Abstract
Purpose
Increasing evidences suggest dysfunctions of microRNAs (miRNAs) are playing important part in tumors. Therefore, the role of miR-802 in osteosarcoma (OS) was exploited. The object was to evaluate the effect of miR-802 and verify its influence on p27 Kip1 (p27) in OS.
Methods
RT-qPCR experiment was used to detect miR-802 and p27 expression in OS tissues and cells. We explored the function of miR-802 through Transwell assays. The phosphoinositide 3-kinase (PI3K)/AKT serine/threonine kinase pathway and epithelial–mesenchymal transition (EMT) was detected by Western blot assays. Luciferase assay was used to testify the target of miR-802.
Results
MiR-802 expression was elevated in OS, which was related to poor clinical outcome in OS patients. MiR-802 overexpression promoted OS migration, invasion and EMT. Further, p27 is a direct target of miR-802. P27 elevation counteracted the promotion effect of OS on EMT, migration and invasion induced by miR-802. In addition, miR-802 overexpression inactivated PI3K/AKT pathway via targeting p27 in OS.
Conclusion
MiR-802 promoted the progress of EMT, migration and invasion in OS via targeting p27. This newly identified miR-802/p27/PI3K/AKT axis may represent potential targets for OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma (OS) is a malignant primary bone tumor affects adolescents [1] and occurs at end of long bone and originates from primitive osteogenic mesenchymal cells [2]. At present, chemotherapy combined with surgical resection has been widely used in the treatment of OS [3, 4]. However, there have no noticeable improvements in the diagnosis of OS and 5-year survival rates of metastasis patients is about 15–30% [5, 6]. Therefore, it is particularly important to explore biomarkers or targets of OS therapy.
The microRNAs (miRNAs) are reported to consist of 18–25 nucleotides [7], which can play a critical role in the pathogenesis of various tumors via negatively regulating gene expression [8]. In the past decades, the miRNAs have been showed exerting very important functions in many types of cancer processes, such as cell proliferation, cell differentiation, cell apoptosis, as well as tumorigenesis [9]. The recent years, many miRNAs (miR-1258, miR-758 and miR-221) have been reported to have an effect on the proliferation, apoptosis, migration and invasion of OS [10,11,12]. In OS, miR-802 has been showed to be relevant to the proliferation using BrdU assays [10]. However, the correlation between miR-802 dysregulation and OS’s deterioration remains unclear.
p27 Kip1 (p27), also called cyclin-dependent kinases (CDKs) inhibitor 1B, was located at chromosome 12p13 [13, 14]. In addition, previous study reported that p27 acted as tumor suppressor through interacting with miRNAs in different human malignancies [15, 16]. For instance, Wang et al. reported that miR-200c could promote gastric cancer progression by inhibiting the expression of p27 Kip1 [17]. Importantly, decreased expression of p27 was associated with poor differentiation and prognosis of OS [18], suggesting that p27 is strongly negatively related to OS carcinogenesis. However, the specific mechanisms and downstream signaling pathways between miR-802 and p27 in OS have not been reported yet.
Here, we found upregulation of miR-802 and decreasing of p27 in OS tissues and cells. It was showed overexpression of miR-802 promoted OS cell migration, invasion, epithelial–mesenchymal transformation (EMT) and PI3K/AKT pathway activation. P27 was direct target of miR-802 and p27 can overturn promoting function of miR-802 on OS. The miR-802/p27/PI3K/AKT axis can regulate OS migration and invasion, and maybe targets of OS therapy.
Materials and methods
Clinical specimens
After approved by the ethics committee of Weifang Weiyi Tumor Hospital, 68 paired of OS tissues and adjacent non-OS tissues were got from the OS patients. OS patients neither obtained chemotherapy nor radiotherapy. The tissue was collected between January 2013 and March 2017. Written informed consent was got from each patient. Then the tissues were stored at −80 °C. Informed consent was obtained from all patients included in the study. All procedures carried out in studies involbing human participants are consistent with the ethical standards of institutions and/or national research councils, as well as with the 1964 Helsinki Declaration and its subsequent amendments or similar ethical standards. Table 1 showed the demographic features and clinicopathologic data.
Cell culture and transfection
Human OS cell lines (143B, HOS, MG-63, U2OS and Saos-2) and hFOB (the normal osteoblast cell) were obtained from Tongpai (Shanghai) biological technology Co., LTD (Shanghai, China). The RPMI 1640 medium (Gibco, USA) contained 10% fetal bovine serum (FBS) (Gibco, Rockville, MD, USA) was used for culturing the cells under the condition of 37 °C with 5% CO2.
The pcDNA3.1 vector was inserting p27 cDNA to obtain p27 overexpression plasmid (pcDNA-p27). The pcDNA-p27 plasmid, miR-802 mimics/inhibitor and their negative control (NC) were obtained by RiBoBio (Guangzhou, China). The total 1 × 105 cells in logarithmic phase were plated in a 24-well plates at 37 °C with 5% CO2 incubator until reaching 90% confluency. Two µl of Lipofectamine™ 2000 (Invitrogen, USA) mixed with 0.8 µg of DNA was added into the cells to transfect the miR-802 mimic/inhibitor/negative control (NC).
RT-qPCR
TRIzol® reagent (Invitrogen; USA) was applied to extract total RNA. The NanoDrop instrument was performed to detect the concentrations of RNA. The A260/280 of RNA at 1.8–2.0 was selected for subsequent experiments. And complementary deoxyribose nucleic acid (cDNA) was performed by PrimeScript RT reagent kit (Takara). RT-qPCR was quantified using SYBR Green system (Takara, China) containing ROX Reference Dye II (final concentration × 0.5) in ABI 7500 Fast instrument. The internal control for miR-802 or p27 is U6 (small nuclear RNA, snRNA) or GAPDH (glyceraldehyde-3-phosphate dehydrogenase). The expression was calculated by 2−△△ct method. Primers were shown in Table 2.
Transwell assays
Transwell assay was applied to detect the migration and invasion in OS. OS cells were conducted using chambers (Costar, NY) with or without Matrigel (Clontech, CA). Then, cells (1 × 105 cells) after transfection were placed into the upper chambers and lower chambers were filled with medium containing with 10% FBS as a chemo-attractant. Cells were fixed with methanol and stained by Giemsa (JRDUN) and then used 1 × PBS washing three times. Finally, stained cells were counted using the microscope.
Protein analysis
OS cells were collected and lysed with radioimmunoprecipitation assay (RIPA, Beyongtime, China). The concentration of protein was detected using Pierce® bicinchoninic acid (BCA) Protein Assay Kit. The proteins were separated in 10–15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and then were transferred to polyvinylidene fluoride (PVDF) membrane. After blocking with 5% non-fat skim milk, the membranes were incubated with primary antibodies (1:1000; p27 Rabbit mAb #3686, vimentin Rabbit mAb #5741, N-Cadherin Rabbit mAb #13116, E-Cadherin Rabbit mAb #3195, GAPDH Rabbit mAb #5174, PI3 Kinase p110α Rabbit mAb #4249, all obtained from CST; p-PI3K Rabbit mAb, ab182651, Abcam, USA) followed by incubation with HRP-linked antirabbit IgG (1:1000; #7074, CST, USA). The protein bands was analysed by Bio-Spectrum Imaging System (UVP, USA).
Luciferase reporter
TargetScan was used for finding targets of miR-802. The wild type or mutant 3′-UTR (WT/MT) of p27 segments was inserted into the pmirGLO (Promega, USA). OS cells were then co-transfected with miRNAs and luciferase reporter vectors (containing WT/MT of p27) by Lipofectamine 3000. Dual luciferase assay system (Promega) was used to analysis luciferase value after 48 h.
Statistical analysis
Statistical results were analyzed by GraphPad Prism 6 software. The data are showed as mean ± SD. The data were analyzed by the χ2 test, Student’s t test or ANOVA analysis. Receiver-operating characteristic (ROC) curve was established to measure the diagnostic value of miR-802 expression level in tumor tissues for OS (TNM stage: I or II/III). Detecting correlation between miR-802 and p27 were using Spearman’s correlation. It was statistically significant when P value of < 0.05.
Results
p27 was downregulated and miR-802 was upregulated in OS
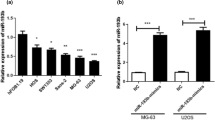
First, the expression of miR-802 and p27 was tested using RT-qPCR in OS tissues and cell lines. The expression of miR-802 was increased in OS tissues (2.41 ± 0.67) than that in the normal tissues (0.77 ± 0.40) as presented in Fig. 1A and Table 3. In Fig. 1B and Table 3, the p27 relative expression was decreased in the OS tissues (1.17 ± 0.44) when compared with normal tissues (2.10 ± 0.48). And then, the miR-802 level was increased in OS cell lines (Saos-2, 1.97 ± 0.13; HOS, 3.07 ± 0.09; U2OS, 3.89 ± 0.10; 143B, 4.80 ± 0.10; MG-63, 5.66 ± 0.16) compared with hFOB cells (1.01 ± 0.46) (Fig. 1C; Table 4). The mRNA expression of p27 in OS cell lines (Saos-2, 0.74 ± 0.05; HOS, 0.52 ± 0.04; U2OS, 0.40 ± 0.05; 143B, 0.29 ± 0.06; MG-63, 0.19 ± 0.05) was significantly lower verse to the hFOB cells (0.99 ± 0.61) (Fig. 1D; Table 4). And interestingly, miR-802 was negatively associated with p27 in OS as shown in Fig. 1E. ROC curves was established to analyze the miR-802 expression in stage I or II/III OS patients and the area under curve (AUC) of miR-802 was 0.848 (95% CI 0.750–0.945) (Fig. 1F). The Youden index of miR-802 expression was 0.722.
Low p27 expression and high miR-802 expression in OS. A miR-802 expression was analysis through RT-qPCR (n = 68). B The p27 expression in OS tissues (n = 68). C The miR-802 expression in OS cells. D The p27 expression in OS cells. E MiR-802 negatively related to p27. *P < 0.05, **P < 0.01, ***P < 0.001. F ROC curve for miR-802 expression in stage I or II/III OS
Subsequently, the association between miR-802 expression and OS clinicopathological features was analyzed by t test and χ2 test in Table 1. Higher miR-802 expression was correlated to TNM stage (P = 0.035 and 0.030), distant metastasis (P = 0.003 and 0.004), response to chemotherapy (P = 0.017 and 0.016) and tumor size (P = 0.029 and 0.026). However, miR-802 had no obvious connection with age (P = 0.519 and 0.343), sex (P = 0.271 and 0.193), familial cancer history (P = 0.325 and 0.227), blood type (P = 0.916 and 0.996) and comorbidities (P = 0.992 and 0.999). Those findings suggested miR-802 have a relationship to OS development.
MiR-802 promoted OS migration and invasion
To explore the regulatory role of miR-802 in OS, the gain- and loss-of-function experiments were used to show it. In Fig. 2A, B, miR-802 expression was enhanced by miR-802 mimic, while it was decreased by miR-802 inhibitor. As presented in Fig. 2C–F, miR-802 mimic transfection significantly promoted migration and invasion of OS cells. On the contrary, miR-802 inhibitor remarkably decreased the migrated and invaded OS cells. These above results confirmed that miR-802 exerted suppressive roles in OS migration and invasion.
MiR-802 directly targeted to p27
To disclose the tumor-suppressive role of miR-802 in OS tumorigenesis, the downstream targets of miR-802 were identified by bioinformatic analysis. p27, a well-known oncogene in OS, was predicated as a target of miR-802. As shown in Fig. 3A, the 3′-UTR of p27 has a putative region for miR-802 binding. We used luciferase reports to confirm the regulatory relationship between miR-802 and p27. MiR-802 evidently inhibited the luciferase activity of WT-p27 group in OS cells; however, it had no suppressive effect in MT- p27 group (Fig. 3B, C). Furthermore, we detected the effects of miR-802 on p27 expression in OS cells in protein and mRNA level. Notably, p27 expression was dramatically reduced by miR-802 mimic in OS cells (143B, 1.40 ± 1.04; MG-63, 1.66 ± 1.43), whereas it was enhanced by miR-802 inhibitor (143B, 0.46 ± 0.10; MG-63, 0.46 ± 0.09) (Fig. 3D–G; Table 5). Therefore, miR-802 directly regulated p27 expression by target its 3’-UTR in OS cells.
MiR-802 promoted OS cell migration and invasion via regulating p27
Since above data has suggested p27 was direct target of miR-802 in OS, p27 might participate miR-802-mediated promotion of OS cells. Therefore, we used miR-802 mimic to overexpress miR-802 in 143B and MG-63 cells and then transfect with p27 plasmid in these cell to reverse p27 expression to detect whether p27 simulate miR-802-induced effects. The mRNA level of p27 was reduced by miR-802 mimic (143B, 0.72 ± 0.33; MG-63, 0.75 ± 0.31) and then reversed by transfecting with p27 plasmid in OS cells (143B, 2.31 ± 0.26; MG-63, 2.01 ± 0.13) (Fig. 4A, B; Table 6). As estimated, miR-802 up-regulation promoted OS cells migration and invasion and p27 plasmid evidently reversed miR-802 induced cell migration and invasion in Fig. 4C, D. In all, p27 joins miR-802-mediating promotion of OS progression.
MiR-802 promoted OS EMT
We want to detect the expression of EMT markers to explore the functions of miR-802 of OS mechanism. The proteins expression of EMT (vimentin, N-cadherin and E-cadherin) in 143B and MG-63 cells were detected by western blot analysis. The results showed that mesenchymal markers (vimentin and N-cadherin) were enhanced by miR-802 overexpression and compromised by p27 elevation. Opposite of this, epithelial marker (E-cadherin) protein expression was reduced by miR-802 augment, while the E-cadherin expression was reversed by p27 elevation (Fig. 5A, B). The above findings indicated miR-802 may promote EMT to regulate OS development in vitro via targeting p27.
MiR-802 promoted PI3K/AKT pathway
We want to explore the possible effect of miR-802 on OS mechanism via PI3K/AKT pathway. Then, western blot was used to detect proteins level of PI3K, p-PI3K, AKT and p-AKT in OS cells. Overexpression of miR-802 increased the phosphorylation of PI3K and AKT expression and p27 elevation could reverse this tendency, indicated that miR-802 overexpression could inactivate the PI3K/AKT pathway via targeting p27 (Fig. 5C, D).
Discussion
OS, a highly aggressive tumor affects young adults and children, is characterized by rapid progress, great transfer potential and high incidence [19, 20]. Moreover, molecular targeted therapies have become a hot spot in tumor treatments [17]. Therefore, identifying the mechanism of OS tumorigenesis is important to develop effective therapeutic approach.
Accumulating reports suggested that dysregulation of miRNAs played crucial roles in the diagnosis and prognosis of OS. Ren et al. [11] revealed that miR-758 upregulation suppressed OS cell growth and metastasis in vitro. MicroRNA-524 targets phosphatase and tensin homolog deleted on chromosome ten (PTEN) and promotes OS cell proliferation [21]. Gao et al. indicated that miRNA-133b can present multiple tumor suppressor activities in OS through targeting Fibroblast Growth Factor Receptor 1 (FGFR1) [22]. MiR-802 participated in the progression of different tumors, such as hepatocellular carcinoma (HCC) [23], cervical cancer [24], gastric cancer [25]. For instance, Chao Jiang et al. found that miR-802 participates in T-cell exhaustion through post-transcriptional inhibition of regulated in development and DNA damage response 1 (REDD1), which might provide the inhibitory effect on HCC progression [23]. Ming Ni et al. showed that miR-802 expression exhibited down-regulated trend in cervical cancer tissues and cells, which suppressed the growth and metastatic-related phenotypes of cervical cancer cell via targeting myosin regulatory light chain interacting protein (MYLIP). Moreover, Zhong-Qing Cao et al. found miR-802 was overexpressed in OS tissues compared with adjacent normal tissues. And the enforced expression of miR-802 could accelerate cell proliferation in U2OS and MG63 cells, meanwhile p27 expression was negatively regulated by miR-802 [26]. However, the function of miR-802 on clinicopathological characteristics and cell migration and invasion in OS remains unclear and the specific mechanisms and downstream signaling pathways between miR-802 and p27 in OS have not been reported yet. Here, we indicated miR-802 was enhanced in OS, which was obviously related to unfavorable clinical outcome. Moreover, the overexpression of miR-802 promoted OS cell migration, invasion and EMT. MiR-802 inhibitor could reverse this trend, confirming that miR-802 has oncogenic effect in OS.
miRNAs may be correlated with the pathogenesis and progression of various tumors through regulating their target genes [27]. This study demonstrated p27 was a candidate target of miR-802 in OS using luciferase reporter assay and online prediction software. The previous research shown p27 is an important inhibitor of CDKs. MiR-200c can accelerate tumor progression of gastric cancer by inhibiting p27 expression, which was similar to our findings in OS. We found p27 low expression in OS and it was negatively related to miR-802 in OS tissues. Functional experiments showed p27 expression was decreased after overexpression of miR-802 and this influence was abolished by mutation of the miR-802. In conclusion, miR-802 mediated the progression of OS via targeting p27.
Zhong-Qing Cao et al. found that miR-802 could deactivate the PI3K/AKT/mammalian target of rapamycin (mTOR) pathway in non-small cell lung cancer (NSCLC) cells in vitro and in vivo [26]. That reports revealed that PI3K/AKT pathway was the downstream signaling pathways of miR-802. To further explore the mechanism between miR-802 and PI3K/AKT, we introduced p27 overexpressed plasmids and found that miR-802 could promote the phosphorylation of PI3K and AKT, while p27 could counteract this phenomenon, which suggested that miR-802 overexpression inactivated PI3K/AKT pathway via targeting p27. In all, these findings suggested miR-802 participated in PI3K/AKT pathway to regulate OS carcinogenesis via targeting p27.
Conclusion
This study indicated that miR-802 was overexpressed in OS, which was related to poor clinical outcome of OS patients. MiR-802 overexpression promoted OS cell migration, invasion, EMT and inactivating PI3K/AKT pathway via targeting p27 in OS. The findings will help us better understand the pathogenesis of OS.
References
Li J, Yang Z, Li Y, et al. Cell apoptosis, autophagy and necroptosis in osteosarcoma treatment. Oncotarget. 2016;7(28):44763–78.
Bhattasali O, Vo AT, Roth M, et al. Variability in the reported management of pulmonary metastases in osteosarcoma. Cancer Med. 2015;4(4):523–31.
Vos HI, Coenen MJ, Guchelaar HJ, Te Loo DM. The role of pharmacogenetics in the treatment of osteosarcoma. Drug Discov Today. 2016;21(11):1775–86.
Amankwah EK, Conley AP, Reed DR. Epidemiology and therapies for metastatic sarcoma. Clin Epidemiol. 2013;5:147–62.
Bishop MW, Janeway KA, Gorlick R. Future directions in the treatment of osteosarcoma. Curr Opin Pediatr. 2016;28(1):26–33.
Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33(27):3029–35.
Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10(10):704–14.
Squadrito ML, Etzrodt M, De Palma M, Pittet MJ. MicroRNA-mediated control of macrophages and its implications for cancer. Trends Immunol. 2013;34(7):350–9.
Zavala-Yoe R, Ramirez-Mendoza RA, Cordero LM. Entropy measures to study and model long term simultaneous evolution of children in Doose and Lennox-Gastaut syndromes. J Integr Neurosci. 2016;15(2):205–21.
Liu W, Zhou Z, Zhang Q, et al. Overexpression of miR-1258 inhibits cell proliferation by targeting AKT3 in osteosarcoma. Biochem Biophys Res Commun. 2019;510(3):479–86.
Ren J, Yang M, Xu F, Chen J. microRNA-758 inhibits the malignant phenotype of osteosarcoma cells by directly targeting HMGA1 and deactivating the Wnt/beta-catenin pathway. Am J Cancer Res. 2019;9(1):36–52.
Hu XH, Zhao ZX, Dai J, et al. MicroRNA-221 regulates osteosarcoma cell proliferation, apoptosis, migration, and invasion by targeting CDKN1B/p27. J Cell Biochem. 2019;120(3):4665–74.
Hnit SS, Xie C, Yao M, et al. p27(Kip1) signaling: transcriptional and post-translational regulation. Int J Biochem Cell Biol. 2015;68:9–14.
Kim TH, Lee HH, Chung SH, et al. Expression of p27 and Jun activation domain-binding protein 1 in endometriosis. Arch Gynecol Obstet. 2015;292(2):377–81.
Shao J, Li S, Palmqvist L, et al. p27(KIP1) and PTEN cooperate in myeloproliferative neoplasm tumor suppression in mice. Exp Hematol Oncol. 2015;5:17.
Montanaro L, Trere D, Derenzini M. Changes in ribosome biogenesis may induce cancer by down-regulating the cell tumor suppressor potential. Biochem Biophys Acta. 2012;1825(1):101–10.
Wang Y, Zeng J, Pan J, et al. MicroRNA-200c is involved in proliferation of gastric cancer by directly repressing p27(Kip1). Biochem Biophys Rep. 2016;8:227–33.
Thomas DM, Johnson SA, Sims NA, et al. Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J Cell Biol. 2004;167(5):925–34.
Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma—connecting aetiology, biology and therapy. Nat Rev Endocrinol. 2017;13(8):480–91.
Lindsey BA, Markel JE, Kleinerman ES. Osteosarcoma overview. Rheumatol Ther. 2017;4(1):25–43.
Zhuang M, Qiu X, Cheng D, et al. MicroRNA-524 promotes cell proliferation by down-regulating PTEN expression in osteosarcoma. Cancer Cell Int. 2018;18:114.
Gao G, Tian Z, Zhu HY, Ouyang XY. miRNA-133b targets FGFR1 and presents multiple tumor suppressor activities in osteosarcoma. Cancer Cell Int. 2018;18:210.
Jiang C, Liu X, Wang M, et al. High blood miR-802 is associated with poor prognosis in HCC patients by regulating DNA damage response 1 (REDD1)-mediated function of T cells. Oncol Res. 2019;27(9):1025–34.
Zhang Q, Lv R, Guo W, Li X. microRNA-802 inhibits cell proliferation and induces apoptosis in human cervical cancer by targeting serine/arginine-rich splicing factor 9. J Cell Biochem. 2019;120(6):10370–9.
Zhang XY, Mu JH, Liu LY, Zhang HZ. Upregulation of miR-802 suppresses gastric cancer oncogenicity via targeting RAB23 expression. Eur Rev Med Pharmacol Sci. 2017;21(18):4071–8.
Cao ZQ, Shen Z, Huang WY. MicroRNA-802 promotes osteosarcoma cell proliferation by targeting p27. APJCP. 2013;14(12):7081–4.
Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314.
Author information
Authors and Affiliations
Contributions
LG conceived and designed the study and drafted the manuscript. SJ and QZ collected, analyzed and interpreted the experimental data. YX, CL and YL revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by Ethical Committee of Weifang Weiyi Tumor Hospital and conducted in accordance with the ethical standards. Approval No. 2012-11.
Research involving human participants and/or animals
All procedures carried out in studies involbing human participants are consistent with the ethical standards of institutions and/or national research councils, as well as with the 1964 Helsinki Declaration and its subsequent amendments or similar ethical standards.
Informed consent
Subjects signed the informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, .F., Jia, S., Zhang, .M. et al. MicroRNA-802 promotes the progression of osteosarcoma through targeting p27 and activating PI3K/AKT pathway. Clin Transl Oncol 24, 266–275 (2022). https://doi.org/10.1007/s12094-021-02683-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02683-w