suggest different options for scheduling [18F]FDG PET/CT scan on the basis of treatment protocol. Commonly, insulin-dependent patients can be scheduled for PET/CT scan in late morning or midday, [18F]FDG should be injected no sooner than 4 h or 6 h after subcutaneous injection of rapid-acting and short-acting insulin respectively after breakfast in the early morning and subsequent fasting. For patients treated with intermediate-acting and/or long-acting insulin, [18F]FDG injection is not recommended on the same day of insulin administration and PET/CT scan should be scheduled in the early morning after insulin injection the evening before and after night fasting. Particular attention needs long-acting insulin management that could interfere with FDG uptake; thus, intermediate-acting replacement is mostly recommended. In the case of continuous insulin infusion, patients should be scheduled in the early morning and the insulin pump stopped for at least 4 h prior [18F]FDG injection.
The basis of this careful attention on insulin administration is the insulin’s affinity for GLUT-4, expressed in the skeletal and cardiac muscle, and in brown and yellow adipose cells (Rea and James 1997): insulin causes the shift of GLUT-4 from intracellular location to the plasma membrane (Huang and Czech 2007; Bryant et al. 2002), promoting both normal glucose and [18F]FDG intracellular uptake and resulting in altered radiotracer biodistribution and suboptimal image quality (Martin and Saleem 2014; Surasi et al. 2014). The aforementioned study by Büsing et al. (2013) also found out that diabetic and insulin treated patients compared to nondiabetics and noninsulin patients presented significant lower mean cerebral SUVmax (p < 0.001) and higher mean muscular SUVmax (p < 0.001).
An increase up to 50% was also observed in average fat tissue SUVmax and myocardial uptake in diabetic patients and insulin patients, respectively.
Despite this known effect of both endogenous and exogenous insulin on [18F]FDG biodistribution, several studies investigated the impact on image quality of insulin administration before [18F]FDG injection to correct hyperglycemia (Fig. 47.6).
In 2006 Turcotte et al. (2006) assessed the impact of intravenous insulin 60 min before [18F]FDG injection on muscular, liver, or lung [18F]FDG uptake. They compared 53 diabetic patients with BGL >7.0 mmol/l vs 53 euglycemic nondiabetic patients and found no significant difference for the SUV calculated on the lung, liver, heart, and skeletal muscles.
A study published in 2009 (Roy et al. 2009) aimed to assess the clinical impact of intravenous administration of short-acting insulin in 63 patients with glycemia greater than 10 mmol/L according to standardized protocol: 2 units for glycemia of 10.0–12.0 mmol/L, 3 units for glycemia of 12.1–14.0 mmol/L, and 4–6 units for glycemia of 14.1 mmol/L and above to reach a glycemia lower than 10.0 mmol/L. In the case of glycemia above 10.0 mmol/L after 30 min, a second insulin dose was given. [18F]FDG was administered at least 60 min after the last insulin administration. After PET/CT scan patients were divided in two groups on the basis of [18F]FDG visual distribution: group A with adequate biodistribution (normal biodistribution, mild muscular uptake or muscular uptake involving more than one muscle group) and group B with altered biodistribution (diffuse muscular uptake of moderate intensity or diffuse, intense muscular uptake resulting in a nondiagnostic examination). [18F]FDG distribution was also semi-quantitatively assessed by SUV mean of liver, gluteal muscles, and myocardium. Group A compared to group B presented a significantly longer delay between insulin and [18F]FDG injections (p < 0.01), higher glycemia reduction after insulin injection (p < 0.01), higher hepatic mean SUV (p < 0.01) and lower gluteal muscular mean SUV (p < 0.01). This study concluded that an interval of at least 90 min between insulin and [18F]FDG administration should be considered and that hepatic and muscular SUVs could be useful tools to define adequate biodistribution of [18F]FDG.
In 2013 Caobelli et al. (2013) evaluated the usefulness and impact on muscular [18F]FDG uptake of a protocol of intravenous insulin administration before [18F]FDG PET/CT scan in 130 diabetic patients. In 20 patients with BGL >180 mg/dl intravenous insulin was administered 30 min before 18F-FDG injection (group 1); in ten patients with BGL >160 mg/dl and <200 mg/dl, no insulin was injected (group 2); 100 euglycemic patients were used as control group. Biodistribution was adequate in group 2, control group, and in 95% of patients in group 1. No significant differences in gluteal muscle SUVmax were found between groups (p = 0.20) and no false negative result was recorded at 6-month follow-up evaluation. In 2013 Song et al. (2013) assessed the impact of intravenous ultra-short insulin administration 60 min before [18F]FDG injection in 105 diabetic patients: 52 patients with BGL >190 mg/dl received 3–5 IU of insulin were compared to the remaining 53 with BGL <190 mg/dl who did not receive insulin and no significant differences in image quality (p = 0.47), hepatic SUVmean (p = 0.13), gluteal muscle, and brain uptake (p = 0.71 and p = 0.16, respectively) were found.
A study conducted by Garcia et al. in 2014 concluded that the quality of [18F]FDG PET/CT scan is not affected by subcutaneous administration of rapid-acting insulin if radiotracer is injected at least 4 h later. They enrolled 120 patients divided in 4 groups on the basis of insulin administration and delay to [18F]FDG injection: 30 diabetic patients with BGL<160 mg/dl without further insulin administration (group 1), 30 diabetic patients with BGL ranging from 168 to 260 mg/dl in which subcutaneous rapid-acting insulin was administered and [18F]FDG injection after a delay of 30–115 min with BGL below 160 mg/dl (group 2), 30 diabetic patients with BGL ranging from 192 to 324 mg/dl in which 18F-FDG was injected 4 h after subcutaneous rapid-acting insulin (group 3), and 30 nondiabetic patients with normal BGL (72–104 mg/dl) (control group). For each patient SUVmax of rectus femoris muscle was calculated: in group 2 SUVmax deviated without relation between BGL and [18F]FDG muscle uptake and the quality of PET-CT scan was suboptimal in 60% of patients in group 2, in 13% of patients in group 1, while it was optimal in all patients of group 3.
Despite several studies investigating protocols of intravenous administration of insulin before [18F]FDG administration in order to reduce BGLs, none has yet been validated. EANM and SNMMI guidelines (Boellaard et al. 2014; Delbeke et al. 2006) recommend, in the case of necessity of insulin to correct hyperglycemia, an appropriate delay between [18F]FDG and insulin administration depending on the type and insulin way of administration. Furthermore EANM guidelines (Boellaard et al. 2014) suggest avoiding insulin administration unless this interval is less than 4 h and preferring rapid-acting insulin subcutaneous injection (effective life 2–4 h), while short-acting, intermediate-acting, or long-acting insulin is not recommended for their longer effective life (3–6 h, 12–18 h and 24 h, respectively).
Conclusions
The management of hyperglycemia in both diabetic and non-diabetic patients represents an issue in PET/CT practice, extensively described by the studies mentioned above. Both hyperglycemia and oral and insulin medications showed advantages and disadvantages on PET/CT scan accuracy: the respect of simple rules guarantees a good quality [18F]FDG PET/CT in staging cancer, and it assumes a pivotal role in patient clinical management.
References
American College of Radiology (2007) ACR–SPR practice guideline for performing FDG-PET/CT in oncology. Available from: http://www.acr.org/;/media/71B746780F934F6D8A1BA5CCA5167EDB.pdf. Published
Barrington SF, Kluge R (2017) FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur J Nucl Med Mol Imaging. 44(Suppl 1):S97–S110
Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W et al (2014) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 42(2):328–354
Brown R, Wahl R (1993) Over expression of glut-1 glucose transporter in human breast cancer: an immunohistochemical study. Cancer. 72:2979–2985
Bryant NJ, Govers R, James DE (2002) Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 3(4):267–277
Büsing KA, Schönberg SO, Brade J, Wasser K (2013) Impact of blood glucose, diabetes, insulin, and obesity on standardized uptake values in tumors and healthy organs on 18F-FDG PET/CT. Nucl Med Biol [Internet]. 40(2):206–213. Available from: https://doi.org/10.1016/j.nucmedbio.2012.10.014
Caobelli F, Pizzocaro C, Paghera B, Guerra UP (2013) Proposal for an optimized protocol for intravenous administration of insulin in diabetic patients undergoing 18F-FDG PET/CT. Nucl Med Commun. 34(3):271–275
Caresia Aroztegui AP, García Vicente AM, Alvarez Ruiz S, Delgado Bolton RC, Orcajo Rincon J, Garcia Garzon JR et al (2017) 18F-FDG PET/CT in breast cancer: evidence-based recommendations in initial staging. Tumor Biol. 39(10):1–23
Carvalho K, Cunha I, Rocha R, Ayala F, Cajaiba M, Begnami M et al (2011) GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clin (Sao Paulo). 66:965–972
Chen Z, Liu M, Li L, Chen L (May 2017) Involvement of the Warburg effect in non-tumor diseases processes. J Cell Physiol. 2017:2839–2849
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E et al (2014) Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: the lugano classification. J Clin Oncol. 32(27):3059–3067
Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA et al (2006) Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med [Internet]. 47(5):885–895. Available from: http://jnm.snmjournals.org/content/47/5/885.short
Di R, Cheng SC, Hudson M, Sakellis C, Van Den Abbeele AD, Kim CK et al (2018) Effects of hyperglycemia on fluorine-18-fluorodeoxyglucose biodistribution in a large oncology clinical practice. Nucl Med Commun 39(5):417–422
Diederichs C, Staib L, Glatting G, Beger H, Reske S (1998) FDG PET: elevated plasma glucose reduces both uptake and detection rate of pancreatic malignancies. J Nucl Med. 39:1030–1033
Eskian M, Alavi A, Khorasanizadeh M, Viglianti BL, Jacobsson H (2019) Effect of blood glucose level on standardized uptake value (SUV) in 18 F- FDG PET-scan: a systematic review and meta-analysis of 20,807 individual SUV measurements. Eur J Nucl Med Mol Imaging. 46:224–237
Gallamini A, Zwarthoed C, Borra A (2014) Positron emission tomography in oncology. Cancers (Basel). 6:1821–1889
Garcia JR, Sanchis A, Juan J, Tomas J, Domenech A, Soler M et al (2014) Influence of subcutaneous administration of rapid-acting insulin in the quality of 18F-FDG PET/CT studies. Nucl Med Commun. 35(5):459–465
Gontier E, Fourme E, Wartski M, Blondet C, Bonardel G, Le Stanc E et al (2008) High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol Imaging. 35(1):95–99
Huang S, Czech MP (2007) The GLUT4 glucose transporter. Cell Metab. 5(4):237–252
Ido T, Wan C, Casella J et al (1978) Labeled 2-deoxy-D-glucose analogs: 18F labeled 2-deoxy-2-fluoro-D-glucose, 2-deoxy-2-fluoro-D-mannose and 14C-2-deoxy-2-fluoro-D-glucose. J Label Compd Radiopharm. 14:175–183
Juweid ME, Cheson BD (2006) Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 354:496–507
Kayano T, Burant C, Fukumoto H, Gould G, Fan Y, Eddy R et al (1990) Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6). J Biol Chem. 265:13276–13282
Larson SM (2006) 18 F-FDG imaging: molecular or functional? J Nucl Med. 47:31N–32N
Lee SH, Jin S, Lee HS, Ryu JS, Lee JJ (2016) Metformin discontinuation less than 72 h is suboptimal for F-18 FDG PET/CT interpretation of the bowel. Ann Nucl Med. 30(9):629–636
Macheda ML, Rogers S, Best JD (2005) Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 202(3):654–662
Madsen PH, Holdgaard PC, Christensen JB, Høilund-Carlsen PF (2016) Clinical utility of F-18 FDG PET-CT in the initial evaluation of lung cancer. Eur J Nucl Med Mol Imaging [Internet]. 43(11):2084–2097. Available from: https://doi.org/10.1007/s00259-016-3407-4
Maher F (1995) Immunolocalization of GLUT1 and GLUT3 glucose transporters inprimary cultured neurons and glia. J Neurosci Res. 42:459–469
Martin J, Saleem N (2014) 18F-FDG PET-CT scanning and diabetic patients: what to do? Nucl Med Commun. 35(12):1197–1203
Massollo M, Marini C, Brignone M, Emionite L, Salani B, Riondato M et al (2013) Metformin temporal and localized effects on gut glucose metabolism assessed using 18F-FDG PET in mice. J Nucl Med [Internet]. 54(2):259–266. Available from: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.112.106666
Moran JK, Lee HB, Blaufox MD (1999) Optimization of urinary FDG excretion during PET imaging, J Nucl Med [Internet]. 40(8):1352–1357. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10450688
Oh JR, Song HC, Chong A, Ha JM, Jeong SY, Min JJ et al (2010) Impact of medication discontinuation on increased intestinal FDG accumulation in diabetic patients treated with metformin. Am J Roentgenol. 195(6):1404–1410
Pauwels E, Sturm E, Bombardieri E, Cleton F, Stokkel M (2000) Positron-emission tomographywith [18F]fluoro- deoxy glucose, part 1. Biochemical uptake mechanism and its implication for clinical studies. J Cancer Res Clin Oncol. 126:549–559
Perng P, Marcus C, Subramaniam RM (2015) 18F-FDG PET/CT and melanoma: staging, immune modulation and mutation-targeted therapy assessment, and prognosis. Am J Roentgenol. 205(2):259–270
Potter M, Newport E, Morten KJ (2016) The Warburg effect: 80 years on. Biochem Soc Trans. 44:1499–1505
Qiao H, Bai J, Chen Y, Tian J (2007) Kidney Modelling for FDG Excretion with PET. Int J Biomed Imaging. 2007:1–4
Randle P, Garland P, Hales C, Newsholme E (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1(7285):785–789
Rea S, James D (1997) Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 46:1667–1677
Rena G, Hardie DG, Pearson ER (2017) The mechanisms of action of metformin. Diabetologia. 60(9):1577–1585
Roy F-N, Beaulieu S, Boucher L, Bourdeau I, Cohade C (2009) Impact of intravenous insulin on 18F-FDG PET in diabetic cancer patients. J Nucl Med [Internet]. 50(2):178–183. Available from: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.108.056283
Shankar L, Hoffman J, Bacharach S et al (2006) Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 47:1059–1066
Song HS, Yoon JK, Lee SJ, Yoon SH, Jo KS, An YS (2013) Ultrashort-acting insulin may improve on18F-FDG PET/CT image quality in patients with uncontrolled diabetic mellitus. Nucl Med Commun. 34(6):527–532
Sprinz C, Zanon M, Altmayer S, Watte G, Irion K, Marchiori E et al (2018) Effects of blood glucose level on 18F fluorodeoxyglucose (18F-FDG) uptake for PET/CT in normal organs: an analysis on 5623 patients. Sci Rep. 8(1):6–11
Surasi DS, Bhambhvani P, Baldwin JA, Almodovar SE, O’Malley JP (2014) 18F-FDG PET and PET/CT patient preparation: a review of the literature. J Nucl Med Technol [Internet]. 42(1):5–13. Available from: http://tech.snmjournals.org/cgi/doi/10.2967/jnmt.113.132621
Szabo Z, Xia J, Mathews W, Brown P (2006) Future direction of renal PET. Semin Nucl Med 36(1):36–50
Theorens B, Sarkar H, Kaback H, Lodish H (1988) Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 55:281–290
Turcotte E, Leblanc M, Carpentier A, Bénard F (2006) Optimization of whole-body positron emission tomography imaging by using delayed 2-deoxy-2-[F-18]fluoro-d-glucose injection following I.V. insulin in diabetic patients. Mol Imaging Biol. 8(6):348–354
Wahl R, Henry C, Ethier S (1992) Serum glucose: effects on tumor and normal tissue accumulation of 2-[F-18]-fluoro-2-deoxy-D-glucose in rodents with mammary carcinoma. Radiology. 183:643–647
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl_1):122S–150S
Warburg OP, Negelein E (1924) Uber den stoffwechsel der carcinomzelle. Biochem Zeitschrift. 152:309–335
Yang J, Wen J, Tian T, Lu Z, Wang Y, Wang Z et al (2017) GLUT-1 overexpression as an unfavorable prognostic biomarker in patients with colorectal cancer. Oncotarget [Internet]. 8(7):11788–11796. Available from: http://www.oncotarget.com/fulltext/14352
Acknowledgements
Reprinted/adapted by permission from “Springer Nature”: Springer Milan, Acta Diabetologica, “Hyperglycemia and 18F-FDG PET/CT, issues and problem solving: a literature review”—Monica Finessi, Gianni Bisi, Désirée Deandreis, DOI 10.1007/s00592-019-01385-8, ISBN 0123456789, ISSN 14325233.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Finessi, M., Liberini, V., Deandreis, D. (2020). Cancer Staging with 18F-FDG PET/CT in Hyperglycemic Patients. In: Faintuch, J., Faintuch, S. (eds) Obesity and Diabetes. Springer, Cham. https://doi.org/10.1007/978-3-030-53370-0_47
Download citation
DOI: https://doi.org/10.1007/978-3-030-53370-0_47
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53369-4
Online ISBN: 978-3-030-53370-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)