Abstract
Introduction: Odontogenic tumors encompass a heterogeneous group of lesions that range from hamartomatous lesions to malignancy. Considerable variation in histologic presentation can mislead their accurate diagnosis and categorization. Ameloblastoma is generally well understood and is easy to diagnose but there has been a constant change in the classification systems ever since Broca classified odontogenic tumors in the year 1867. Over the years, it has been modified by the World Health Organization with many additions and omissions. This dynamic change is based on the result and conclusions of molecular and genetic studies with the last modification in 2017. Case Report: We present two cases of females aged 32 and 60 years who reported with facial swellings, revealed the presence of distinct histopathological findings and were diagnosed as ameloblastoma with dentinoid or adenoid ameloblastoma. Literature search revealed dearth of distinct forms of ameloblastoma that show the formation of duct like structures and dentinoid. Conclusion: It is interesting to highlight such cases as the biological behavior is still unexplored due to paucity of relevant studies and follow up of patients. Understanding the pathogenesis and the histopathological characteristics of the newer entities will enable the prompt diagnosis, treatment plan and expanding the spectrum of the lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ameloblastoma is a benign but locally invasive tumor of odontogenic epithelial origin which is devoid of hard tissue formation [1]. Ameloblastoma rarely cause diagnostic difficulties due to classic histopathological presentation [2]. However, variation in conventional histologic presentations is not unusual [3]. Brannon (1994) defined Adenoid ameloblastoma (AA) as a variant of ameloblastoma whose histopathological features include a follicular or plexiform ameloblastoma with tubular structures [2]. These tubular structures resemble those seen in Adenomatoid odontogenic tumor (AOT); thus, the name adenoid ameloblastoma. Evans et al. in 2004 first introduced the term “adenoid ameloblastoma with dentinoid” as a neoplasm with histopathological features similar to ameloblastoma and AOT along with hard tissue formation [4]. AA is a rare histological variant in the unusual presentation category which can pose problem in diagnosis due to the presence of cribriform architecture, adenoid structures and varying degrees of dentinoid. Adenoid structures in odontogenic tumors like AOT and calcifying epithelial odontogenic tumor is commonly seen, however glandular or ductal structures are unusual in ameloblastoma. Jayasooriya PR et al. in their recent paper stated that any tumor which is a variant of ameloblastoma, has AOT-like areas with presence of dentinoid could be diagnosed as AA [5]. The present cases depicted all the features listed by various authors for AA. We report two cases with varied histopathological presentation that revealed existence of follicular/plexiform ameloblastoma with adenoid and dentinoid changes. Through this paper we intend to highlight two institutional cases of AA. The review of previous cases diagnosed as AA have also been tabulated (Table 1).
Case Presentation
Case 1
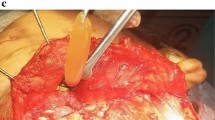
A 32 years old female reported to our institution with complaint of pain and swelling on right side face from past 8 months which was increasing in size. No history of trauma was elicited. The patient underwent biopsy in 2015 although the report was inconclusive. Swelling resolved and spontaneously re-occurred on the same site. Extraoral examination revealed bony hard, non-tender 5 × 5 cm swelling on right side which extended from infraorbital region to lower border of mandible (Fig. 1a). Intraoral examination revealed buccal and lingual cortical expansion which extended from 43 to 45 region. Radiographic examination revealed an expansile lesion with cortical perforation in the buccal cortex and condylar neck region (Fig. 1b-c). The incisional biopsy was reported as acanthomatous ameloblastoma due to areas of squamous metaplasia in the tumor islands. The stroma also showed the presence of numerous hemorrhagic areas. Based on this, hemi mandibulectomy with reconstruction was performed with the goal to eradicate pathology, prevention of recurrence and to restore form, function and esthetics of the patient (Fig. 1d). The excised tissue specimen revealed numerous cystic structures lined by odontogenic epithelium with peripheral ameloblast like and central stellate reticulum like cells (Fig. 2a-b). Odontogenic epithelium proliferated in the form of interconnecting strands with adjacent areas of abundant homogenous eosinophilic dentinoid like material deposition that stained positive (pink) for van gieson (Fig. 2c-d). Adjoining multiple ductal structures lined by cuboidal to low columnar cells were also evident. Intervening stroma showed moderate inflammation, extensive hemorrhagic areas and multiple small and large vascular channels engorged with red blood cells. Based on clinical, radiological and histopathological examination final diagnosis of follicular ameloblastoma with dentinoid/AA was made. This intriguing histopathological diagnosis led us to retrospect another case which had revealed similar findings.
A collage of Case 1 with (a) Extraoral photograph revealing bony hard, non-tender swelling on right side of face, (b) Computed Tomography which shows an expansile lesion, (c) Radiographic examination (CT) reveals cortical perforation in the buccal cortex and condylar neck region, (d) Resected gross specimen after hemi mandibulectomy
A collage of the histopathologic findings of Case 1 with (a) Photomicrograph reveals numerous cystic structures lined by odontogenic epithelium with tall columnar peripheral palisading of ameloblast like cells [4x/H&E], (b) shows centrally placed network like arrangement recapitulating stellate reticulum formation[10x/H&E], (c&d) Photomicrographs of tissue sections showing odontogenic epithelium proliferating in the form of interconnecting strands with adjacent areas showing deposition of abundant homogenous eosinophilic dentinoid like material stained amorphous pink. [10x/Van Gieson]
Case 2
A 60-year-old female had visited the college with complaint of swelling in the lower jaw for 5 years with no history of trauma (Fig. 3a). The swelling gradually increased to present size with referred pain to ear and head. Intraoral examination revealed solitary, firm, fluctuant and nontender swelling of size 14 × 12 cm that extended from right to left angle region obliterating the buccal vestibule (Fig. 3b). Radiographic findings revealed a well-defined multilocular radiolucency with radiopacity causing expansion and thinning of cortex. The biopsy tissue upon sectioning showed intraosseous odontogenic neoplasm with tumor cells proliferating as interconnected plexus. Peripheral cells were cuboidal to low columnar with central stellate reticulum like areas with perivascular hyalinization (Fig. 4a). Formation of dentinoid like material was also evident which stained positive for van gieson (Fig. 4b). Stroma showed rich vascularity with areas of cystic degeneration (Fig. 4c). Significant sheet like proliferation of epithelial cells was noted along with glandular and adenoid like areas (Fig. 4d). Thus, a diagnosis of plexiform ameloblastoma with dentinoid changes/ AA was recapitulated. In consideration of both cases, it is suggested that AA could essentially result from any variant of ameloblastoma whether follicular or plexiform due to inductive changes and dentinoid formation.
A collage of the histopathologic findings of Case 2 with (a) Photomicrograph revealing interconnected strands and cords of odontogenic epithelium with tall columnar peripheral palisading ameloblast like cells diagnosed as plexiform ameloblastoma [10x/H&E], (b) Photomicrograph showing deposition of abundant homogenous eosinophilic dentinoid like material stained amorphous pink. [10x/Van Gieson], (c) Photomicrograph depicting extensive decongested vessels [10x/H&E], (d) Photomicrograph showing proliferation of spindle shaped cells forming rosette like appearance[40x/H&E]
Discussion
Ameloblastoma generally do not show any evidence of induction; however, rare cases associated with hard tissue formation have been reported [6]. Dentinoid is defined as non-mineralized substance that resembles dentin, but which neither contains tubules nor fulfils the criteria for atubular dentin. It is located in a close anatomical relationship to odontogenic epithelium [7]. It is also hypothesized that the excessive stimulation of angiogenesis during tumor development may also lead to an inductive process that might aid in dentinoid formation in the adjacent connective tissue [8]. At times, AOT-like areas predominate, which may overshadow the ameloblastomatous areas leading to benign diagnosis and conservative treatment, which will ultimately result in recurrence [3]. AA is considered as a neoplasm with potential for extension and recurrence. Thus, decision of proper patient care is determined by accurate diagnosis of the tumor. In consideration with the previous reports, the histologic findings of the present cases resembled strongly those reported by Orlowski et al., Matsumoto et al., and Evans et al. so new term “adenoid ameloblastoma with dentinoid” was coined. It is also intriguing to note that the reported cases of longstanding or recurrent ameloblastoma never exhibited the formation of dentinoid helping us to conclude that ameloblastoma with dentinoid formation is not a secondary phenomenon in a pre-existing conventional ameloblastoma rather it arises de novo [8]. AA is demographically similar to conventional ameloblastoma but with histopathological differences and a higher rate of recurrence. AOT shows mutation in KRAS p.G12V and p.G12R gene whereas ameloblastoma shows BRAF p V600 gene mutation which makes them genetically distinct. In a recent study, nine AA cases were screened by TaqMan allele-specific qPCR to assess BRAF p.V600E, ameloblastoma signature mutation, and KRAS p.G12V and p.G12R, AOT signature mutations. Neither BRAF nor KRAS mutations were identified in any of the case. The molecular outcome supported AA as an entity distinct from AOT and ameloblastoma [9]. Further both our cases revealed rich vascularity with endothelial lined blood vessels and areas of cystic degeneration resembling hemangiomatous ameloblastoma. Possible explanation for vascularity is similar to Lucas RB et al. findings which stated that vascular component is purely a secondary change and opined that cystic degeneration occurs in the stroma of plexiform ameloblastoma. During the cystic formation, some blood vessels persist and dilate instead of diminishing resulting in vascular component that can present as diagnostic dilemma [8]. Thus, we aimed to highlight these two cases of a newly recognized entity separate from the ameloblastoma group of tumors, defined as an epithelial neoplasm characterized by cribriform architecture and duct-like structures, with dentinoid presently diagnosed as AA [10].
Conclusion
The recently updated 5th edition WHO odontogenic tumor classification (2022) has also included AA as a separate entity in the benign tumor category. Our cases will add on to the already published cases depicting it as unique standalone tumor due to its rarity, unusual behavior and prognosis. Thus, it is pertinent to diagnose it accurately and further studies incorporating genetic analysis should be done to shed light on its biological aggressiveness.
Data Availability (data transparency)
Data Available.
Code Availability (software application or custom code)
NA.
References
Kumar K, Shetty DC, Wadhwan V, Dhanapal R, Singh HP (2013 Jan) Dentinoameloblastoma with ghost cells: a rare case report with emphasis on its biological behavior. Dent Res J (Isfahan) 10(1):103–107. https://doi.org/10.4103/1735-3327.111809PMID: 23878572; PMCID: PMC3714810
Saxena K, Jose M, Chatra LK, Sequiera J (2012 May) Adenoid ameloblastoma with dentinoid. J Oral Maxillofac Pathol 16(2):272–276. https://doi.org/10.4103/0973-029X.99088PMID: 22923903; PMCID: PMC3424947
Adorno-Farias D, Muniz VRVM, Soares AP, Cury PR, Rabelo RG, Fernández-Ramires R, de Azevedo RA, Dos Santos JN (2018 Jul) Ameloblastoma with adenoid features: a series of eight cases. Acta Histochem 120(5):468–476. https://doi.org/10.1016/j.acthis.2018.05.006Epub 2018 May 21. PMID: 29799420
Evans BL, Carr RF, Phillipe LJ (2004) Nov;98(5):583-8 Adenoid ameloblastoma with dentinoid: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. doi: https://doi.org/10.1016/j.tripleo.2004.02.077. PMID: 15529131
Jayasooriya PR, Abeyasinghe WAMUL, Liyanage RLPR, Uthpali GN, Tilakaratne WM (2022 Jun) Diagnostic Enigma of Adenoid Ameloblastoma: Literature Review based evidence to consider it as a new sub type of Ameloblastoma. Head Neck Pathol 16(2):344–352. https://doi.org/10.1007/s12105-021-01358-wEpub 2021 Jul 19. PMID: 34282559; PMCID: PMC9187834
Rai HK, Pai SM, Dayakar A, Supriya H (2017 May-Aug) Adenoid ameloblastoma with dentinoid: a rare hybrid variant. J Oral Maxillofac Pathol 21(2):319. https://doi.org/10.4103/jomfp.JOMFP_53_15PMID: 28932051; PMCID: PMC5596692
Slabbert H, Altini M, Crooks J, Uys P (1992) Ameloblastoma with dentinoid induction: Dentinoameloblastoma. J Oral Pathol Med 21:46–48 [PubMed] [Google Scholar]
Pandiar D, Ramani P, Shameena PM, Krishnan RP, Monica K (2022 Feb) Adenoid ameloblastoma: a neglected variant of ameloblastoma or a separate entity? Oral oncol. 125:105681. https://doi.org/10.1016/j.oraloncology.2021.105681. Epub 2021 Dec 28. PMID: 34971882
Jofre SA, Roth M, Lahouti AH, Gersten A, Azad AK, Kelsch RD, Asiry S, Goldstein Y, Naeem R, Rao R (2022 Jun) Ameloblastoma with adenoid features: Case report with cyto-histopathologic correlation and molecular findings. Diagn Cytopathol 50(6):E140–E145. https://doi.org/10.1002/dc.24929Epub 2022 Jan 25. PMID: 35077030
WHO Classifcation of Tumours Editorial Board. Head and neck tumours. Lyon (France): International Agency for Research on Cancer; 2022. (WHO classifcation of tumours series, 5th ed.; vol. 9). https://publications.iarc.fr/
Funding
NA.
Author information
Authors and Affiliations
Contributions
Guarantor of the integrity of the study: Sharma G.
Literature research: Sharma G, Kamboj M.
Data acquisition: Sharma G and Kamboj M.
Diagnostics and management of patient: Sharma G, Kamboj M, and Singh V.
Manuscript preparation: Sharma G and Kamboj M.
Manuscript editing: Narwal A, Devi A.
Manuscript review: Sharma G and Singh V.
Corresponding author
Ethics declarations
Conflicts of interest/Competing Interests (include appropriate disclosures)
NA.
Ethics Approval (include appropriate approvals or waivers
NA.
Consent to Participate (include appropriate statements)
NA.
Consent for publication (include appropriate statements)
NA.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, G., Kamboj, M., Narwal, A. et al. Demystifying Histologic Conundrum of Adenoid Ameloblastoma: Case Report with Literature Review. Indian J Otolaryngol Head Neck Surg 75, 2432–2437 (2023). https://doi.org/10.1007/s12070-023-03534-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-023-03534-6