Abstract
The biological role of miR-26a involved in the carcinogenesis of prostate cancer (PC) has been controversial. Besides, the underlying mechanism by which miR-26a plays a role in PC has been unclear. To investigate the role of miR-26a-5p in the PC, miR-26a-5p was detected and statistically analyzed in clinical PC tissues and a panel of PC cell lines. Using bioinformatics analysis, we found that serpine1 messenger RNA (mRNA) binding protein 1 (SERBP1) was a potential downstream target of miR-26a-5p. Using luciferase reporter and western blot, we identified that miR-26a-5p negatively regulated SERBP1 on the PC cell line level. It was confirmed that miR-26a-5p was markedly downregulated in PC tissues compared with normal controls whose reduced expression was significantly associated with metastasis and poor overall prognosis and found that miR-26a-5p was able to prevent proliferation and motility of PC cells in vitro. Additionally, SERBP1 was identified as a downstream target of miR-26a-5p. Moreover, it was observed that SERBP1 was markedly upregulated in prostate cancer tissues and was significantly associated with tissue metastasis and Gleason score. Taken together, our results for the first time demonstrate that the loss of miR-26a-5p promotes proliferation, migration, and invasion through targeting SERBP1 in PC, supporting the tumor-suppressing role of miR-26a-5p in PC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PC) is the most common noncutaneous cancer and the fifth most common cancer affecting men of all ages in China [1]. PC, being increasing rapidly in metropolitan areas, becomes a major public health issue in China. The patients hospitalized were often diagnosed as advanced PC which was characterized by metastasis, the movement of cancer cells from the point of origin within the prostate gland to multiple distant organ sites throughout the body [2]. However, the underlying mechanism of metastasis of PC remains largely unknown.
It has been well-accepted that microRNAs (miRNAs) play a crucial role in the tumorigenesis in the way of either promotion or suppression of cancer, depending on the different tumor types [3]. miR-26a has been reported to be anti-oncogenic or plays a tumor-suppressing role in breast cancer [4–7], pancreatic cancer [3], nasopharyngeal carcinoma [8], bladder cancer [9], gastric cancer [10], and hepatocellular carcinoma [11]; however, with the exception of glioblastoma [12], lung cancer [13], and ovarian cancer [14] where miR-26a was reported to be oncogenic or play a tumor-promoting role, suggesting that the role of miR-26a was controversial in the context of different cancers. Even in the same type of PC, the role of miR-26a involved in the carcinogenesis was reported to be in contrast [15, 16]. Thus, the role of miR-26a in PC remains to be studied.
In the present study, to investigate the role of miR-26a-5p in PC, miR-26a-5p was detected and statistically analyzed in clinical PC tissues and a panel of PC cell lines. It was shown that miR-26a-5p was pronouncedly downregulated in PC tissues as compared with normal controls and significantly associated with metastasis and Gleason score of PC. Using bioinformatics analysis, we found that serpine1 messenger RNA (mRNA) binding protein 1 (SERBP1) was a potential downstream target of miR-26a-5p. Using luciferase reporter and western blot, we identified that miR-26a-5p negatively regulated SERBP1 on the PC cell line level. Furthermore, It was found that SERBP1 expression was not only significantly associated with metastasis but also with overall prognosis. Taken together, our results for the first time demonstrate that the loss of miR-26a-5p promotes proliferation, migration, and invasion through negatively regulating SERBP1 in the setting of prostate cancer.
Materials and methods
PC clinical tissues
One hundred forty pairs of fresh PC tissues and corresponding normal control tissues were retrieved and recruited from the Department of Urology collected from 2008 to 2015. The present study was approved by the Medical Ethics Committee of Zhujiang Hospital, Southern Medical University, and signed informed consent was obtained from each patient before undergoing prostatectomy. None of the recruited patients received chemo- or radio-therapeutic treatment before surgery, and clinicopathological information of all patients was available through retrieval of the hospital information system in Zhujiang Hospital. Representative hematoxylin and eosin (H&E)-stained slides from each patient were retrospectively reviewed blindly and separately by two pathologists. All fresh tissues were separately excised by experienced pathologists and were frozen in liquid nitrogen within 15 min after prostatectomy and stored at −80 °C until analysis.
PC cell lines and transfection
The human PC cell lines VCaP, 22RV1, LNCaP, and DU-145 as well as normal prostate cell lines PREC and RWPE were all obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Human embryonic kidney 293 cells, also often referred to as HEK293, were from the Gefanbio Company (Gefanbio, Shanghai, China). These cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum (FBS) and penicillin/streptomycin in a humidified incubator at 37 °C with an atmosphere of 5 % CO2, unless otherwise stated. Transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instruction. The stably transfectant VCaP cell line overexpressing SERBP1 was established using puromycin screen (800 ng/ml) for 4 weeks.
Plasmids
The plasmid pCMV-miR-26a-5p harboring the mature sequence of miR-26a-5p and its empty vector control was purchased from the OriGene Company (#SC400939, Rockville, MD, USA). pMirTarget-SERBP1-3′-UTR (#SC213902) harboring the full length of 3′-untranslated region (UTR) of homo SERBP1 (NM_014282) and its mutation control (PS100062) were also purchased from the OriGene Company. In order to establish the stable transgenic PC cell line whose endogenous miR-26a can be artificially changed, the full length of pre-miR-26a sequence was subcloned into the pcDNA6.2(−)-myc/his blank vector (Invitrogen, Carlsbad, CA, USA) and the Sgf I and Mlu I restriction sites present upstream and downstream of the pre-miR-26a-5p sequence in the pCMV-miR-26a expression vector were mutated to a BamHI and HindIII restriction site using the following PCR primers: forward: 5′-GGATCCGTGATATCACAAGGTCCCAG-3′ (BamHI) and reverse: 5′-AAGCTTCTACATGCAAAGGGCAGGAG-3′ (HindIII). A short hairpin RNA (shRNA) interference vector of miR-26a-5p was constructed by Genepharm Company (Shanghai, China). Specific small interfering RNA (siRNA) against SERBP1 was designed and synthesized also by Genepharm Company (Shanghai, China), and the detailed sequence is listed in Supplementary Table 1.
RNA extraction and quantitative real-time reverse transcription PCR (qRT-PCR)
Total RNA of the cultured cells was extracted using a TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The quality and quantity of RNA was determined using a NanoDrop® ND-1000 spectrophotometer (Thermal Fisher, Wilmington, DE, USA). Reverse transcription (RT) was performed using random primers of the SuperScript III First-Strand Synthesis SuperMix kit (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s protocols. All samples were with SYBR Green PCR Master Mix (Takara, Dalian, China). U6 expression was used for normalization, and miR-26a-5p relative gene expression was determined by the comparative delta-delta CT method (2−ΔΔCt) using IQ5 software. The expression of miR-26a-5p was normalized with U6. Both the primers involved were designed and synthesized by Shangon Company (Shanghai, China). The miR-26a-5p RT primer was 5′-GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GATACGACAGCCTA-3′, the miR-26a-5p forward primer was 5′-GCG GCG GTT CAA GTA ATC CAGG-3′, and the miR-26a-5p reverse primer was 5′-ATC CAG TGC AGG GTC CGA GG-3′.
Cell viability assay
Cell viability was examined by the methylthiazolyl blue tetrazolium (MTT) assay (Shangon, Shanghai, China), according to the standard protocol after transfection for 24, 48, 72, and 96 h. PC cells were plated in 96-well plates at a density of 5 × 103 cells per well. After transfection, cell proliferation was dynamically monitored using a spectrometer at every 24 h. Cells were incubated for 4 h with 20 μl MTT at 37 °C. The color was developed by incubating the cells in 150 μl dimethyl sulfoxide (DMSO); the absorbance was detected at 490 nm wavelength. The data were obtained from three independent experiments.
Clonogenic assay
VCaP and LNCaP cells were plated to medium plates at a confluence of 3 × 105 cells and transfected for 48 h. The cells were then trypsinized, re-suspended in the media, and counted. The cells were re-seeded (500 cells per medium plate) and incubated for 10 days. Fresh media were added on the fifth day. On the tenth day, the media were removed from the dishes and washed once with ice-cold PBS. The colonies were stained with 0.1 % (w/v) crystal violet for 20 min on a rocking platform. The dishes were rinsed three times with PBS and air-dried, and the colonies were counted.
Migration and invasion assays in vitro
Cell migration ability was calculated by the wound healing assay. PC cells were plated in a six-well plate at a concentration of 4 × 105 cells/well and allowed to form a confluent monolayer for 24 h. After transfection, the monolayer was scratched with a sterile pipette tip (10 μl), washed with a serum-free medium to remove floated and detached cells, and photographed (time 0 and 48 h) by an inversion fluorescence microscope (Olympus, Japan). Cell culture inserts (24-well, pore size 8 μm; BD Biosciences) were seeded with 5 × 103 cells in 100 μl of the medium with 0.1 % FBS. Inserts pre-coated with Matrigel (40 μl, 1 mg/ml; BD Biosciences) were used for invasion assays. The medium with 10 % FBS (400 μl) was added to the lower chamber and served as a chemotactic agent. Noninvasive cells were wiped from the upper side of the membrane, and cells on the lower side were fixed in cold methanol (−20 °C) and air-dried. Cells were stained with 0.1 % crystal violet (dissolved in methanol) and counted using the inverted microscope. Each individual experiment had triplicate inserts, and four microscopic fields were counted per insert.
Western blotting
Cell lysates were prepared in RIPA lysis buffer (BioTeke, Beijing, China). An equal amount of total cell lysates was separated by 10 % SDS-PAGE and transferred to NC membranes incubated with primary antibodies against SERBP1 (sc-367) and GAPDH (sc-25778) overnight, which were from Santa Cruz Biotechnology (CA, USA). Membranes were subsequently probed with AP-conjugated secondary antibodies, and the blots were visualized with a Western Breeze Kit (WB7105, Invitrogen Life Technologies, CA, USA).
Immunohistochemistry (IHC)
Hematoxylin and eosin-stained slides and unstained slides for immunohistochemical analysis were prepared from formalin-fixed, paraffin-embedded blocks of CRC tissues. Immunohistochemical stains were performed using heat-induced epitope retrieval, an avidin-biotin complex method. The rabbit anti-SERBP1 antibody (sc-367, Santa Cruz Biotechnology, CA, USA) was diluted with 1:100. The sections were evaluated by light microscopic examination, and cellular localization of the protein and immunostaining level in each section was assessed by two pathologists. The staining was scored as follows: negative, weak (less than 15 % of cells with positive staining), medium (more than 30 % but less than 60 % of cells with positive staining), and strong (more than 60 % of cells with positive staining) according to the signal intensity. Both negative and weak immunostaining were defined as low expression, whereas moderate and strong staining were categorized into high expression.
Luciferase reporter assay
HEK293 cells were maintained in a 24-well plate and co-transfected with pCMV-miR-26a-5p, pMirTarget-SERBP1-3′-UTR, or its mutation 3′-UTR of SERBP1 control as well as empty vector. Forty-eight hours later, cell lysates were prepared and the relative dual-luciferase activity was examined with the Dual-Luciferase Reporter Assay System (Promega, Wisconsin, WI, USA).
Statistics
Data were expressed as mean ± SD and were analyzed by two-tailed independent sample Student’s t test, one-way ANOVA, and chi-square test using SPSS for Windows version 17.0 (SPSS, Chicago, USA). Kaplan-Meier survival curves were plotted, and log rank test was done. Graphs were carried out with Prism 5.0 software (GraphPad Software, San Diego, CA, USA). P value <0.05 was defined as statistically significant in comparison with the paired control group (*p < 0.05; **p < 0.01; ***p < 0.001).
Results
miR-26a-5p was significantly expressed to be lower in PC tissues compared with controls
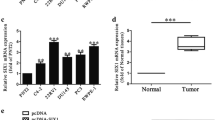
Firstly, to investigate the expression of miR-26a-5p in PC (tumor, abbreviated as T) and paired normal control (normal, abbreviated as N), miR-26a-5p was detected using qRT-PCR technique in 140 cases of PC and its paired normal control tissues. The clinicopathological significance of miR-26a-5p expression in 140 cases is shown in Table 1. As determined by qRT-PCR, the miR-26a-5p expression was pronouncedly lower in the PC tissues than in matched normal control tissues (p < 0.001) (Fig. 1a). The T/N ratios of miR-26a-5p expression were found to be statistically related to the angiolymphatic invasion, lymph node metastasis, and clinical stage (Table 1). The tumors with advanced clinical stage, with T3–4 grade, or with lymph node metastasis expressed lower levels of miR-26a-5p compared with controls. In addition, miR-26a-5p expression was significantly correlated with the invasion degree of four different kinds of PC cell lines in vitro (VCaP, 22RV1, LNCaP, and DU-145) (Fig. 1b).
miR-26a-5p was pronouncedly downregulated in PC tissues as well as PC cell lines compared with normal controls. a The basal expression of miR-26a-5p was detected using quantitative real-time reverse transcription PCR (qRT-PCR) in 140 paired HCC tissues and its paired normal controls. Total RNA was extracted using a TRIzol reagent followed by reverse transcription into cDNA. The relative expression of miR-26a-5p, normalized to U6, was calculated using the formula 2−ΔΔCt (relative expression). ***p < 0.001, in comparison with normal control; b In the same way, the expression of miR-26a-5p was detected using qRT-PCR as did on clinical tissues in six different kinds of PC cell lines involved. *p < 0.05; **p < 0.01, versus PREC
Loss of miR-26a-5p promotes the proliferation, migration, and invasion of PC cells
To investigate the role of miR-26a-5p on the cell growth of PC cells, we performed MTT and subsequently confirmed it by clonogenic assay. Based on the basal expression of miR-26a-5p in vitro PC cell lines, VCaP and LNCaP cell lines were chosen as two extremes. The VCaP cell line was transfected with anti-miR-26a-5p vectors, and the LNCaP cell line was transfected with miR-26a-5p overexpression vectors. It can be seen that knockdown of miR-26a-5p can significantly promote proliferation (Fig. 2a) and clonogenic ability (Fig. 2b) in VCaP cells whereas re-expression of miR-26a-5p can suppress proliferation (Fig. 2b) and clonogenic ability (Fig. 2d) in LNCaP cells compared to the control group. In addition, the effects of miR-26a-5p on cell migration and invasion abilities of PC cell lines were examined by the wound healing assay and Transwell assays in vitro, respectively. Inhibition of miR-26a-5p was able to markedly promote migration (Fig. 2b) and invasion (Fig. 2c) in VCaP cells whose miR-26a expression level was comparatively higher than that of other five kinds of PC cell lines. Likewise, re-expression of miR-26a-5p can significantly suppress migration (Fig. 2b) and invasion (Fig. 2c) in LNCaP cells whose miR-26a expression level was comparatively lower than that of other five PC cell lines involved. All the results we obtained in this section suggested that miR-26a-5p could suppress the proliferation, migration, and invasion of PC cells in vitro.
Re-expression of miR-26a-5p was able to markedly prevent the proliferation and motility of PC cells. a Proliferative variation was assayed using the MTT approach in VCaP and LNCaP cell lines which were transfected with pcDNA6.2-sh-miR-26a-5p and pcDNA6.2-miR-26a-5p vectors, respectively, for 0, 24, 48, 72, and 96 h. b Migratory variation was evaluated by the wound healing assay in VCaP and LNCaP cell lines which were transfected with pcDNA6.2-sh-miR-26a-5p and pcDNA6.2-miR-26a-5p vectors, respectively, for 24 h. c Similarly, invasive variation was detected using the Transwell assay in VCaP and LNCaP cell lines which were transfected with pcDNA6.2-sh-miR-26a-5p and pcDNA6.2-miR-26a-5p vectors, respectively, for 72 h. d Clonogenic assay of VCaP and LNCaP cells after transfection with pcDNA6.2-sh-miR-26a-5p and pcDNA6.2-miR-26a-5p. The images of migratory cells from the scratched boundary were observed and acquired with a light microscope (×100), whereas the images of Transwell chamber were taken with a light microscope (×400). Similar results were obtained from three independent experiments, and shown are representative figures. **p < 0.01; ***p < 0.001, compared with the control group
SERBP1 was a direct downstream target of miR-26a-5p and can be negatively regulated by miR-26a-5p
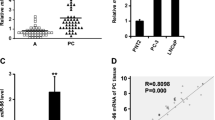
To explore the underlying mechanism through which miR-26a-5p played a role in the suppression of proliferation, migration, and invasion of PC cells in vitro, we were in an attempt to unravel it from the angle of miRNA-mRNA regulation and control, which was the classical working mode of miRNA. Therefore, to identify the potential downstream target of miR-26a-5p, TargetScan 6.2 was used to search the possible target of miR-26a-5p. It was discovered that serpine1 mRNA binding protein 1, abbreviated as SERBP1, contained potential binding sites of miR-26a-5p (Fig. 3a). Luciferase activity assay showed that miR-26a-5p significantly inhibited luciferase activity of the wild-type (WT) 3′-UTR of SERBP1 but not mutation (MUT) 3′-UTR of SERBP1 or empty vector control (Fig. 3b). Either re-expression or knockdown of miR-26a-5p can significantly decrease or increase the SERBP1 expression level (Fig. 3c) on the PC cell line level. Furthermore, we have also detected the endogenous expression of SERBP1, and it was found that SERBP1 expression increased with the increasing invasive ability of PC cell lines (Fig. 3d), which was in stark contrast with miR-26a-5p expression.
SERBP1 was negatively regulated by miR-26a-5p. a Conserved seed sequence of miR-26a-5p in common mammalian animals other than mice and rat. Bioinformatic prediction using TargetScan 6.2 (http://www.targetscan.org/): SERBP1-3′-UTR-containing mature seed sequence of miR-26a-5p. wt wild type, mt mutation type. b Putative regulation of miR-26a-5p over SERBP1 was confirmed using the luciferase reporter assay in HEK293 cells. HEK293 cells were co-transfected with wild-type (WT) reporter plasmid, mutation (MUT) reporter plasmid and empty vector, as well as pCMV-miR-26a-5p plasmid, followed by relative fluorescent intensity examined with the Dual-Luciferase Reporter Assay System (Promega, Wisconsin, WI, USA). c Expression of SERBP1 was qualitatively and semi-quantitatively analyzed by western blot after miR-26a-5p being overexpressed and downregulated in VCaP and LNCaP cells, respectively. d Basal protein level of SERBP1 in PREC, RWPE, VCaP, 22RV1, LNCaP, and DU-145 was qualitatively and semi-quantitatively determined by western blot. GAPDH was used as the internal control, and representative figures from triple independent experiments are shown here. *stands for p < 0.05, **means p < 0.01
SERBP1 expression was remarkably upregulated in PC tissues in comparison with paired normal control
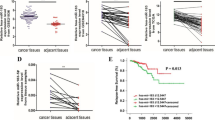
Having discovered that SERBP1 was a putative downstream target of miR-26a-5p, with the help of bioinformatics prediction and luciferase reporter assay, we were determined to investigate the level of SERBP1 expression in PC as well as its paired normal control tissues. SERBP1 was detected using an IHC approach (Fig. 4a), and it can be seen that the expression of SERBP1 was highly heterogeneous, with its expression being strong positive, moderate positive, and weak positive and some cases being hardly detectable in PC tissues. Whereas weak positive immunostaining of SERBP1 was commonly seen in the majority of paired normal control tissues, with a few being moderate positive staining. In terms of sublocalization of SERBP1, immunostaining of SERBP1 was both membranous and cytoplasmic in PC and normal control cells. After the IHC experiment was completed, we have carried out a statistical analysis of SERBP1 expression, finding that SERBP1 was significantly higher in PC tissues than in paired normal control tissues (Table 2).
SERBP1 as well as miR-26a-5p were markedly associated with overall prognosis in patients with PC. a The expression of SERBP1 detected using an IHC method in PC tissues. SERBP1 was highly heterogeneous with its expression status being strong (scored as +++), moderate (scored as ++), weak (scored as +), and negative (scored as none). The immunostaining of SERBP1 was mainly membranous and cytoplasmic, whatever in cancerous or normal prostate tissues. Kaplan-Meier survival curve of b SERBP1 expression and c miR-26a expression. Of note, the cutoff value was the half of the mean expression (0.058) of miR-26a in PC tissues, above which (>0.029) was defined as higher expression whereas below (<0.029) as low expression. The log rank test was used in the analysis of Kaplan-Meier survival curve using SPSS 17.0 software
SERBP1 was pronouncedly associated with metastasis and poor overall prognosis of PC
To understand the clinicopathological significance of SERBP1 expression, we have carried out the cross-table statistical analysis. It was found that SERBP1 expression level was not only significantly associated with metastasis but also associated with Gleason score (Table 2). Subsequently, to observe the overall prognostic significance of SERBP1 expression, Kaplan-Meier survival curve was performed. It was shown that there was a remarkably significant difference between patients with high SERBP1 expression and patients with low SERBP1 expression (Fig. 4b). Furthermore, we have also performed the Kaplan-Meier survival curve regarding miR-26a-5p, and it was shown that there was also a significant difference between patients with high expression of miR-26a-5p and patients with low miR-26a-5p (Fig. 4c), indicating that both miR-26a-5p and SERBP1 could be used as a prognostic biomarker for the overall prognosis of patients with PC. In addition, given that many factors involved were pronouncedly associated with SERBP1 expression, to evaluate the weight of clinicopathological factors involved as well as SERBP1 expression in prognosis, we have performed both univariate and multivariate survival analyses (Table 3). Univariate Cox regression analysis showed that SERBP1 expression (p = 0.023), N classification (p = 0.017), angiolymphatic invasion (p = 0.008), and Gleason score (p = 0.006) were prognostic predicators for patients with PC. By using multivariate analysis, we further stringently examined prognostic parameters of PC that were found to be significant in univariate analysis. It can be seen that SERBP1 (p = 0.045) and Gleason score (p = 0.032) were independent prognostic factors influencing the 5-year overall survival, indicating that SERBP1 expression can be used as an independent prognostic predictor for PC.
SERBP1 negatively regulated by miR-26a-5p was able to promote the proliferation of PC cells in vivo
Having found that miR-26a-5p was capable of negatively regulating the SERBP1 expression, we have next wondered whether SERBP1 could play a role in the proliferation of PC cells in vivo. Based on the basal expression level of SERBP1 in the panel of PC cells involved (Fig. 3d), we have established the stable transfectant PC cells using the VCaP cell line whose SERBP1 was artificially overexpressed (Fig. 5a). Then, proliferative variation was evaluated in vitro after transfection with pcDNA6.2-SERBP1 into VCaP cells. It was shown that proliferation of VCaP cells transfected with pcDNA6.2-SERBP1 was markedly increased relative to control group (Fig. 5b). Moreover, to further observe the effect of overproliferation in vivo, we have generated the xenografted nude mice model with the stably transfectant VCaP cell line, finding that SERBP1 was remarkably able to promote the proliferation of VCaP cells in vivo (Fig. 5d, e). Meanwhile, we have also evaluated the influence of overproliferation by miR-26a-5p in vivo using nude mice xenografted with stably transfectant LNCaP and VCaP cell lines whose endogenous miR-26a-5p was downregulated or upregulated, respectively, finding that miR-26a-5p can significantly suppress the proliferation of PC cells in vivo (Fig. 5c, f).
SERBP1 negatively regulated by miR-26a-5p was found to be markedly able to promote the proliferation of PC cells in vivo. a PC cells stably overexpressing SERBP1 were established with success in the VCaP cell line, as exemplified by western blot. b Proliferative variation was evaluated using the MTT approach in two different stable cell line subclones of VCaP, termed as 1# and 2#, and the control cell line was transfected with pcDNA6.2. c Quantitative assay of tumor volume from nude mice xenografted with LNCaP cells transfected with pcDNA6.2 and pcDNA6.2-miR-26a-5p, with each group having seven nude mice. d Representative tumor lesions dissected from nude mice xenografted with VCaP cells transfected with pcDNA6.2 and pcDNA6.2-SERBP1. e Quantitative assay of tumor weight dissected from nude mice xenografted with VCaP cells transfected with pcDNA6.2 and pcDNA6.2-SERBP1. f Tumor lesions dissected from nude mice xenografted with LNCaP cells whose basal miR-26a-5p was upregulated or not and VCaP cells whose miR-26a-5p was significantly downregulated. ***p < 0.001, in comparison with the control group using two-tailed independent sample Student’s t test
SERBP1 has capacity for promoting the migration and invasion of PC cells in vitro
Subsequent to the observation that artificial upregulation of SERBP1 can promote the proliferation of VCaP cells whose basal level of SERBP1 was hardly detectable, we continued to explore the effect exerted by the upregulation of SERBP1 over migration and invasion in VCaP cells. It can be found that the overexpression of SERBP1 can remarkably promote the migration and invasion of VCaP cells (Fig. 6a, b). To further verify, transient knockdown using siRNA was employed. On the basis of successful knockdown of SERBP1, as exemplified by western blot (Fig. 6c), the effects of overproliferation, invasion, and migration were evaluated using MTT, Transwell, and wound healing assays, respectively, in LNCaP and VCaP cells. It was observed that transient knockdown of SERBP1 was pronouncedly able to suppress the proliferation (Fig. 6d), invasion (Fig. 6e), and migration (Fig. 6f) of LNCaP and VCaP cells. All the results obtained in this section suggested that SERBP1 was capable of promoting proliferation, migration, and invasion of PC cells in vitro.
SERBP1 has the capacity for promoting the migration and invasion of PC cells in vitro. a Upregulation of SERBP1 was observed to be capable of promoting the migration of VCaP cells stably overexpressing SERBP1 with two different subclonal cells (named as SERBP1-1# and SERBP1-2#, respectively. b Similarly, the upregulation of SERBP1 could also enhance the invasive ability of VCaP cells stably overexpressing SERBP1 using two different subclones of cells. c The transient knockdown effect of specific siRNA against SERBP1 in LNCaP and VCaP cells, as exemplified by western blot. d Knockdown of SERBP1 can suppress the proliferation of PC cells in LNCaP and VCaP cells, as shown using the MTT method. e Knockdown of SERBP1 was able to inhibit the migration of PC cells in LNCaP and VCaP cells using the Transwell assay. f Silencing of SERBP1 can also prevent the migration of PC cells using the wound healing assay. All the experiments were carried out independently in triplicate, and representative figures are shown here. The two-tailed independent sample T test was employed when dealing with the statistical difference between two different groups. **p < 0.01; *p < 0.05, in comparison with the control group
Discussion
In the present study, it was found that miR-26a-5p was significantly decreased in PC tissues in comparison with normal controls and that miR-26a-5p expression level was significantly associated with metastasis and invasion. SERBP1 was downstream target of and negatively regulated by miR-26a-5p and whose expression was not only significantly with metastasis but also with poor overall prognosis. Therefore, our results supported the tumor-suppressing role of miR-26a-5p in the setting of PC, suggesting that re-expression of miR-26a-5p could be used as a potential therapeutic strategy for the suppression of metastasis of PC.
Despite the great advancement of clinical practice in the management of patients with advanced PC [17], patients hospitalized in effect benefited little from chemo- or radiotherapy and had a poor life quality and dismal prognosis, owing to metastasis that is a typical characterization and mortality reason for advanced PC [18]. Our experimental results provided an important and possible therapeutic strategy, indicating that miR-26a-5p could be used as a potential therapeutic target for patients with PC. As re-expression of miR-26a-5p in vitro was found to be able to effectively prevent the proliferation and motility of PC cell lines through negatively regulating SERBP1 that was defined as an oncoprotein in the present study.
The role of miR-26a involved in the carcinogenesis has been controversial and seems to be different in different types of cancers. miR-26a has been extensively reported to play an anti-oncogenic role in breast carcinoma [4–7], colorectal cancer [19], and liver cancer [20, 21], where miR-26a was found to be able to suppress the metastasis and growth of cancer cells, which was totally in agreement with our observations in PC cell lines in terms of inhibition of growth and motility. In addition, some studies carried out previously in the background of PC [22–25], where miR-26a was screened using microchip, have discovered that miR-26a was one of the significantly downregulated PC-related miRNAs in PC tissues [24], which was fully congruent with our findings on PC tissue level and was suggestive of its putative tumor-suppressing role in PC. As for the reason why miR-26a was commonly observed to be downregulated in PC, it remains unknown in the present study that deserves to be further investigated. But, it is highly likely to be owing to the fact that the promoter of miR-26a was found to be hypermethylated in prostate cancer [26] and breast cancer [27, 28]. Based on the previous relevant reports, hypermethylation of the promoter of miR-26a could result in the remarkably downregulation on the mRNA level of miR-26a, possibly accounting for the reason why miR-26a was commonly observed as downregulated or even diminished in PC cancer tissues in comparison with paired normal control. However, Tian and associates [15] found that miR-26a in combination with miR-19b, miR-23b, and miR-92a could promote the proliferation of prostate cancer cells by co-regulating the expression of PTEN, PI3K/Akt pathway, and cyclin D1 in vitro, indicating that miR-26a was tumor-promoting, which was in stark contrast with our observations in PC cell lines in vitro that miR-26a alone was found to be capable of preventing the proliferation of PC cells. Consider that the same hairpin RNA structure may give birth to mature products from each strand, termed miR-5p and miR-3p, respectively [29–31], which can bind different mRNAs, thereby leading to different working patterns despite from the same hairpin RNA. Moreover, the majority of previous literatures regarding miR-26a in the setting of cancer did not clearly state whether miR-26a-3p or miR-26a-5p was involved. This therefore may in part account for the discrepant working patterns of the same miR-26a in the same type of PC. The underlying reason why the conflicting phenotype of miR-26a in the same type of cancer remains to be further studied. In our study, we have just focused on the role mediated by miR-26a-5p in PC cells, whereas miR-26a-3p has not been paid attention. Therefore, whether the roles mediated by miR-26a-5p and miR-26a-3p, respectively, are the same or not remains unknown that left to be further investigated in the following.
In our study, we for the first time found that the loss of miR-26a promotes proliferation, migration, and invasion through targeting SERBP1, with the exception of several working targets and signaling pathways mentioned and found previously in PC [15, 16, 24]. The original report regarding SERBP1 involved in the setting of cancer emerged in epithelial ovarian cancer (EOC) [32]; then it was extended in breast cancer [33] and colon cancer [34]. Until now, there have been few reports regarding SERBP1 expression in prostate cancer. In our study, we found that SERBP1 was significantly higher in PC tissues compared with normal controls, which was inconsistent with the observation made by Serce et al.’s report [33] in which SERBP1 was not differentially expressed in breast carcinoma relative to normal breast tissue, at both the mRNA and protein levels. Furthermore, in terms with prognosis, our result was wholly in disagreement with Serce et al.’s finding in breast carcinoma that the overexpression of SERBP1 was pronouncedly associated with better overall prognosis. Besides, we have also found that the expression of SERBP1 was also markedly correlated with metastasis and invasion after clinicopathological analysis, which was in full agreement with the observation made by Koensgen and colleagues [32] in EOC. In our study, not only did we find that SERBP1 expression was significantly associated with lymph node metastases and Gleason score but also we found that SERBP1 expression can be used as an independent prognostic indicator for patients with PC through multivariate Cox regression analysis. The limited cases of clinical samples enrolled [35] and different primary antibodies used [36] against SERBP1 between our current study and earlier relevant studies may account for the probability resulting in the disagreement between our study and previous other’s in the case of prognostic relevance of SERBP1 expression. Furthermore, in vitro PC cell lines, we have discovered that SERBP1 has the capacity for promoting both the proliferation and motility of PC cancer cells, which was in stark contrast with one previous report conducted by Costa et al. [34] in HEK293 T cells in vitro that SERBP1 could inhibit the growth of HEK293 T cells. Besides there having been no more relevant evidence concerning the role mediated by SERBP1 in the malignant behavior of cancer cells, the role the SERBP1 played in prostate cancer cells remains to be further studied and confirmed. With regard to the underlying mechanism through which SERBP1 worked in cancer cells, it remains unknown but one recent study [37] found that SERBP1 could be in complex with liver receptor homolog-1 (LRH1) in the regulation of pathways implicated in cancer.
Taken together, to our knowledge, this is the first report that the loss of miR-26a promotes proliferation, migration, and invasion through targeting SERBP1 in PC, supporting the tumor-suppressing role of miR-26a in PC.
References
Hu Y, Zhao Q, Rao J, Deng H, Yuan H, Xu B. Longitudinal trends in prostate cancer incidence, mortality, and survival of patients from two Shanghai City districts: a retrospective population-based cohort study, 2000-2009. BMC Public Health. 2014;14(1):356.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Deng J, He M, Chen L, Chen C, Zheng J, Cai Z. The loss of miR-26a-mediated post-transcriptional regulation of cyclin E2 in pancreatic cancer cell proliferation and decreased patient survival. PLoS One. 2013;8(10):e76450.
Gao J, Li L, Wu M, Liu M, Xie X, Guo J, Tang H, Xie X. MiR-26a inhibits proliferation and migration of breast cancer through repression of MCL-1. PLoS One. 2013;8(6):e65138.
Ichikawa T, Sato F, Terasawa K, Tsuchiya S, Toi M, Tsujimoto G, Shimizu K. Trastuzumab produces therapeutic actions by upregulating miR-26a and miR-30b in breast cancer cells. PLoS One. 2012;7(2):e31422.
Jansen MP, Reijm EA, Sieuwerts AM, Ruigrok-Ritstier K, Look MP, Rodriguez-Gonzalez FG, Heine AA, Martens JW, Sleijfer S, Foekens JA, et al. High miR-26a and low CDC2 levels associate with decreased EZH2 expression and with favorable outcome on tamoxifen in metastatic breast cancer. Breast Cancer Res Treat. 2012;133(3):937–47.
Zhang B, Liu XX, He JR, Zhou CX, Guo M, He M, Li MF, Chen GQ, Zhao Q. Pathologically decreased miR-26a antagonizes apoptosis and facilitates carcinogenesis by targeting MTDH and EZH2 in breast cancer. Carcinogenesis. 2011;32(1):2–9.
Yu L, Lu J, Zhang B, Liu X, Wang L, Li SY, Peng XH, Xu X, Tian WD, Li XP. miR-26a inhibits invasion and metastasis of nasopharyngeal cancer by targeting EZH2. Oncol Lett. 2013;5(4):1223–8.
Lin Y, Chen H, Hu Z, Mao Y, Xu X, Zhu Y, Wu J, Li S, Mao Q, Zheng X, et al. miR-26a inhibits proliferation and motility in bladder cancer by targeting HMGA1. FEBS Lett. 2013;587(15):2467–73.
Deng M, Tang HL, XH L, Liu MY, XM L, YX G, Liu JF, He ZM. miR-26a suppresses tumor growth and metastasis by targeting FGF9 in gastric cancer. PLoS One. 2013;8(8):e72662.
Zhang Y, Zhang B, Zhang A, Li X, Liu J, Zhao J, Zhao Y, Gao J, Fang D, Rao Z. IL-6 upregulation contributes to the reduction of miR-26a expression in hepatocellular carcinoma cells. Braz J Med Biol Res. 2013;46(1):32–8.
Guo P, Lan J, Ge J, Nie Q, Guo L, Qiu Y, Mao Q. MiR-26a enhances the radiosensitivity of glioblastoma multiforme cells through targeting of ataxia-telangiectasia mutated. Exp Cell Res. 2014;320(2):200–8.
Liu B, Wu X, Wang C, Liu Y, Zhou Q, Xu K. MiR-26a enhances metastasis potential of lung cancer cells via AKT pathway by targeting PTEN. Biochim Biophys Acta. 2012;1822(11):1692–704.
Shen W, Song M, Liu J, Qiu G, Li T, Hu Y, Liu H. MiR-26a promotes ovarian cancer proliferation and tumorigenesis. PLoS One. 2014;9(1):e86871.
Tian L, Fang YX, Xue JL, Chen JZ. Four microRNAs promote prostate cell proliferation with regulation of PTEN and its downstream signals in vitro. PLoS One. 2013;8(9):e75885.
Zhao S, Ye X, Xiao L, Lian X, Feng Y, Li F, Li L. MiR-26a inhibits prostate cancer progression by repression of Wnt5a. Tumour Biol. 2014;35(10):9725–33.
Rodriguez-Antolin A, Gomez-Veiga F, Alvarez-Osorio JK, Carballido-Rodriguez J, Palou-Redorta J, Solsona-Narbon E, Sanchez-Sanchez E, Unda M. Factors that predict the development of bone metastases due to prostate cancer: recommendations for follow-up and therapeutic options. Actas Urol Esp. 2014;38(4):263–9.
Rove KO, Crawford ED. Evolution of treatment options for patients with CRPC and bone metastases: bone-targeted agents that go beyond palliation of symptoms to improve overall survival. Oncology (Williston Park). 2011;25(14):1362–70 .1375-1381, 1387
Konishi H, Fujiya M, Ueno N, Moriichi K, Sasajima J, Ikuta K, Tanabe H, Tanaka H, Kohgo Y. MicroRNA-26a and -584 inhibit the colorectal cancer progression through inhibition of the binding of hnRNP A1-CDK6 mRNA. Biochem Biophys Res Commun. 2015;467(4):847–52.
Zhang X, Cheng SL, Bian K, Wang L, Zhang X, Yan B, Jia LT, Zhao J, Gammoh N, Yang AG, et al. MicroRNA-26a promotes anoikis in human hepatocellular carcinoma cells by targeting alpha5 integrin. Oncotarget. 2015;6(4):2277–89.
Ma DN, Chai ZT, Zhu XD, Zhang N, Zhan DH, Ye BG, Wang CH, Qin CD, Zhao YM, Zhu WP, et al. MicroRNA-26a suppresses epithelial-mesenchymal transition in human hepatocellular carcinoma by repressing enhancer of zeste homolog 2. J Hematol Oncol. 2016;9(1):1.
Erdmann K, Kaulke K, Thomae C, Huebner D, Sergon M, Froehner M, Wirth MP, Fuessel S. Elevated expression of prostate cancer-associated genes is linked to down-regulation of microRNAs. BMC Cancer. 2014;14:82.
Darshan M, Zheng Q, Fedor HL, Wyhs N, Yegnasubramanian S, Lee P, Melamed J, Netto GJ, Trock BJ, De Marzo AM, et al. Biobanking of derivatives from radical retropubic and robot-assisted laparoscopic prostatectomy tissues as part of the prostate cancer biorepository network. Prostate. 2014;74(1):61–9.
Song H, Liu Y, Pan J, Zhao ST. Expression profile analysis reveals putative prostate cancer-related microRNAs. Genet Mol Res. 2013;12(4):4934–43.
Mahn R, Heukamp LC, Rogenhofer S, von Ruecker A, Muller SC, Ellinger J. Circulating microRNAs (miRNA) in serum of patients with prostate cancer. Urology. 2011;77(5):1265 .e1269-1216
Borno ST, Fischer A, Kerick M, Falth M, Laible M, Brase JC, Kuner R, Dahl A, Grimm C, Sayanjali B, et al. Genome-wide DNA methylation events in TMPRSS2-ERG fusion-negative prostate cancers implicate an EZH2-dependent mechanism with miR-26a hypermethylation. Cancer Discov. 2012;2(11):1024–35.
Sandhu R, Rivenbark AG, Mackler RM, Livasy CA, Coleman WB. Dysregulation of microRNA expression drives aberrant DNA hypermethylation in basal-like breast cancer. Int J Oncol. 2014;44(2):563–72.
Sandhu R, Rivenbark AG, Coleman WB. Loss of post-transcriptional regulation of DNMT3b by microRNAs: a possible molecular mechanism for the hypermethylation defect observed in a subset of breast cancer cell lines. Int J Oncol. 2012;41(2):721–32.
Zhou C, Lu Y, Li X. miR-339-3p inhibits proliferation and metastasis of colorectal cancer. Oncol Lett. 2015;10(5):2842–8.
Guo L, Yu J, Yu H, Zhao Y, Chen S, Xu C, Chen F. Evolutionary and expression analysis of miR-#-5p and miR-#-3p at the miRNAs/isomiRs levels. BioMed Res Int. 2015;2015:168358.
Guo L, Zhang H, Zhao Y, Yang S, Chen F. Selected isomiR expression profiles via arm switching? Gene. 2014;533(1):149–55.
Koensgen D, Mustea A, Klaman I, Sun P, Zafrakas M, Lichtenegger W, Denkert C, Dahl E, Sehouli J. Expression analysis and RNA localization of PAI-RBP1 (SERBP1) in epithelial ovarian cancer: association with tumor progression. Gynecol Oncol. 2007;107(2):266–73.
Serce NB, Boesl A, Klaman I, von Serenyi S, Noetzel E, Press MF, Dimmler A, Hartmann A, Sehouli J, Knuechel R, et al. Overexpression of SERBP1 (plasminogen activator inhibitor 1 RNA binding protein) in human breast cancer is correlated with favourable prognosis. BMC Cancer. 2012;12:597.
Costa FC, Saito A, Goncalves KA, Vidigal PM, Meirelles GV, Bressan GC, Kobarg J. Ki-1/57 and CGI-55 ectopic expression impact cellular pathways involved in proliferation and stress response regulation. Biochim Biophys Acta. 2014;1843(12):2944–56.
Mayer B, Muche R. Formal sample size calculation and its limited validity in animal studies of medical basic research. Tierarztliche Praxis Ausgabe K, Kleintiere/Heimtiere. 2013;41(6):367–74.
Baker M. Reproducibility crisis: blame it on the antibodies. Nature. 2015;521(7552):274–6.
Mari Y, West GM, Scharager-Tapia C, Pascal BD, Garcia-Ordonez RD, Griffin PR. SERBP1 is a component of the liver receptor homologue-1 transcriptional complex. J Proteome Res. 2015;14(11):4571–80.
Acknowledgments
The study was supported by the Guangdong Natural Science Foundation (No. S201301004644) and the Southern Medical University Supporting Foundation (No. PX2015N015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Electronic supplementary material
Supplementary table 1
Listed were the siRNA sequences involved against SERBP1. (DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Guo, K., Zheng, S., Xu, Y. et al. Loss of miR-26a-5p promotes proliferation, migration, and invasion in prostate cancer through negatively regulating SERBP1. Tumor Biol. 37, 12843–12854 (2016). https://doi.org/10.1007/s13277-016-5158-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5158-z