Abstract
Background
Recently, a new odontogenic tumor has been described, the so-called adenoid ameloblastoma (AdAM). The aim of this review was to determine the clinical and imaging features of AdAM and to describe its main histopathological findings.
Methods
The systematic review included published cases with a diagnosis of AdAM in the gnathic bones, which had sufficient clinical, imaging, and histopathological data to confirm its diagnosis. The following histopathological diagnostic criteria were adopted: presence of ameloblastoma-like components, duct-like structures, spiral cellular condensations, and a cribriform architecture.
Results
Fifteen articles, corresponding to 30 cases of AdAM, were selected. Most cases affected men (63.3%), with a slight preference for the mandible (16:14) and the posterior region of gnathic bones was the most commonly affected site. The mean age at diagnosis was 40.8 years. Clinically, the lesions usually presented as a swelling (53.3%) and, radiographically, as a well-defined radiolucency (33.4%). Surgical resection (40%) was the most frequently adopted treatment and recurrence occurred in 30% of cases. Microscopic examination showed cribriform areas in most AdAM cases (93.3%); duct-like structures and spiral cellular condensations were seen in 100% of the cases.
Conclusion
The small number of reported cases, the existence of erroneous diagnoses, and the adoption of initial conservative management make it difficult to determine whether AdAM has a higher risk of recurrence or more aggressive biological behavior than conventional ameloblastomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ameloblastoma is a benign epithelial odontogenic tumor [1] originally described in 1827 by Cuzack and the publication of further details in 1868 by Broca [2]. It accounts for 13 to 58% of all odontogenic tumors [3], with an incidence rate of 0.92 cases/million people/year. Ameloblastoma shows a peak incidence in the third decade of life and a slight male preference. The mandible is the most affected site. The follicular and plexiform subtypes are the most common histopathological patterns [4, 5].
Clinically, ameloblastoma presents as a painless swelling of slow and expansive growth, which may imply significant morbidity for the patient [4, 6, 7]. Although being considered a benign neoplasm, ameloblastoma tends to be locally aggressive and the recurrence rates are high [8,9,10]. The adopted treatment depends on the clinicopathological type and the patient’s systemic condition, with surgical excision being the mainstay treatment [11].
In 2022, with the update of the World Health Organization (WHO) Classification of Head and Neck Tumors, the term “conventional” was reintroduced and a new entity was recognized, the so-called adenoid ameloblastoma (AdAM) [12, 13]. The latter is characterized histopathologically by a cribriform architecture, the presence of duct-like structures, and the formation of dentinoid material [13]. Concerning their mutational profile, the absence of the BRAF mutation and the scarcity of investigations on other already known mutations in conventional ameloblastomas, such as SMO, represent a research gap in new studies on the molecular profile of these lesions [14, 15].
Several studies have reported cases of AdAM in the gnathic bones. However, further characterization of this tumor is necessary to better understand its etiopathogenesis and biological behavior. Thus, the present systematic review aimed to investigate the clinical and imaging findings of AdAM, as well as to describe its main histopathological findings reported in scientific literature.
Materials and Methods
This systematic review was registered in PROSPERO (Number: CRD42022344636) and was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16].
Data Sources and Search Strategies
An electronic literature search, with no time or language restrictions, was performed between July and August 2022 in the following databases: PubMed/Medline, Web of Science, Science Direct, Scopus, Embase, and Cochrane Collaboration Library. We also searched the gray literature in Opengrey, ProQuest, and Google Scholar. In addition, hand searches were performed by crossing the reference lists of the included articles. After identification, duplicate references were removed.
Following the population, intervention, comparison, and outcome (PICO) model, the following research question was formulated for this systematic review: “What are the most common clinical, imaging, and histopathological features of AdAM?”.
The search strategy was based on the combination of the following keywords: (“Adenoid ameloblastoma”[tw] OR “Adenomatoid ameloblastoma”[tw] OR “Dentinoameloblastoma”[tw] OR “Adenoid ameloblastoma with dentinoid”[tw] OR “Adenoid ameloblastoma with dentinoid”[tw] OR “Plexiform ameloblastoma with dentinoid”[tw] OR “Atypical plexiform ameloblastoma with dentinoid”[tw] OR “Dentinoameloblastoma with ghost cells”[tw]).
Eligibility Criteria and Study Selection
All published studies that described cases diagnosed as AdAM in the gnathic bones were eligible. The studies had to provide sufficient clinical, imaging, and histological data so that the diagnosis of AdAM could be confirmed. Randomized controlled clinical trials, cohort studies, case–control studies, cross-sectional studies, case series, and case reports were included. In vitro studies, cytological studies, review articles, and letters to the editor were excluded, unless any of these categories reported cases with sufficient clinical, imaging, and histological information to confirm the diagnosis.
The histopathological diagnostic criteria adopted for inclusion of the articles in the systematic review were the presence of (i) an ameloblastomatous component, (ii) duct-like structures, (iii) spiral cellular condensations, and (iv) cribriform architecture [12].
Four authors (HGFM, RICG, CSOC, and HFP) independently screened the titles and abstracts of all articles identified by the electronic searches. Next, the full texts of the studies that met the inclusion criteria the inclusion criteria were read. Six authors (HGFM, RICG, CSOC, HFP, RPM, and EFM) thoroughly evaluated the clinical, imaging, and histopathological features, as well as molecular findings, in order to confirm the diagnosis of AdAM. In the case of disagreement, the final diagnosis was reached by consensus. If the disagreement persisted, two other experienced pathologists evaluated the cases (MCCM and RAF).
Data Extraction and Analysis
Three of the authors independently assessed the articles eligible for data extraction (HGFM, RIG, and CSOC) and any disagreement was resolved by discussion. The following data were extracted from each article: (i) author(s); (ii) publication year; (iii) country where the study was conducted; (iv) number of reported cases; (v) age and sex of patient(s); (vi) tumor location; (vii) clinical features; (viii) imaging features; (ix) histopathological features; (x) molecular features; (xi) treatment; (xii) recurrence, and (xiii) follow-up period.
The methodological quality of the articles was evaluated according to the CARE guidelines (for CAse REports), a qualitative checklist for observational studies and case reports [17].
Results
Study Selection
The search strategy retrieved 118,536 articles from the different databases, including those identified by hand searching. After reading the titles and abstracts, 107 articles were potentially eligible and their full texts were assessed by six evaluators (HGFM, RIGG, CSOC, HFP, RPM, and EFM). After reading the full text of the pre-selected articles, 15 studies met all inclusion criteria, and were selected for this systematic review, with a total number of 30 reported cases of AdAM in the gnathic bones. Figure 1 shows the flowchart of the article selection process. All studies were published in English. The 30 reported cases selected for this review were from seven countries, covering four of the five continents, except for Oceania. Brazil was the country with the largest number of reported cases (Fig. 2). Detailed information on the studies selected for this systematic review is available in Supplementary Tables 1 and 2.
Clinical and Radiographic Features
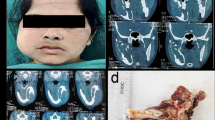
Male patients were more commonly affected by AdAM (63.3%). The mean age was 40.8 years, ranging from 15 [18] to 70 years [19]. Only one patient was under 18 years of age [18]. The mandible was the most affected site, with a mandible/maxilla ratio of 16/14, and most tumors involved the posterior region of the mandible (Fig. 3).
Swelling was a frequent clinical finding (53.3%) in the AdAM cases included in this systematic review, whereas pain (13.3%) and paresthesia (10%) were less commonly described. Considering all cases, 30% of the tumors were larger than 3 cm and tooth displacement was reported in only 10% of the cases. Radiographically, AdAM mainly appeared as a well-defined radiolucency (33.4%). There was only one case of peripheral AdAM without bone involvement [20]. In addition, 20% of the cases showed cortical bone destruction.
Surgical resection was the most common treatment (40%) in the included studies. In two of the cases reported by Loyola et al. [21], radiotherapy was used as adjuvant therapy and neck dissection was also performed in one of these cases. Recurrence was a common finding, observed in 30% of cases. Table 1 describes the clinical and imaging data of all AdAM cases included in the present study.
Histopathological and Molecular Features
Regarding histopathological features, 50% of the AdAM cases in our review exhibited the plexiform subtype as the predominant morphological pattern, followed by the concomitant presence of the plexiform and follicular patterns (36.7%). Histopathological features such as odontogenic epithelium resembling the stellate reticulum (93.3%) and a cribriform pattern (90%) were present in most of the included cases, while duct-like structures and spiral cellular condensations were found in 100% of the cases. Dentinoid material was present in 70% of the cases. Distinct cell morphologies were also reported, including the presence of clear cells (43.4%), ghost cells (23.3%), spindle cells (16.7%), and multinucleated giant cells (20%) (Table 2). Figure 4 illustrates some of the histopathological features described in our systematic review, which refer to a case of AdAM diagnosed at our oral pathology service.
Histopathological features of adenoid ameloblastoma. a Presence of an ameloblastomatous plexiform pattern and cribriform arrangement in solid areas (100×). b Spiral epithelial structures and clear cells (200×). c Duct-like structures containing scant eosinophilic material (400×). d Dentinoid material near some clear cells (400×)
Only 10 cases (33.3%) [19, 22] had their molecular profile investigated. Somatic mutations in FGFR2 and SMO were found in only one case (0.03%) [19] (Supplementary Table 2).
Quality Assessment
The results of the quality assessment of the selected studies are presented in Supplementary Table 3. Most articles adequately described the clinical characteristics (60%), diagnostic techniques used (100%), and therapy modalities (96.7%). However, only 46.7% of the articles reported follow-up data.
Discussion
Odontogenic tumors are an uncommon group of neoplasms that originate from odontogenic tissues and their remnants and that show a heterogeneous biological behavior. The correct diagnosis of these tumors is of the utmost importance for adequate treatment. However, despite advances in the diagnostic methods for odontogenic lesions, controversies regarding their etiopathogenesis, the paucity of information on histopathological and molecular features, and their unclear clinical behavior can make the diagnosis difficult [5, 9].
After several studies have endorsed AdAM to be a distinct entity [11, 22,23,24,25,26,27], the WHO recognized this tumor in its recent classification [13]. Underreporting of AdAM may exist and many cases may have been diagnosed as either ameloblastoma or adenomatoid odontogenic tumor (AOT), depending on the predominance of microscopic features of these tumors. The study by Loyola et al. [21] supports this possibility; in that study, 45 cases initially diagnosed as ameloblastomas had their histopathological slides reassessed and four of them were reclassified as AdAM. The current recognition of AdAM as a distinct entity and the better characterization of its histopathological and molecular features are expected to raise the awareness of pathologists and to increase the accurate identification of AdAM. Thus, we believe that more cases of AdAM will be reported over the next years.
This systematic review allowed to identify the clinicopathological and imaging findings of AdAM cases reported in the literature so far, which can be summarized as follows: AdAM was more frequent in male patients, with a mean age of 40.8 years, and the mandible was the most affected anatomical site, with a mandible/maxilla ratio of 16/14. Among cases with mandibular involvement, most occurred in the posterior region. Radiographically, a well-delimited radiolucent appearance predominated, but diffuse and multilocular features were also observed. It is noteworthy that the clinical and imaging features of AdAM are not pathognomonic. Therefore, thorough histopathological analysis is essential for a correct diagnosis.
The histopathological findings considered to be essential for inclusion of the studies in our systematic review were the presence of an ameloblastomatous component, duct-like structures, spiral epithelial cellular condensations (resembling morulae), and a cribriform architecture. Dentinoid material, clear cells, ghost cells, and areas of calcifications may also be present in AdAM. The formation of dentinoid material in these cases is probably due to an inducing effect of the odontogenic epithelial component. Moreover, a reduction in vascular supply can promote degenerative changes, inducing the formation of a pseudoglandular pattern in large AdAMs [28].
Previous studies have described the molecular profile of AdAM. Immunohistochemical analysis usually shows overexpression of the Ki-67 proliferative marker. Furthermore, mutations in the BRAF and p.V600E genes, commonly identified in ameloblastomas, are absent in AdAM [11, 21]. These findings podem sugerir the view that AdAM is an odontogenic tumor distinct from conventional ameloblastoma. A recent study reported mutations in the SMO and FGFR2 genes in a single case of AdAM [19]. These mutations have been previously detected in some ameloblastomas [29, 30].
Sweeney et al. [14] identified SMO mutations in 39% of ameloblastomas analyzed and 46% harbored BRAF mutations, which tend to be mutually exclusive. The authors suggest that these alterations might define two independent genetic etiologies for ameloblastoma. However, further investigations are needed to conclude whether those mutations influence the development of AdAM and if they are sufficient to justify its recognition as a new entity according to its molecular profile.
Malignant transformation of AdAM has not been reported so far. However, Jayasooriya et al. [11] described a recurrent AdAM case with atypia and hypercellularity, which led to a diagnostic challenge between AdAM or odontogenic carcinoma with dentinoid. However, the mere presence of cellular atypia is not enough to confirm a malignant background [8]. Odontogenic carcinoma with dentinoid is another rare and poorly characterized odontogenic tumor that should be included in the differential diagnosis of AdAM. This low-grade malignant odontogenic neoplasm is histopathologically characterized by the presence of cords and islands of eosinophilic or clear epithelial cells associated with dentinoid material and, less commonly, duct-like structures [31]. Atypia and perineural invasions are occasionally reported. The absence of epithelium similar to conventional ameloblastoma distinguishes it from AdAM.
Like ameloblastoma itself, AOT is the main differential diagnosis of AdAM. AdAM and AOT share some microscopic features, although the former can be distinguished by the presence of a typical ameloblastomatous component. Furthermore, in contrast to AOT, AdAM does not harbor the KRAS mutation [22, 32]. Thus, the importance of the differential diagnosis between the two tumors must be highlighted, especially because of the aggressiveness of AdAM as opposed to the indolent behavior of AOT.
In our systematic review, 30% of the cases diagnosed as AdAM relapsed. In particular, all cases reported by Loyola et al. [21] underwent surgical resection as primary therapy and the mean time to first recurrence was 9 months. Furthermore, Evans et al. [25] reported a case of recurrence 9 years after initial treatment. However, most recurrences occurred due to misdiagnosis of the lesion as AOT and subsequent conservative treatment. The likelihood of an inaccurate diagnosis can be attributed to the predominance of AOT-like areas upon histopathological analysis, leading to conservative treatment [21]. The biological behavior, prognosis, and appropriate treatment strategies for AdAM remain uncertain and careful and long-term follow-up is therefore essential.
Our results suggest that AdAM is a rare odontogenic tumor, with 30 cases reported to date and included in this study. It mostly affects men in a wide age range, commonly presenting as an asymptomatic and intraosseous growth, with a predilection for the posterior region of the mandible. Radiographically, they predominantly appear as a well-defined radiolucency. Histopathologically, AdAm may present different ameloblastoma morphological patterns with epithelial islands in a spiral arrangement, duct-like structures, and, more rarely, rosette-like structures, associated with dentinoid.
Conclusion
The differential diagnosis, especially with ameloblastoma and AOT, is essential for accurate diagnosis and, consequently, for the correct management of the tumor. Thus, a detailed histopathological analysis of the tumor is imperative considering the findings presented here. Since AdAM is a lesion recently recognized by the WHO (2022) and with few cases reported, this systematic review will help to consolidate information available in the literature. Despite that, it may be early to implement definitive standardized criteria for the diagnosis of AdAM. Eight cases developed multiple recurrences even after wide excision of the tumor, indicating an aggressive behavior, although this needs further investigation. The few reported cases, erroneous diagnoses, and initial conservative treatment impair a clear conclusion about the actual behavior of AdAM. With the increasing number of cases reported over the last decade and the current recognition of AdAM as a separate entity by the WHO, longitudinal studies on this tumor evaluating its clinical behavior, as well as histopathological and molecular studies elucidating the genetic features of the neoplastic process, will be necessary. Such studies should provide surgeons and pathologists with the data needed for the correct diagnosis, management, and prognosis of this newly recognized tumor.
References
Laborde A, Nicot R, Wojcik T et al (2017) Ameloblastoma of the jaws: management and recurrence rate. Eur Ann Otorhinolaryngol Head Neck Dis 134:7–11. https://doi.org/10.1016/j.anorl.2016.09.004
Almeida RAC, Andrade ES, Barbalho JC et al (2016) Recurrence rate following treatment for primary multicystic ameloblastoma: systematic review and meta-analysis. Int J Oral Maxillofac Surg 45:359–367. https://doi.org/10.1016/j.ijom.2015.12.016
Oginni FO, Stoelinga PJ, Ajike SA et al (2015) A prospective epidemiological study on odontogenic tumours in a black african population, with emphasis on the relative frequency of ameloblastoma. Int J Oral Maxillofac Surg 44:1099–1105. https://doi.org/10.1016/j.ijom.2015.03.018
Hendra FN, Natsir Kalla DS, Van Cann EM et al (2019) Radical vs conservative treatment of intraosseous ameloblastoma: systematic review and meta-analysis. Oral Dis 25:1683–1696. https://doi.org/10.1111/odi.13014
Hendra FN, Van Cann EM, Helder MN et al (2020) Global incidence and profile of ameloblastoma: a systematic review and meta-analysis. Oral Dis 26:12–21. https://doi.org/10.1111/odi.13031
Yang R, Liu Z, Peng C et al (2017) Maxillary ameloblastoma: factors associated with risk of recurrence. Head Neck 39:996–1000. https://doi.org/10.1002/hed.24720
Kelppe J, Hagström J, Sorsa T (2019) Ameloblastoma: a retrospective single institute study of 34 subjects. Acta Odontol Scand 77:82–87. https://doi.org/10.1080/00016357.2018.1532530
El-Naggar AK, Chan JKC, Grandis JR et al (2017) Odontogenic and maxillofacial bone tumours. WHO classification of head and neck tumours, 4th edn. IARC, Lyon, pp 205–260
Kreppel M, Zöller J (2017) Ameloblastoma-Clinical, radiological, and therapeutic findings. Oral Dis 24:63–66. https://doi.org/10.1111/odi.12702
Gneep DR, Bishop JA (2020) Gnepp’s diagnostic surgical pathology of the head and neck, 3rd edn. Elsevier, Amsterdam
Jayasooriya PR, Abeyasinghe WAMUL, Liyanage RLPR et al (2022) Diagnostic Enigma of Adenoid Ameloblastoma: Literature Review based evidence to consider it as a new sub type of Ameloblastoma. Head Neck Pathol 16:344–352. https://doi.org/10.1007/s12105-021-01358-w
WHO Classification of Tumours Editorial Board. Head and neck tumours (2022) Lyon (France): International Agency for Research on Cancer. WHO classification of tumours series, 5th ed. Vol. 9 https://publications.iarc.fr/
Vered M, Wright JM (2022) Update from the 5th edition of the World Health Organization classification of head and neck tumors: odontogenic and maxillofacial bone tumours. Head Neck Pathol 16:63–75. https://doi.org/10.1007/s12105-021-01404-7
Sweeney RT, McClary AC, Myers BR et al (2015) Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat Genet 47(1):97
Soluk-Tekkesin M, Wright JM (2022) The World Health Organization classification of odontogenic lesions: a summary of the changes of the 2022, 5th Edn. Turk Patoloji Derg 38:168–184. https://doi.org/10.5146/tjpath.2022.01573
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
Gagnier JJ, Kienle G, Altman DG et al (2013) The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med 2:38–43. https://doi.org/10.7453/gahmj.2013.008
Adorno-Farias D, Muniz VRVM, Soares AP et al (2018) Ameloblastoma with adenoid features: a series of eight cases. Acta Histochem 120:468–476. https://doi.org/10.1016/j.acthis.2018.05.006
Jofre SA, Roth M, Lahouti AH et al (2022) Ameloblastoma with adenoid features: case report with cyto-histopathologic correlation and molecular findings. Diagn Cytopathol 50:E140–E145. https://doi.org/10.1002/dc.24929
Khalele BAO, Al-Shiaty RA (2016) Adenoid ameloblastoma with dentinoid and cellular atypia: a rare case report. Ital J Med 10:238–240. https://doi.org/10.4081/itjm.2016.639
Loyola AM, Cardoso SV, de Faria PR et al (2015) Adenoid ameloblastoma: clinicopathologic description of five cases and systematic review of the current knowledge. Oral Surg Oral Med Oral Pathol Oral Radiol 120:368–377. https://doi.org/10.1016/j.oooo.2015.05.011
Coura BP, Dos Santos JN, Fonseca FP et al (2021) Adenoid ameloblastoma with dentinoid is molecularly different from ameloblastomas and adenomatoid odontogenic tumors. J Oral Pathol Med 50:1067–1071. https://doi.org/10.1111/jop.13243
Tajima Y, Sakamoto E, Yamamoto Y (1992) Odontogenic cyst giving rise to an adenomatoid odontogenic tumor: report of a case with peculiar features. J Oral Maxillofac Surg 50:190–193. https://doi.org/10.1016/0278-2391(92)90370-f
Matsumoto Y, Mizoue K, Seto K (2001) Atypical plexiform ameloblastoma with dentinoid: adenoid ameloblastoma with dentinoid. J Oral Pathol Med 30:251–254. https://doi.org/10.1034/j.1600-0714.2001.300410.x
Evans BL, Carr RF, Phillipe LJ (2004) Adenoid ameloblastoma with dentinoid: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 98:583–588. https://doi.org/10.1016/j.tripleo.2004.02.077
Ide F, Mishima K, Saito I, Kusama K (2009) Diagnostically challenging epithelial odontogenic tumors: a selective review of 7 jawbone lesions. Head Neck Pathol 3:18–26. https://doi.org/10.1007/s12105-009-0107-4
Saxena K, Jose M, Chatra LK, Sequiera J (2012) Adenoid ameloblastoma with dentinoid. J Oral Maxillofac Pathol 16:272–276. https://doi.org/10.4103/jomfp.JOMFP_53_15
Pandiar D, Gopinath D (2022) Adenoid ameloblastoma: the histological paradox. Head Neck Pathol 16:538–539. https://doi.org/10.1007/s12105-021-01372-y
Brown NA, Rolland D, McHugh JB et al (2014) Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res 20:5517–5526. https://doi.org/10.1158/1078-0432.CCR-14-1069
Sweeney RT, McClary AC, Myers BR et al (2014) Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat Genet 46:722–725. https://doi.org/10.1038/ng.2986
Gondak RO, Mariano FV, de Sousa SF et al (2020) CTNNB1 and APC mutations in odontogenic carcinoma with dentinoid. Oral Surg Oral Med Oral Pathol Oral Radiol 129:e249–e256. https://doi.org/10.1016/j.oooo.2019.08.017
Gomes CC, de Sousa SF, de Menezes GH et al (2016) Recurrent KRAS G12V pathogenic mutation in adenomatoid odontogenic tumours. Oral Oncol 56:e3–e5. https://doi.org/10.1016/j.oraloncology.2016.03.001
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Contributions
HGFM: conceptualization, data curation, formal analysis, investigation, methodology resources, software, validation, visualization, roles/writing—original draft, and writing—review and editing. RICG: data curation, formal analysis, visualization, and roles/writing—original draft. CSOC: data curation, formal analysis, visualization, and roles/writing—original draft. HFP: investigation and roles/writing—original draft. RPM: writing—review and editing, investigation, and roles/writing—original draft. EFM: writing—review and editing, investigation, and roles/writing—original draft. MCCM: project administration, visualization, and writing—review and editing. RAF: formal analysis, investigation, methodology resources, visualization, and writing—review and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study informed consent is not required.
Consent for Publication
For this type of study consent for publication is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12105_2023_1574_MOESM2_ESM.docx
Supplementary Table 2.Summary of histopathological and molecular data of the studies included in thesystematic review (DOCX 31.6 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Farias Morais, H.G., Gonçalo, R.I.C., de Oliveira Costa, C.S. et al. A Systematic Review of Adenoid Ameloblastoma: A Newly Recognized Entity. Head and Neck Pathol 17, 688–696 (2023). https://doi.org/10.1007/s12105-023-01574-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-023-01574-6