Abstract
Adenoid ameloblastoma is a hybrid odontogenic tumour showing histopathological features of both ameloblastoma and adenomatoid odontogenic tumour (AOT), with approximately 40 cases reported in the literature. The aims of the report are to illustrate the diagnostic challenges of adenoid ameloblastoma using three new cases and to analyze evidence in literature to consider adenoid ameloblastoma as a new sub type of ameloblastoma. A literature review was performed with the key words—adenoid ameloblastoma, hybrid/composite odontogenic tumours, hybrid ameloblastoma and adenomatoid odontogenic tumour, ameloblastoma with inductive changes, dentinoid and dentinoma to select the cases compatible with the diagnosis of adenoid ameloblastoma. Out of the 40 cases reported in literature, 31 cases with sufficient information and 3 new cases were analyzed. Out of the 34 adenoid ameloblastomas majority of tumours (76.5%) occurred in adults with age ranging from 25 to 55 years. Slight female predilection with a male:female ratio of 0.9:1 was observed. Approximately, 64.7% occurred in the mandible. Radiologically, 82.4% of adenoid ameloblastomas presented as radiolucent lesions while 47.1% occurred with ill-defined margins and cortical perforation at diagnosis. Histopathologically, 70.8% of tumours presented as plexiform ameloblastomas, while duct like structures/glandular structures were the commonest feature supportive of adenomatoid odontogenic tumour observed in overwhelming majority of 95.9% of adenoid ameloblastomas. 91.6% of tumours showed inductive change in the form of dentinoid. Further, 45.4% of the tumours developed at least one recurrence following surgical excision. The report presents literature review based evidence to show the existence of adenoid ameloblastoma, which is demographically similar to conventional ameloblastoma but with histopathological differences and presenting with higher rate/multiple recurrences, indicating its biological aggressiveness. Thus, we would like to propose the inclusion of adenoid ameloblastoma as a sub type of ameloblastoma in the next revision of the WHO odontogenic tumour classification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Odontogenic tumours are a heterogeneous group of tumours that occur exclusively in the jaw bones and or gingival mucosa. These tumours show wide range of biological behaviour requiring different degrees of surgical treatment from simple excision and enucleation to radical resections [1,2,3,4]. As for any other tumour category, odontogenic tumours are also widely classified using World Health Organization (WHO) classification system [1,2,3,4].
The WHO classification of odontogenic tumours has evolved over the years from the initial classification released in 1971 to subsequent updates released in 1992, 2005 and 2017 [1,2,3,4]. Although, majority of the odontogenic tumours can be diagnosed based on criteria given in WHO classification on head and neck tumours, occasionally diagnostically challenging odontogenic tumours that defy classification based on current knowledge are encountered. Hybrid odontogenic tumours presenting with features of ameloblastomas and adenomatoid odontogenic tumours with dentinoid also known as adenoid ameloblastoma is one such entity [5,6,7,8].
The aim of the report is to present a literature review based introduction of adenoid ameloblastoma, considering three new diagnostically challenging odontogenic tumours to illustrate the features that support the diagnosis of adenoid ameloblastoma. Thus the present report was compiled to show evidence for the existence of adenoid ameloblastoma, with the aim of considering it as a separate clinicopathological entity in the WHO odontogenic tumour classification at the next revision.
Materials and Method
A literature review was performed with the key words—adenoid ameloblastoma, hybrid/composite odontogenic tumours, hybrid ameloblastoma and adenomatoid odontogenic tumour, ameloblastoma with inductive changes, dentinoid and dentinoma to select the cases compatible with the diagnosis of adenoid ameloblastoma. Out of the 19 articles downloaded, 31 cases compatible with the diagnosis of adenoid ameloblastoma, with adequate information was selected [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. Thus the final review contained 31 cases of adenoid ameloblastoma diagnosed from 1994 to 2020 and 3 new cases from the archives of the Department of Oral Pathology, Faculty of Dental Sciences, University of Peradeniya and National Dental Hospital, Sri Lanka.
For the literature review on adenoid ameloblastoma, information on age at diagnosis, gender, site and location of the lesion, clinical, radiological and histopathological features of the lesion, treatment and number of recurrences were extracted from the articles and entered in to excel sheets.
Results
Table 1 shows the clinicopathological features of 34 cases of adenoid ameloblastoma.
Accordingly, adenoid ameloblastoma is a tumor that occurs predominantly (76.5%) in adults aged between 25 and 55 years. A slight female predilection with a male:female ratio of 0.9:1 was observed. Approximately, 64.7% occurred in the mandible, while in both jaws posterior regions were involved in 52.9% of the cases. Radiologically, 82.4% of adenoid ameloblastomas presented as purely radiolucent lesions in spite of containing dentinoid while 47.1% occurred with ill-defined margins and cortical perforation at diagnosis. Histopathologically, 70.8% of tumours had plexiform ameloblastoma as the ameloblastomatous component, while duct like structures/glandular structures were the commonest feature supportive of adenomatoid odontogenic tumour observed in overwhelming majority of 91.9% of adenoid ameloblastomas. 91.5% of tumours showed inductive change in the form of dentinoid. All lesions were surgically excised though both conservative and radical excision had been performed for 29.4 and 23.4% of cases respectively. Further, 45.4% of the tumours showed at least one recurrence. Out of the 10 lesions that recurred three had been radically excised while the rest were conservatively excised.
The clinicopathological features of the three new cases of adenoid ameloblastomas are as follows;
Case 1
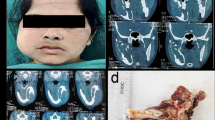
A 38 year old female presented with a radiolucent swelling in the mandible extending from 2nd premolar to 1st-molar region in 2013. The primary lesion was conservatively excised and the histopathology revealed a tumour composed of odontogenic epithelium arranged in to interconnected strands (Fig. 1a). Two atypical features identified in the tumour were (1) focal aggregations of dentinoid (Fig. 1b), (2) hypercellularity with cells exhibiting hyperchromatic nuclei, minimal cytoplasms and occasional mitoses. The lesion was diagnosed as an atypical plexiform ameloblastoma with dentinoid. Four years after the occurrence of the initial lesion the patient presented with a recurrent lesion which was radiologically identified as two separate multilocular radiolucencies (Fig. 2). Histopathologically, the anterior lesion exhibited a cystic lesion extending in to soft tissue. The cyst was lined by odontogenic epithelium, with an ameloblast like cell layer situated towards the capsule and hyper cellular epithelial whorls and stellate reticulum like cells elsewhere in the epithelial lining (Fig. 3a). A few duct like structures were also evident. The posterior lesion exhibited multiple foci showing hypercellularity (Fig. 3b) with 3 mitotic figures per 10HPF with dentinoid. Thus, the lesion was diagnosed as an adenoid ameloblastoma with dentinoid. Subsequently, lesion was radically treated with a mandibulectomy. Histopathology of the excision revealed similar features to the incision confirming the diagnosis of adenoid ameloblastoma.
Case 2
A 40 year old female presented with a recurrent swelling in the posterior mandible of 6 × 4 cm in size. Histopathologically 1st to 3rd recurrences showed unencapsulated odontogenic tumours composed of small epithelioid cells with hyperchromatic nuclei and minimal cytoplasms diffusely infiltrating in to bone (Fig. 4a). The epithelial component showed rosettes, epithelial whorls and small duct like structures (Fig. 4b). A few areas of the tumour showed vague ameloblast like tall columnar cells with reverse polarity nuclei (Fig. 4c). Approximately, 2 mitotic figures per 10HPF were observed. Dentinoid and woven bone formation was also observed. Some areas of the biopsy showed areas reminiscent of Homer-Wright rosettes (Fig. 4d). Differential diagnoses considered were atypical AOT, odontogenic carcinoma with dentinoid and Ewing’s sarcoma/primitive neuroendocrine tumour (ES/PNET). Immunohistochemical investigations with CD99, NSE and cytokeratin (MNF116) were performed and ES/PNET was excluded as tumour cells only showed positivity with cytokeratin while other markers were negative. Finally the lesion was diagnosed as a recurrent adenoid ameloblastoma even with the absence of a prominent ameloblastomatous component as the tumour did not show overt malignant features supportive of odontogenic carcinoma with dentinoid.
a A tumours composed of small epithelioid cells with hyperchromatic nuclei and minimal cytoplasms diffusely infiltrating in to bone. H&E × 4. b A photomicrograph showing epithelial whorls. H&E × 40. c Ameloblast like tall columnar cells with reverse polarity nuclei present throughout the tumour. H&E × 40. d A section showing areas reminiscent of Homer-Wright rosettes. H&E × 10
Case 3
A 42 year old female presented with a gradually enlarging left maxillary swelling of 6 month duration. Occipito-mental view showed radio-opacity in the left side maxillary antrum with evidence of bone erosion. Histological analysis of excisional biopsy revealed a cystic odontogenic tumour with peripheral fibrous wall and a thin rim of reactive bone in most areas. Epithelial lining showed basally located layer of ameloblast like cells with reversed nuclear polarity (Fig. 5a, b). Solid areas of the tumour comprised of clear cells, stellate reticulum like cells and cuboidal cells, some of which were arranged into ductal structures. A few foci showed whorling of cells reminiscent of adenomatoid odontogenic tumour (Fig. 5c). Scattered eosinophilic secretory material most likely to be dentinoid was present in relation to tumour cells. Focal aggregates of ameloblastomatous islands were present in the cyst wall. The lesion was conservatively excised with no evidence of recurrence for the 6 month follow up period.
Discussion
Irrespective of the tumour, the correct diagnosis is extremely important for the management of the patient. However, occasionally diagnostically challenging lesions are encountered. Thus the present report based on three diagnostically demanding odontogenic tumours suggestive of the diagnosis of adenoid ameloblastoma is presented to highlight the deficiencies that exist in the WHO odontogenic tumour classification [4] with some suggestions for its modification.
The first case was initially reported as an atypical plexiform ameloblastoma with dentinoid. Dentinoid is defined as generally non-mineralized substance which resembles dentin, but which neither contains tubules nor fulfils the criteria for atubular dentin, and which is located in a close anatomical relationship to odontogenic epithelium [9]. Literature review revealed a similar case reported by Matsumoto et al. [5]. However, they also considered the lesion as an adenoid ameloblastoma [5]. By definition, an adenoid ameloblastoma is an odontogenic tumour showing histopathological features of both ameloblastoma and AOT along with hard tissue formation [6,7,8]. Initially it was not possible to diagnose our case 1 as an adenoid ameloblastoma with dentinoid since the primary tumour described did not show features of AOT, such as duct like structures or glandular differentiation. However, the anterior lesion that recurred showed features supportive of AOT, hence the diagnosis of adenoid ameloblastoma was given for the recurrent lesion. To the best of our knowledge, to date malignant transformation has not been reported in adenoid ameloblastoma. However, at the time of recurrence, case-1 of the present report showed atypia and hypercellularity which resulted in a diagnostic dilemma of whether to diagnose it an adenoid ameloblastoma or an odontogenic carcinoma with dentinoid. Though initially ameloblastic carcinoma, was considered as a differential diagnosis, it was excluded due to the presence of dentinoid. Although, odontogenic carcinoma with dentinoid is not a diagnosis that has been included in the WHO classification [4], it was the main lesion that was considered in the differential diagnosis due to the presence of neoplastic epithelial component and dentinoid in the recurrent lesion, which was also supportive of the lesion described by Mosqueda-Taylor et al. [9]. However, as the present lesion did not contain a prominent clear cell component or a basaloid cell component as per Table 2 [9] which excluded odontogenic sarcoma. In addition due to the fact that WHO classification [4] indicates that increased mitosis and hypercellularity per se are not indicative of malignancy, final diagnosis achieved for the lesion was adenoid ameloblastoma.
Table 1 shows a literature review based summary of clinico-pathological features of adenoid ameloblastoma [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. Accordingly, age at presentation, gender and site distribution of the adenoid ameloblastoma is more compatible with conventional ameloblastoma than AOT [4, 24]. In contrast to conventional ameloblastoma which commonly shows follicular differentiation [24], adenoid ameloblastoma predominantly presents with plexiform growth pattern (Table 1). Radiologically, adenoid ameloblastoma may show mixed radio-dense lesions. Furthermore, adenoid ameloblastoma seem to be more aggressive in behavior due to presence of recurrences in a high number of cases (Table 1) compared to conventional ameloblastoma [22]. Thus, the problem of whether to classify adenoid ameloblastoma as a sub type of conventional ameloblastoma or as a separate entity arises. Considering the number of cases reported in the literature, there is sufficient evidence to classify adenoid ameloblastoma as a sub type of ameloblastoma. Further if the presence of dentinoid preclude it being considered as a variant of ameloblastoma, the example of AOT which may also contain dentine can be used to justify the present argument.
Typical AOTs are benign odontogenic tumours that behave almost like hamartomas [4]. These tumours occur predominantly in females in the second/third decade of life, in the anterior maxilla while histopathologically majority are encapsulated and recurrences are extremely uncommon [4]. With reference to case-2, it was diagnosed as adenoid ameloblastoma because majority of the clinico-pathological features were unusual for typical AOTs. Namely, the patient was in the fourth decade of life when the initial lesion occurred in an unusual site for AOT which was the posterior mandible. Further, the tumour was unencapsulated and diffusely infiltrated in to surrounding bone while three recurrences occurred within a period of 6 years. Although the lesion exhibited ameloblast like cells in a few foci, typical growth patterns of conventional ameloblastoma (including the plexiform type) was not observed. Literature reports, approximately eleven cases of aggressive AOTs which are histopathologically identical to non aggressive AOT but presenting with clinically aggressive features such as rapid enlargement [25] (Table 2). Although, majority (8 cases) of the aggressive AOTs were also observed in the mandible [25], our case was not diagnosed as such because histopathologically it exhibited some unusual features not identified in typical AOT. Another diagnosis that was considered was AOT with inductive change, which had been considered as a new entity and discussed as an aggressive tumour compared to conventional AOT, during the 2017 revision of the WHO classification. However, it was excluded as AOT with inductive change was not described in the new edition [4]. In addition, although, an extensive literature review failed to reveal malignant AOTs, it is worthwhile to consider malignant transformation for the present tumour, because of the infiltrative nature, mitotic activity, hypercellularity and multiple recurrences. Although, individually none of these features are truly supportive of the diagnosis of malignancy collectively they flash a red light as typical AOT would not exhibit these features. However, finally the tumour was diagnosed as adenoid ameloblastoma and not as a malignant AOT or odontogenic sarcoma as individual features supportive of malignancy was not evident in the tumour.
From the literature review and case reports main diagnostic criteria that need to be fulfilled to diagnose adenoid ameloblastoma include, presence of
-
1.
any histopathological subtype of ameloblastoma; or a tumour showing ameloblast like cells and or stellate reticulum like cells, without the characteristic architectural arrangement of ameloblastoma;
-
2.
adenomatoid odontogenic tumour showing at least one or more features such as duct like structures, glandular differentiation or epithelial whorls with local invasion;
-
3.
dentinoid in a mature fibrous stroma.
It is not always essential to identify all architectural characteristics supportive of ameloblablastoma or AOT as such a finding would result in a diagnosis of hybrid tumour or a collision tumour. In addition, in the case of AOT, the most important finding is the local invasion that is not seen in conventional AOTs.
It would have been ideal if molecular characterization of adenoid ameloblastoma was carried out to find whether there is a resemblance with ameloblastoma by showing BRAF mutation [26] or a resemblance with AOT by showing KRAS mutation [27].
However, it was not possible due to lack of resources and therefore, the authors suggest that further molecular biological studies should be undertaken to identify the genetics of adenoid ameloblastoma. Further discussion may be required to confirm the diagnosis of adenoid ameloblastoma in the following scenarios
-
1.
Tumours that show architectural characteristics of ameloblastoma or adenomatoid odontogenic tumour with dentinoid;
-
2.
Adenomatoid odontogenic tumour without local invasion.
In conclusion the present report highlights the existence of adenoid ameloblastoma and discusses the difficulties encountered during its diagnosis and when excluding malignant counterparts of odontogenic tumours.
Data Availability
Patient information and microscopic slides are available in respective author’s institution archives.
References
Pindborg JJ, Kramer IRH, Torloni H. Histological typing of odontogenic tumours: jaw cysts and allied lesions. 1st ed. Geneva: WHO; 1971.
Kramer IRH, Pindborg JJ, Shear M. Histological typing of odontogenic tumours. In: WHO international histological classification of tumours, 2nd edn. London: Springer; 1992.
Philipsen HP, Nikai H. Odontogenic tumours: adenomatoid odontogenic tumour. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours, pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. p. 304–5.
El-Naggar, Chan JKC, Grandis JR, Takata T, Slootweg P, editors. Chapter 8: odontogenic and maxillofacial bone tumours. In: WHO classification of head and neck tumours, 4th edn. Lyon: IARC; 2017. p. 205–60.
Matsumoto Y, Mizou K, Seto K. Atypical plexiform ameloblastoma with dentinoid: adenoid ameloblastoma with dentinoid. J Oral Pathol Med. 2001;30:251–4.
Ide F, Mishima K, Saito I, Kusama K. Diagnostically challenging epithelial odontogenic tumors: a selective review of 7 jawbone lesions. Head Neck Pathol. 2009;3:18–26.
Evans BL, Carr RF, Phillipe LJ. Adenoid ameloblastoma with dentinoid: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:583–8.
Raj HK, Pai SM, Dayakar A, Supriya H. Adenoid ameloblastoma with dentinoid: a rare hybrid variant. J Oral Maxillofac Pathol. 2017. https://doi.org/10.4103/jomfp.JOMFP_53_15.
Mosqueda-Taylor A, Neville BW, Tatemoto Y, Ogawa I, Takata T. Odontogenic carcinoma with dentinoid: a new odontogenic carcinoma. Head Neck Pathol. 2014;8(4):421–31.
Salehinejad J, Gholami M, Eshghpour M, Mehri T. An infrequent histopathological subtype of ameloblastoma: adenoid granular cell ameloblastoma with dentinoid. Dent Res J (Isfahan). 2016. https://doi.org/10.4103/1735-3327.187878.
Loyola AM, Cardoso SV, de Faria PR, Servato JP, Eisenberg AL, Dias FL, Thavaraj S, Gomes CC, Gomez RS. Adenoid ameloblastoma: clinicopathologic description of five cases and systematic review of the current knowledge. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2015;120:368–77.
Saxena K, Jose M, Chatra LK, Sequiera J. Adenoid ameloblastoma with dentinoid. J Oral Maxillofac Pathol. 2012;16:272–6. https://doi.org/10.4103/0973-029X.99088.
Ghasemi-Moridani S, Yazdi I. Adenoid ameloblastoma with dentinoid: a case report. Arch Iran Med. 2008;11:110–2.
Slabbert H, Altini M, Crooks J, Uys P. Ameloblastoma with dentinoid induction: dentinoameloblastoma. Oral Pathol Med. 1992;21:46–8.
Orlowski WA, Doyle JL, Salb R. Unique odontogenic tumour with dentinogenesis and unicystic plexiform ameloblastoma. Oral Surg Oral Med Oral Pathol. 1991;72:91–4.
Sonone A, Hande A, Chaudhary M, Bonde R, Sheorain A, Agni N. Adenoid ameloblastoma with dentinoid and ghost cells: a composite odontogenic tumour: a rare case report and review of the literature. Oral Surg. 2011;4:77–81. https://doi.org/10.1111/j.1752-248X.2010.01109.x.
Allen CM, Neville BW, Hammond HL. Adenomatoid dentinoma: report of four cases of an unusual odontogenic tumour. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:313–7.
Takeda Y. So called immature dentinoma: a case presentation and histopathological comparison with ameloblastic fibrodentinoma. J Oral Pathol Med. 1994;22:92–6.
Tajima Y, Sakamoto E, Yamamoto Y. J Odontogenic cyst giving rise to an adenomatoid odontogenic tumour: report of a case with peculiar features. Oral Maxillofac Surg. 1992;50:190–3.
Kumar P, Jayam C, Patil S, Zingade J. Mixed odontogenic tumour with dentinoid and ghost cells. BMJ Case Rep. 2015. https://doi.org/10.1136/bcr-2015-211867.
Khan N, Bavle RM, Paremala K, Makarla S, Venugopal R. Induction with various histological patterns in adenomatoid odontogenic tumour. J Clin Diagn Res. 2017;11(7):ZJ01–2. https://doi.org/10.7860/JCDR/2017/28803.10235 (Epub 1 July 2017).
de Arruda JAA, Noronha MS, Aberu LG, de Lacerda JCT, Silva TA, Mesquita RA. Adenoid ameloblastoma in the posterior maxilla: a case report and review of literature. Oral Maxillofac Surg. 2020. https://doi.org/10.1007/s10006-020-00830-1.
Adorno-Farias D, Muniz VRVM, Soares AP, Cury PR, Rabelo RG, Fernandez-Ramires R, de Azevedo RA, Santos JN. Ameloblastoma with adenoid features: a series of 8 cases. Acta Histochem. 2018;120:468–76.
Gunawardana KSDN, Jayasooriya PR, Rambukewella IK, Tilakaratne WM. A clinico-pathological comparison between maxillary and mandibular ameloblastomas. J Oral Pathol Med. 2010;39(236):41.
Shaikh S, Bansal S, Desai RS, Ahmad I. Aggressive adenomatoid odontogenic tumor of the mandible: a rare case report and review of the literature. J Oral Maxillofac Pathol. 2018;22(Suppl 1):S11–5. https://doi.org/10.4103/jomfp.JOMFP_69_15.
Seki-Soda M, Sano T, Ito K, Yokoo S, Oyama T. An immunohistochemical and genetic study of BRAF(V600E) mutation in Japanese patients with ameloblastoma. Pathol Int. 2020;70(4):224–30. https://doi.org/10.1111/pin.12899 (Epub 13 Jan 2020).
Coura BP, Bernardes VF, de Sousa SF, França JA, Pereira NB, Pontes HAR, Batista AC, da Cruz Perez DE, Albuquerque Junior RLC, de Souza LB, Martins MD, Diniz MG, Gomez RS, Gomes CC. KRAS mutations drive adenomatoid odontogenic tumor and are independent of clinicopathological features. Mod Pathol. 2019;32(6):799–806. https://doi.org/10.1038/s41379-018-0194-4 (Epub 14 Jan 2019).
Funding
No funding obtained.
Author information
Authors and Affiliations
Contributions
PRJ: Concept, Oral Pathologist reporting on case 1, manuscript preparation. WAMULA: Literature review, compiling data for Table 1. PL: Oral Pathologist reporting on case 3, critical review of the manuscript. NU: Literature review, compiling results. WMT: Oral Pathologist reporting on case 2, critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest to disclose.
Ethical Approval
At the time of taking biopsies, patients have given consent to use their information for research purposes following de-identification.
Informed Consent
Patients have given consent to publish de-identified information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jayasooriya, P.R., Abeyasinghe, W.A.M.U.L., Liyanage, R.L.P.R. et al. Diagnostic Enigma of Adenoid Ameloblastoma: Literature Review Based Evidence to Consider It as a New Sub Type of Ameloblastoma. Head and Neck Pathol 16, 344–352 (2022). https://doi.org/10.1007/s12105-021-01358-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-021-01358-w