Abstract
Objective
The goal of this study was to investigate differential expression of the cell cycle signaling proteins p27 and Jun activation domain-binding protein 1 (Jab1) in benign ovarian cysts of patients with endometriosis compared to controls.
Methods
Ovarian tissues of 26 endometriosis and 28 non-endometriosis patients were used to evaluate expression of p27 and Jab1 by immunohistochemistry.
Results
There are no differences in clinical characteristics between the endometriosis and non-endometriosis group. In the endometriosis group, CD10, Jab1 and p27 were positive in 50, 48.3 and 57.7 % of cases, respectively, compared to 14.3, 17.9 and 50 %, respectively, in the non-endometriosis group. Jab1 and p27 were positive in 69.2 and 84.6 %, respectively, of the definite endometriosis cases.
Conclusions
These results demonstrate that Jab1 expression was increased in endometriosis, while p27 expression was similar in the endometriosis and non-endometriosis groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometriosis is a chronic inflammatory disease defined as ectopic endometrium outside the uterus. It is associated with dysmenorrhea, dyspareuria and infertility, and the prevalence increases with reproductive age. However, the pathophysiologic causes of endometriosis remain unknown despite much effort, although coelomic metaplasia, retrograde menstruation, and exposure of endocrine disruptors have been implicated. p27 and Jun activation domain-binding protein (Jab) 1 were expressed in epithelial tumors [1]. The expression of Jab1, but not p27, was higher in borderline compared to benign tumors, suggesting that the roles of cell cycle regulators, such as Jab1, in ovarian disease should be further investigated.
Various positive and negative regulators maintain the cell cycle. Jab1 is homologous to the COP9 signal some subunit 5 and is involved in cell cycle regulation. Jab1 was initially identified as a transcriptional co-activator of the gene-regulatory activator protein, which stimulates tumor progression when activated [2]. p27 is a well-known negative regulator of cell cycle progression, acts as a cyclin-dependent kinase inhibitor (CDKI), and inhibits the G1 to S phase checkpoint transition during normal cell cycle progression. Thus, p27 is considered a tumor suppressor with anti-proliferative action that promotes apoptosis and cell cycle arrest. [3]. Jab1 directly interacts with p27 to induce subsequent degradation by stimulating phosphorylation, and loss or downregulation of p27 is related to malignancy [4]. In vivo and in vitro studies showed that expression of Jab1 and p27 was inversely correlated in various cancers, and overexpression of Jab1 and low expression of p27 were associated with poorer prognosis or advanced disease [5–8]. Previous reports have indicated that Jab1-induced suppression of p27 is an important mechanism of regulation of human oncogenesis.
The proto-oncogene c-Jun is a transcription factor that forms a variety of dimeric complexes and can induce cell proliferation and tumor progression. There are two main mechanisms how the c-Jun protein can stimulate cell cycle progression: (1) induction of genes coding for components of the cell cycle clock machinery, such as cyclin D1, and (2) repression of tumor suppressor genes, such as p53 [9].
Endometriotic cells of endometriosis undergo proliferation, adhesion and have lower sensitivity than eutopic endometrium to apoptosis [10]. Although endometriosis is histologically benign, the disease process leads to progressive, recurring destructive lesions, similar to cancer. Therefore, this study focused on Jab1-induced cell cycle regulation during the pathogenesis of endometriosis. We investigated the correlation between Jab1 and p27 protein expression in ovarian endometriosis by comparing diseased tissue with that of other types of benign ovarian tumor.
Materials and methods
This case–control study enrolled 54 patients (26 endometriosis and 28 non-endometriosis) who underwent an oophorectomy, ovarian cystectomy or salpingo-oophorectomy in a tertiary university hospital from August 2011 to December 2013. This study was approved by the Institutional Review Board (IRB) and informed consent was obtained from all patients before the operation. Information on each patient’s background, such as age, body weight, height, coexisting diseases, and drug prescriptions, was obtained from electronic medical records. Clinical stages of endometriosis were characterized according to the revised American Fertility Society (AFS) classification, and were graded as minimal (Stage I), mild (Stage II), moderate (Stage III) and severe (Stage IV). The obtained tissues were immediately sent to the laboratory for analyses.
Immunohistochemical analyses
All ovarian tissues were fixed in 10 % formaldehyde, embedded in paraffin blocks, and then cut into 5-µm thick sections. Serial sections were mounted on glass slides coated with poly-l-lysine. Each section was de-paraffinized in xylene and rehydrated in a graded ethanol series. For antigen retrieval, slides were heated in a microwave oven at 90–100 °C with 0.1 M sodium citrate buffer (pH 6.0) for 10 min and cooled for 1 h at room temperature. The slides were then washed three times for 5 min each in Tris-buffered saline (TBS; pH 7.4). Endogenous peroxidases were blocked by treating the sections with 0.3 % hydrogen peroxide for 30 min, followed by washing three times with TBS. The slides were then incubated in a humidified chamber with blocking buffer (5 % normal goat serum + 0.5 % Tween-20 in TBS) for 1 h at room temperature. The slides were incubated with primary antibody added to a moist chamber overnight at 4 °C. The following panel of antibodies was used: (1) Jab1 (sc-13157, 1:200, Santa Cruz Biotechnology, Inc., CA, USA); (2) Anti-p27 KIP 1 (ab7961, 1:250, Abcam Inc., Cambridge, MA, USA); (3) CD10 (sc-46656, 1:100, Santa Cruz Biotechnology, Inc., CA, USA). Immunostaining was performed using an EXPOSE mouse- and rabbit-specific horseradish peroxidase (HRP)/3′,3-diaminobenzidine (DAB) detection immunohistochemistry (IHC) kit (Abcam Inc., Cambridge, MA, USA) based on the manufacturer’s protocol. Similar tissue sections were immunostained with non-specific IgG as negative controls. Only tissues with brown-colored staining were considered positive; no background staining was observed in any of the cases.
Statistical analysis
We inputted data into Microsoft 2007 Office Excel and performed statistical analyses using the Statistical Package for the Social Sciences (SPSS) version 12.0 (SPSS Inc., Chicago, IL, USA). Parametric data are presented as means (standard deviation, [SD]) and non-parametric data as medians (interquartile range [IQR]). Continuous variables were analyzed using Student’s t test or Mann–Whitney U test and discrete variables were analyzed using the chi-square test or Fisher’s exact test. A p < 0.05 was considered to indicate statistical significance.
Results
Subjects
Clinical characteristics of ovary tissues with or without endometriosis are summarized in Table 1. There was no difference in age and weight between the groups, although height was slightly higher in the endometriosis than in the non-endometriosis group. However, the difference was minimal and had no clinical meaning. The body mass index (BMI) was 21.8 kg/m2 in the endometriosis group and 21.9 kg/m2 in the non-endometriosis group. In most endometriosis subjects, the revised AFS stage was severe, with 19.2 % at stage III and 80.7 % at stage IV.
CD10, Jab1 and p27 expression
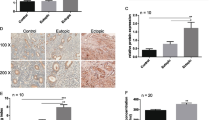
Results of IHC are shown in Fig. 1. Approximately 50 % of the endometriosis samples expressed CD10, as a marker of endometrial stroma, Jab1 and p27 (Fig. 1a–c). However, Jab1 expression was not localized to the CD10-positive area (white-colored arrow. In the non-endometriosis cases, almost all samples were negative for CD10 and Jab1, and 50 % expressed p27 (Fig. 1d–f, arrow). These IHC results are summarized in Table 2. The CD10 and Jab1 positive ratio in endometriosis patients was significantly higher than in non-endometriosis patients (p = 0.005 and p = 0.012, respectively). There was no significant difference in p27 expression between the two groups.
Immunohistochemical evaluation of CD10, Jab1, and p27 expression in endometriosis samples (a–c) benign ovarian cysts (d–f). CD10 and Jab1 were expressed in endometriosis samples but not in non-endometriosis samples. Both sample types expressed p27. Black-colored arrows indicate CD10 (+), Jab1 (+), and p27 (+) expression, while the white-colored arrows indicate CD10 (-), Jab1 (+), and p27 (+) expression. The double-headed arrow in (F) indicates p27 (+)
Table 3 shows Jab1 and p27 expression in the definite endometriosis and non-endometriosis groups. The term ‘definite’ was defined as confirmed surgical inspection and immunohistological CD10 conformity. Definite endometriosis was associated with positive immunoreactivity for Jab1 (69.2 %) and p27 (84.6 %). In contrast, definite non-endometriosis was associated with negative Jab1 immunoreactivity (87.5 %). However, these differences were not significant because of the small sample size.
Table 4 shows data suggesting that Jab1 is a biomarker of endometriosis. Jab1 expression was similar to that of CD10 (positive predictive value: CD10 = 76.4 % vs. Jab l = 72.2 %). However, the sample size was insufficient to calculate significance.
Discussion
In this study, we compared the expression of Jab1 and p27 in endometriotic and non-endometriotic tissue by IHC. The results indicated that Jab1 was expressed with increased frequency in endometriotic tissue compared to non-endometriotic tissue, although there were no significant differences in p27 expression.
The diagnosis of endometriosis may be suspected clinically or by surgical inspection. However, the positive predictive value may be as low as 45 % [11]. To improve the diagnostic sensitivity of endometriosis, the cell-surface metalloendopeptidase CD10 has been used adjunctively. CD10 is expressed in normal and ectopic endometrial stroma [12–14], endometrial stromal neoplasms [12, 15], and adenomyosis [15]. CD10 increased the sensitivity of endometriosis diagnosis by improving recognition of ectopic stromal cells. Despite the small sample size, the current study suggested Jab1 to be a possible biomarker for endometriosis since its expression was similar to CD10. This result could be confirmed by testing suspected endometriosis tissue using CD10 and Jab1.
The pathophysiology of endometriosis has remained unclear, particularly the role of the cell cycle. Schor et al. [16] reported that p27 was downregulated in the endometrium of endometriosis patients and Camargo-Kosugi et al. [17] found that the V109G polymorphism of the p27 gene was associated with development of endometriosis. In our study, the p27 positive rate was similar in both endometriosis and non-endometriosis patients, although the expression level was relatively low compared to that reported by Lee [1]. p27 is thought to be negatively involved in the progression of peritoneal endometriosis [18], and could have a negative effect on the glandular cells of eutopic endometrium [19].
There were several limitations to this study. First, peritoneal lesions should have been included because peritoneal endometriosis is thought to have a different etiology than ovarian endometriosis [10]. Second, the number of definite endometriotic ovarian samples was relatively small. Moreover, it is unclear whether expression of Jab1 and p27 affects the severity of endometriosis. However, to our knowledge, this was the first study of Jab1 expression in endometriosis.
Although endometriosis is more prevalent and results in many reproductive and gynecologic complications, it has been investigated less extensively than ovarian cancer and borderline tumors. This study suggested the possibility of Jab1 as a biomarker. Although the significance must be investigated further, Jab1 expression was similar to that of CD10.
References
Lee W, Park E, Kim D, Kim T, Lee H, Chung S (2011) Expression of p53, p27 and Jab1 protein in epithelial ovarian tumors. Eur J Gynaecol Oncol 33:358–362
Emberley ED, Niu Y, Leygue E, Tomes L, Gietz RD, Murphy LC et al (2003) Psoriasin interacts with Jab1 and influences breast cancer progression. Cancer Res 63:1954–1961
Borriello A, Bencivenga D, Criscuolo M, Caldarelli I, Cucciolla V, Tramontano A et al (2011) Targeting p27Kip1 protein: its relevance in the therapy of human cancer. Expert Opin Ther Targets 15:677–693
Masciullo V, Sgambato A, Pacilio C, Pucci B, Ferrandina G, Palazzo J et al (1999) Frequent loss of expression of the cyclin-dependent kinase inhibitor p27 in epithelial ovarian cancer. Cancer Res 59:3790–3794
Rassidakis GZ, Claret FX, Lai R, Zhang Q, Sarris AH, McDonnell TJ et al (2003) Expression of p27(Kip1) and c-Jun activation binding protein 1 are inversely correlated in systemic anaplastic large cell lymphoma. Clin Cancer Res 9:1121–1128
Kouvaraki MA, Korapati AL, Rassidakis GZ, Tian L, Zhang Q, Chiao P et al (2006) Potential role of Jun activation domain-binding protein 1 as a negative regulator of p27kip1 in pancreatic adenocarcinoma. Cancer Res 66:8581–8589
Jeon JH, Lee KN, Hwang CY, Kwon KS, You KH, Choi I (2005) Tumor suppressor VDUP1 increases p27(kip1) stability by inhibiting JAB1. Cancer Res 65:4485–4489
Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tai Y et al (2001) Jab1 expression is associated with inverse expression of p27(kip1) and poor prognosis in epithelial ovarian tumors. Clin Cancer Res 7:4130–4135
Shaulian E, Karin M (2002) AP-1 as a regulator of cell life and death. Nat Cell Biol 4:E131–E136
Beliard A, Noel A, Foidart JM (2004) Reduction of apoptosis and proliferation in endometriosis. Fertil Steril 82:80–85
Walter AJ, Hentz JG, Magtibay PM, Cornella JL, Magrina JF (2001) Endometriosis: correlation between histologic and visual findings at laparoscopy. Am J Obstet Gynecol 184:1407–1413
Chu P, Arber DA (2000) Paraffin-Section Detection of CD10 in 505 Nonhematopoietic Neoplasms Frequent Expression in Renal Cell Carcinoma and Endometrial Stromal Sarcoma. Am J Clin Pathol 113:374–382
Sumathi V, McCluggage W (2002) CD10 is useful in demonstrating endometrial stroma at ectopic sites and in confirming a diagnosis of endometriosis. J Clin Pathol 55:391–392
Groisman GM, Meir A (2003) CD10 is helpful in detecting occult or inconspicuous endometrial stromal cells in cases of presumptive endometriosis. Arch Pathol Lab Med 127:1003–1006
McCluggage W, Sumathi V, Maxwell P (2001) CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms. Histopathology 39:273–278
Schor E, da Silva ID, Sato H, Baracat EC, Girao MJ, de Freitas V (2009) P27Kip1 is down-regulated in the endometrium of women with endometriosis. Fertil Steril 91:682–686
Camargo-Kosugi CM, da Silva ID, Sato H, D’Amora P, Carvalho CV, Nogueira-de-Souza NC et al (2009) The V109G polymorphism in the p27 gene is associated with endometriosis. Eur J Obstet Gynecol Reprod Biol 145:180–183
Matsuzaki S, Canis M, Murakami T, Dechelotte P, Bruhat MA, Okamura K (2001) Expression of the cyclin-dependent kinase inhibitor p27Kip1 in eutopic endometrium and peritoneal endometriosis. Fertil Steril 75:956–960
Shiozawa T, Nikaido T, Nakayama K, Lu X, Fujii S (1998) Involvement of cyclin-dependent kinase inhibitor p27Kip1 in growth inhibition of endometrium in the secretory phase and of hyperplastic endometrium treated with progesterone. Mol Hum Reprod 4:899–905
Acknowledgments
This work was supported in part by the Soonchunhyang University Research Fund (20130617). This work was partly supported by Jeil Pharmacy.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, TH., Lee, HH., Chung, SH. et al. Expression of p27 and Jun activation domain-binding protein 1 in endometriosis. Arch Gynecol Obstet 292, 377–381 (2015). https://doi.org/10.1007/s00404-015-3642-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3642-0