Abstract
Objective
To determine the role of miR-26a-5p in tumor invasion and metastasis in hepatocellular carcinoma (HCC).
Methods
We evaluated miR-26a-5p expression in HCC tissues by quantitative PCR and then analyzed its clinical significance using a Cox regression model. Transwell and nude mouse models were used to examine tumor metastasis in vitro and in vivo, respectively. The relationship between miR-26a-5p and epithelial-mesenchymal transition was also investigated by q-PCR and western blot.
Results
Strong downregulation of miR-26a-5p was observed in tumor tissues compared to paired adjacent normal tissues. Moreover, patients with low miR-26a-5p expression had a significantly poorer prognosis than those with high expression. The multivariate analysis indicated that miR-26a-5p expression was an independent prognostic indicator. The experimental transwell model and athymic mouse model revealed that miR-26a-5p depressed tumor metastasis in vitro and in vivo, respectively. In addition, the decreased miR-26a-5p level observed in HCC was associated with reduced E-cadherin expression and upregulation of vimentin, which affects the molecular mechanism of EMT.
Conclusion
Downregulation of miR-26a-5p promotes tumor metastasis by targeting EMT and influences the prognosis of HCC patients. Therefore, miR-26a-5p has potential as a new biomarker and therapeutic target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common and aggressive human malignancies in the world and is the third leading cause of cancer-related mortality worldwide and the principal cause of fatality among patients with cirrhosis. Furthermore, HCC is not easy to diagnose in its early stages, and determining effective therapies is difficult [1, 2]. Some aspects of liver tumor cell migration and invasion are well understood. However, HCC metastasis is a complex process, with many intrinsic cellular factors and extrinsic microenvironmental factors influencing the metastatic potential of HCC cells [3], and the molecular mechanisms that mediate the metastatic cascade remain largely unknown. To promote the development of effective therapies targeting metastasis and improve the overall prognosis of patients with HCC, we need to increase our understanding of the molecular mechanisms of HCC invasion and metastasis.
miRNAs are single-stranded non-coding RNAs of 19–25 nucleotides in length that are processed from longer endogenous hairpin transcripts. A single miRNA can target more than 100 transcripts, and research has indicated that more than 60% of human protein-coding genes are modulated by miRNAs, suggesting that they serve as master modulators of gene expression [4]. A general characteristic of tumor tissues is downregulation of most miRNAs in comparison to normal tissues [5–9]. The dysregulation of miRNA expression in cancer cells may be due to genetic alterations, modification of epigenetic patterns, and transcriptional and post-transcriptional regulation, as has been observed for other genes that are dysregulated in cancer cells. Importantly, dysregulation of individual miRNAs and miRNA signatures have been used to classify HCCs according to specific biological and clinical parameters [10]. Particular miRNA signatures are correlated with a poor outcome and tumor relapse, indicating that a set of miRNAs and the associated network of probably hundreds of target transcripts may define a migratory phenotype. Based on these facts, we believe that improving our understanding of the influence of miRNAs in HCC and using miRNAs as noninvasive biomarkers will aid in the diagnosis and treatment of hepatocellular carcinoma [11–13].
As we know, many studies revealed that the miR-26 family (including miR-26a and miR-26b) is commonly downregulated in multiple types of cancer, including HCC, breast cancer [14, 15]. In this study, we focused on miR-26a-5p. Its unregulated expression in cancers was demonstrated to be related to a variety of tumor behaviors and functions in different cancers [16, 17] and it is also downregulated in hepatocellular carcinoma [18], but reports of its function in tumor invasion and metastasis are limited. Furthermore, miR-26a-5p is a potential biomarker for HCC, and determining its molecular mechanisms in HCC tissues and cells may aid in earlier diagnosis and increased treatment efficacy.
Methods
Tissue samples and clinical data
In this study, HCC tumor tissues and paired adjacent non-tumor tissues from each included patient were analyzed. We included 50 tumor tissue samples and paired adjacent normal tissue samples preserved in our Tumor Specimen Bank; the samples were obtained from 50 patients who underwent surgical resection at our department between August 2012 and July 2014. The diagnosis of each HCC case was histopathologically confirmed. The protocols used for this study were approved by the Human Subjects Committee of Zhongnan Hospital, and written informed consent was obtained from all patients. All included medical cases had no history of preoperative therapy or other tumors. The tissue samples were well-preserved at −80 °C until they were used for the experiments. In regard to clinical data, we collected age, gender, liver function, BCLC stage, HBV infection, cirrhosis status, AFP, and Child-Pugh score for every patient. In addition, 2 years of follow-up data (through August 2016) were collected for each medical case. The overall survival rate (OS) and recurrence-free survival rate (RFS) were also included in the statistical analysis.
Cell culture
The cell lines used for this study included the normal human liver cell line L02 and the hepatoma cell lines Huh7, Hep3B, HepG2 and HCCLM9. They were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) where they were characterized by mycoplasma detection, DNA fingerprinting, isozyme detection, and determination of cell viability. The cells were incubated at 37 °C in a humidified chamber supplemented with 5% CO2.
Quantitative polymerase chain reaction (q-PCR)
Total tissue and cellular RNA were extracted using the TRIzol reagent (Invitrogen, USA) according to the manufacturer’s protocol to analyze the levels of the mRNAs of interest. Extracted total RNA was quantified using a Nanodrop™ spectrophotometer (Thermo Scientific) at 260 and 280 nm. Reverse transcription of mRNA was performed using the OligodT primer. Quantitative real-time PCR was performed using an iQ5 Quantitative PCR System (Bio-Rad, USA). β-actin was used for normalization of expression, and 2−ΔΔCT values were normalized to the β-actin levels.
Western blot
Western blot was used to analyze total cellular protein samples. The samples were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes (Millipore, USA). The membranes were incubated with primary antibodies overnight at 4 °C. Then, the membranes were washed and incubated for 1.5 h with secondary antibodies. Finally, the PVDF membranes were subjected to immunoblotting analysis using the ECL immunoblotting kit (Beyotime Institute of Biotechnology, China) according to the manufacturer’s protocol. Each band was normalized with respect to its corresponding GAPDH band.
Inhibitor assay construction and transfection
The miR-26a-5p-inhibitor and control-inhibitor sequences were synthesized. The various cell lines were transfected with the inhibitors using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific Inc.) and Opti-MEM (Thermo Fisher Scientific) according to the manufacturer’s protocol. The cells were lysed at 48 h after transfection.
Transwell assay
Cell migration was evaluated by transwell experiments. The quantitative cell migration assays were performed using 24-well plates containing chambers with 8 μm polycarbonate filters. The cells that invaded through the membrane into the lower surface after 24 h were fixed, stained and counted at 37 °C.
Athymic mouse model
The athymic mouse model (4- to 6-week-old mice) was established by injecting 100 µl of HCCLM9 cells that had been stably transfected with miR-26a-5p-inhibitor and suspended at 2 × 107 cells/ml into the tail vein. Normal HCCLM9 cells were injected into the tail veins of nude mice to create a control athymic mouse model. Overall survival (OS) was compared between the experimental and control athymic mouse models. We also compared the number of metastatic nodes in the lung tissues of the two athymic mice models by HE staining at 7 weeks after injection.
Statistical analysis
Continuous and dichotomous variables are presented as the mean ± standard deviation and discrete numbers, respectively. The Student’s t test, χ 2 test and Fisher’s exact test were performed to make comparisons between groups using SPSS 22.0 software (IBM, Chicago, IL, USA). The Kaplan–Meier test was used to estimate OS and RFS. Differences with P values <0.05 were considered statistically significant. The Cox regression model was used to determine independent factors influencing prognosis.
Results
miR-26a-5p was strongly downregulated in HCC
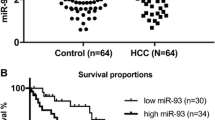
We tested 50 tumor and related adjacent tissues from 50 HCC patients by q-PCR. The results revealed that miR-26a-5p expression was downregulated in 76% (38/50) of HCC tissues (Fig. 1a). The overall comparison of tumor and adjacent tissues demonstrated that miR-26a-5p expression was downregulated in the tumor tissues (P < 0.05) (Fig. 1b).
Clinical data according to miR-26a-5p level. a miR-26a-5p expression level in tumor tissues compared to paired adjacent tissues from 50 patients tested by q-PCR. b Related miR-26a-5p expression in all 50 HCC tissues and adjacent normal regions. c Kaplan–Meier curves and log-rank test present the differences in overall (left panel) and recurrence-free (right panel) survival between groups with high and low miR-26a-5p in tumor tissues. *P < 0.05
Level of miR-26a-5p was associated with HCC progression
We divided the 50 patients into two groups according to the miR-26a-5p expression level: the 25 patients with the highest expression were classified as the high expression group, and the remaining 25 patients were classified as the low expression group. The comparison of the general data for these two groups showed that they were well-matched (Table 1). Then, we collected prognostic data and conducted a survival analysis. The results showed that high miR-26a-5p expression was associated with higher overall and recurrence-free survival rates (Fig. 1c). Based on these findings, we performed a Cox regression analysis and found that miR-26a-5p expression, preoperative Child-Pugh grade, and preoperative viral load were independent prognostic indicators (Table 2).
Different miR-26a-5p expression levels between cancer and normal hepatocellular cell lines
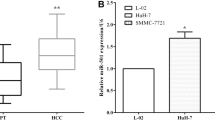
Using q-PCR, we detected the miR-26a-5p expression levels in multiple cell lines, including the normal human liver cell line L02 and several hepatoma cell lines. We found that miR-26a-5p expression was significantly lower in the hepatoma cell lines than in the normal hepatocellular cell line (Fig. 2a).
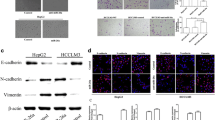
Expression and function of miR-26a-5p in vitro. a miR-26a-5p expression levels in HCC cell lines and a normal human liver cell line. b Cell number below the membrane of the transwell after transfection with miR-26a-5p-inhibitor, control-inhibitor and no inhibitor. c Images of cells transfected with miR-26a-5p-inhibitor, control-inhibitor and no inhibitor fixed on the lower layer of the membrane after invasion. d Difference in expression of vimentin and E-cadherin after transfection with miR-26a-5p-inhibitor and control-inhibitor in the HCCLM9 cell line tested by q-PCR. e Difference in expression of vimentin and E-cadherin after transfection of HCCLM9 cells with miR-26a-5p-inhibitor tested by western blot. *P < 0.05
Lower miR-26a-5p expression promotes tumor invasion and metastasis in vitro
We compared cell invasion between HCCLM9 cells transfected with miR-26a-5p-inhibitor and control-inhibitor (Table 3) in vitro using a transwell assay. The results showed that decreased miR-26a-5p expression increased cell invasion (Fig. 2c), and this finding was statistically significant (Fig. 2b).
miR-26a-5p regulated tumor cell invasion and metastasis in vitro by targeting epithelial-mesenchymal transition
The expression levels of vimentin and E-cadherin were compared between HCCLM9 cells transfected with miR-26a-5p-inhibitor, control-inhibitor, and no inhibitor by q-PCR (primers was presented in Table 3) and Western blot. We found that low expression of miR-26a-5p upregulated vimentin expression and downregulated E-cadherin expression (Fig. 2d, e).
miR-26a-5p regulated tumor metastasis in vivo and may influence prognosis
As mentioned above, we injected HCCLM9 cells stably transfected with miR-26a-5p-inhibitor into the tail veins of athymic mice to create an experimental mouse model and compared the survival rate and tumor metastasis among those mice with those among control nude mice injected with normal HCCLM9 cells. The results revealed that low expression of miR-26a-5p in vivo was associated with a poorer survival rate (Fig. 3c) and upregulated tumor metastasis in lung tissues; both of these findings were statistically significant (Fig. 3a, b).
Expression and function of miR-26a-5p in vivo. a Images of metastatic nodules from the lungs of nude mice stained with HE. Metastatic nodules are indicated by black arrows (scale bars 50 μm). b Metastatic nodule numbers in lung tissues of mice injected with HCCLM9 cells stably transfected with miRNA inhibitor compared with control. c Kaplan–Meier survival analysis of mice injected with HCCLM9 cells stably transfected with miRNA inhibitor compared with control. *P < 0.05
Discussion
In recent years, HCC diagnosis and treatment have greatly improved, resulting in an increased survival rate. However, HCC is still considered a high malignancy cancer in comparison to others cancer types. Currently, the molecular mechanisms underlying HCC carcinogenesis and tumor promotion are unknown. In general, tumor suppressor genes protect normal cells from converting into cancer cells. However, in cancer cells, these genes often have genetic mutations and aberrant epigenetic modifications [19–21]. Some miRNAs have been reported as tumor suppressors, but the roles of other miRNAs in cancer have not yet been identified. Currently, miRNA dysregulation is detectable in the early stages of liver tumorigenesis, and a number of studies have illustrated the relevance of aberrant miRNA expression in multiple aspects of hepatocarcinogenesis and HCC cell biology [22, 23]. Based on these facts, we believe that miRNAs play a very important role in HCC tumor promotion via molecular mechanisms. miR-26a-5p was previously demonstrated to be a tumor suppressor in some tissues [24–28], but its function and molecular mechanism in HCC were still unclear.
In this study, we demonstrated that miR-26a-5p expression is decreased in tumor tissues and HCC tumor cell lines compared to normal adjacent tissues (Fig. 1a, b) and normal liver cells (Fig. 2a), respectively. In regard to the clinical data analysis, we divided the 50 included patients into a high expression group (25 cases) and a low expression group (25 cases) according to the miR-26a-5p level in the tumor tissue. Based on the well-matched general data, we found that high miRNA-26a-5p level significantly increased the overall and recurrence-free survival rates (Fig. 1c). Furthermore, the results of the Cox regression analysis demonstrated that miR-26a-5p level is an independent prognostic indicator (P = 0.001, Table 2).
We transfected miR-26a-5p-inhibitor into HCCLM9 cells and examined its effect on epithelial-mesenchymal transition (EMT) regulation in vitro. The q-PCR and Western blot results revealed that miRNA-26a-5p influences EMT. The EMT is defined as follows: epithelial cells lose their polarity and cohesiveness and transform into spindle-shaped cells with a more fibroblast- or myofibroblast-like phenotype [29–33]. EMT and metastasis are generally considered late events in tumorigenesis, and acquiring a mesenchymal phenotype allows a malignant epithelial cell to detach from the primary tumor. Additionally, EMT has been shown to be critical in the early events of tumor cell metastatic dissemination, during which cells become more motile and exhibit invasive potential. EMT can also be reactivated in cancer to promote the tumorigenic progression of epithelial cells; for example, it can increase migration and invasion and inhibit apoptosis and senescence [34, 35]. As mentioned above, EMT is defined as the loss of epithelial phenotypes and cell polarity and the acquisition of a mesenchymal phenotype, which includes attenuation of E-cadherin, N-cadherin, vimentin, and b-catenin [36–38]. Vimentin plays a significant role in supporting and anchoring the position of the organelles in the cytosol, and the regulation of vimentin reduces the potential for tumor cells to migrate, suggesting that this protein influences the cellular invasive phenotype. Vimentin is considered one of the most significant markers of EMT, and its expression has been measured in a series of neoplasms [39–41]. E-cadherin has also been shown to be an EMT marker, and both E-cadherin and vimentin serve as prognostic markers [42]. In this study, we demonstrated that miR-26a-5p is strongly associated with vimentin and E-cadherin expression and is a potential biomarker that can be used to target EMT to act as a tumor suppressor (Fig. 2d, e). Additionally, an in vitro transwell assay (Fig. 2b, c) and in vivo (Fig. 3) nude mouse model demonstrated that downregulation of miR-26a-5p expression can promote tumor metastasis and indicate poor prognosis.
The results of our study allow us to propose the role and function of miR-26a-5p in HCC. First, miR-26a-5p was decreased in HCC tissues and cells. Although 12 tissues did not reveal downregulation (even four tissues exhibited upregulation), but most of the clinical samples (76%) are shown to be the same regulation trend as the cells. This may be related to the sensitivity and accuracy of the diagnosis in the population. In addition, the difference in the expression level of miR-26a-5p between tumor and normal tissues (Fig. 1b) indicates that miR-26a-5p is a potential biomarker for early HCC diagnosis. Second, the Cox regression analysis of the results of the nude mouse model experiments demonstrated that the miR-26a-5p expression level was an independent prognostic predictor for HCC. Moreover, the transwell assay and the analysis of lung tissue samples from nude mice provided supplementary evidence of the involvement of miR-26a-5p in tumor invasion and metastasis. These findings indicated that upregulation of miR-26a-5p may suppress tumor invasion and metastasis. Thus, miR-26a-5p could be a new therapeutic target for HCC. Finally, we determined that downregulation of miR-26a-5p increases vimentin expression and decreases E-cadherin expression. This finding could partially explain the molecular mechanism of miR-26a-5p in tumor metastasis, as E-cadherin and vimentin are both involved in EMT.
To the best of our knowledge, miR-26 family, especially miR-26b-5p, is reported as a potential biomarker and tumor suppressor in HCC [43, 44]. In this study, miR-26a-5p expression was strongly associated with EMT, which may be the molecular mechanism through which miR-26a-5p impacts tumor invasion and metastasis. Therefore, miR-26a-5p may be a new biomarker and therapeutic target for HCC. This study had several limitations. Because we only included 50 paired tissues in the clinical data analysis, the results of the clinical analysis may not be sufficiently robust to draw any conclusions. However, we compared miR-26a-5p expression in multiple cell lines and in an athymic mouse model to confirm the results of the clinical analysis. Furthermore, we demonstrated the miR-26a-5p regulates tumor invasion and metastasis by targeting EMT; however, the specific molecular mechanisms and pathways involved in this regulation are still unclear and need to be discovered in the future.
Despite several limitations of this study, our data strongly suggest that downregulation of miR-26a-5p targets EMT to promote tumor metastasis and influences the prognosis of HCC patients. miR-26a-5p is a new potential biomarker and therapeutic target for HCC.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13.
Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8.
Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314.
Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W, et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem. 2006;99:671–8.
Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, et al. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955–63.
Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY, Zhang JF, et al. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Res. 2010;70:9798–807.
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–58.
Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B, et al. miR-221 overexpression contributes to liver tumorigenesis. Proc Natl Acad Sci USA. 2010;107:264–9.
Anwar SL, Lehmann U. MicroRNAs: emerging novel clinical biomarkers for hepatocellular carcinomas. J Clin Med. 2015;4:1631–50.
Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;6:e23937.
Chen YP, Jin X, Xiang Z, Chen SH, Li YM. Circulating microRNAs as potential biomarkers for alcoholic steatohepatitis. Liver Int. 2013;33:1257–65.
Chen YJ, Zhu JM, Wu H, Fan J, Zhou J, Hu J, et al. Circulating microRNAs as a fingerprint for liver cirrhosis. PLoS One. 2013;8:e66577.
Shen G, Lin Y, Yang X, Zhang J, Xu Z, Jia H. MicroRNA-26b inhibits epithelial-mesenchymal transition in hepatocellular carcinoma by targeting USP9X. BMC Cancer. 2014;14:393.
Verghese ET, Drury R, Green CA, Holliday DL, Lu X, Nash C, et al. MiR-26b is down-regulated in carcinoma-associated fibroblasts from ER-positive breast cancers leading to enhanced cell migration and invasion. J Pathol. 2013;231:388–99.
Guo K, Zheng S, Xu Y, Xu A, Chen B, Wen Y. Loss of miR-26a-5p promotes proliferation, migration, and invasion in prostate cancer through negatively regulating SERBP1. Tumour Biol. 2016;37:12843–54.
Ghanbari R, Mosakhani N, Asadi J, Nouraee N, Mowla SJ, Yazdani Y, et al. Downregulation of plasma MiR-142-3p and MiR-26a-5p in patients with colorectal carcinoma. Iran J Cancer Prev. 2015;8:e2329.
Tan Y, Ge G, Pan T, Wen D, Chen L, Yu X, et al. A serum microRNA panel as potential biomarkers for hepatocellular carcinoma related with hepatitis B virus. PLoS One. 2014;9:e107986.
Floquet C, Deforges J, Rousset JP, Bidou L. Rescue of non-sense mutated p53 tumor suppressor gene by aminoglycosides. Nucleic Acids Res. 2011;39:3350–62.
Zhang J, Fan D, Jian Z, Chen GG, Lai PB. Cancer specific long noncoding RNAs show differential expression patterns and competing endogenous RNA potential in hepatocellular carcinoma. PLoS One. 2015;10:e141042.
Cerkevich TJ. Transactional analysis for the physician: stroking hunger and time structure. J Med Assoc State Ala. 1975;45:36–8.
Yang Z, Cappello T, Wang L. Emerging role of microRNAs in lipid metabolism. Acta Pharm Sin B. 2015;5:145–50.
Baffy G. MicroRNAs in Nonalcoholic Fatty Liver Disease. J Clin Med. 2015;4:1977–88.
Hromadnikova I, Kotlabova K, Hympanova L, Krofta L. Gestational hypertension, preeclampsia and intrauterine growth restriction induce dysregulation of cardiovascular and cerebrovascular disease associated microRNAs in maternal whole peripheral blood. Thromb Res. 2016;137:126–40.
Gasparini P, Cascione L, Landi L, Carasi S, Lovat F, Tibaldi C, et al. microRNA classifiers are powerful diagnostic/prognostic tools in ALK-, EGFR-, and KRAS-driven lung cancers. Proc Natl Acad Sci USA. 2015;112:14924–9.
Ghanbari R, Mosakhani N, Asadi J, Nouraee N, Mowla SJ, Yazdani Y, et al. Downregulation of plasma MiR-142-3p and MiR-26a-5p in patients with colorectal carcinoma. Iran J Cancer Prev. 2015;8:e2329.
Hromadnikova I, Kotlabova K, Hympanova L, Krofta L. Cardiovascular and cerebrovascular disease associated microRNAs are dysregulated in placental tissues affected with gestational hypertension, preeclampsia and intrauterine growth restriction. PLoS One. 2015;10:e138383.
Jiang W, Min J, Sui X, Qian Y, Liu Y, Liu Z, et al. MicroRNA-26a-5p and microRNA-23b-3p up-regulate peroxiredoxin III in acute myeloid leukemia. Leuk Lymphoma. 2015;56:460–71.
Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Investig. 2003;112:1776–84.
Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–83.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig. 2009;119:1420–8.
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Investig. 2009;119:1438–49.
Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Investig. 2009;119:1429–37.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90.
Sanchez-Tillo E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, Darling DS, et al. EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci. 2012;69:3429–56.
Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–26.
Vasko V, Espinosa AV, Scouten W, He H, Auer H, Liyanarachchi S, et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci USA. 2007;104:2803–8.
Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–66.
Wu Y, Zhou BP. New insights of epithelial-mesenchymal transition in cancer metastasis. Acta Biochim Biophys Sin (Shanghai). 2008;40:643–50.
Singh S, Sadacharan S, Su S, Belldegrun A, Persad S, Singh G. Overexpression of vimentin: role in the invasive phenotype in an androgen-independent model of prostate cancer. Cancer Res. 2003;63:2306–11.
Li M, Zhang B, Sun B, Wang X, Ban X, Sun T, et al. A novel function for vimentin: the potential biomarker for predicting melanoma hematogenous metastasis. J Exp Clin Cancer Res. 2010;29:109.
Masferrer E, Ferrandiz-Pulido C, Masferrer-Niubo M, Rodriguez-Rodriguez A, Gil I, Pont A, et al. Epithelial-to-mesenchymal transition in penile squamous cell carcinoma. J Urol. 2015;193:699–705.
Wang Y, Sun B, Zhao X, Zhao N, Sun R, Zhu D, et al. Twist1-related miR-26b-5p suppresses epithelial-mesenchymal transition, migration and invasion by targeting SMAD1 in hepatocellular carcinoma. Oncotarget. 2016;26:24383–401.
Wang Y, Sun B, Sun H, Zhao X, Wang X, Zhao N, et al. Regulation of proliferation, angiogenesis and apoptosis in hepatocellular carcinoma by miR-26b-5p. Tumour Biol. 2016;37:10965–79.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Ethical statement
This study was approved by the Research Ethics Committee of Zhongnan Hospital of Wuhan University. The study was conducted in full accordance with the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Chang, L., Li, K. & Guo, T. miR-26a-5p suppresses tumor metastasis by regulating EMT and is associated with prognosis in HCC. Clin Transl Oncol 19, 695–703 (2017). https://doi.org/10.1007/s12094-016-1582-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-016-1582-1