Abstract
The primary cause of tumor-related death in breast cancer is still represented by distant metastasization. The dissemination of tumor cells from the primary tumor to distant sites through bloodstream cannot be early detected by standard imaging methods. Circulating tumor cells (CTCs) play a major role in the metastatic spread of breast cancer. Different analytical systems for CTCs isolation and detection have been developed and novel areas of research are directed towards developing assays for CTCs molecular characterization. This review describes the current state of art on CTCs detection techniques and the present and future clinical implications of CTCs enumeration and characterization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Circulating tumor cells (CTCs) are defined as tumor cells circulating in the peripheral blood of patients, shed from the primary tumor or from its metastases [1]. CTCs may be present at the circulatory system for analysis via fragile tumor vessels to the main bloodstream early in tumorigenesis [2–4]. The detection of CTCs in peripheral blood of cancer patients holds great promise, and many exciting technologies have been developed over the past few years. Two factors make the detection and isolation of CTCs challenging: (a) CTCs are rare in the circulation of cancer patients and (b) there is no one marker that can reliably and efficiently distinguish these cells from other blood cells [5]. It has been estimated that in metastatic cancer patients, CTCs are present at a frequency of approximately 1 CTC per 105−7 white blood cells, and in localized disease this frequency may be lower (1 CTC per 108 white blood cells) [6]. Their identification and characterization require extremely sensitive and specific analytical methods, which are usually a combination of enrichment and detection procedures [7].Several technologies exist for CTC detection, but cell search (Veridex, Raritan, NJ, USA), which relies on EpCAM-based immunomagnetic separation, is the only one that has received Food and Drug Administration (FDA) clearance to be used as an aid in monitoring patients with metastatic breast, colorectal and prostate cancer [8].
The detection and enumeration of CTCs has shown to be useful in breast cancer diagnosis, estimating prognosis, monitoring disease recurrence and response to anticancer therapy. Moreover, characterization of CTCs offers new perspectives to understand the biological issues of CTCs and the mechanism of metastasis and drug resistance.
This review will address the current knowledge on CTCs in breast cancer patients with focus on the technology and clinical relevance of these cells.
Strategies for CTCs analysis
The majority of CTCs analysis methods require two steps; an enrichment step and a detection step. The enrichment step can be subdivided into two major classes of techniques; cell size/density-based approaches and immunomagnetic separation-based approaches (Fig. 1).
Enrichment methods
CTCs enrichment strategies are based on technologies that can distinguish CTCs among the surrounding hematopoietic cells, according to their physical (size, density, electric charge, deformability) and biological (cell surface protein expression, viability) characteristics [9]. The enrichment should be considered as a preliminary sample preparation step, by which the majority of blood cells are removed from the sample to improve the relative concentration of CTCs. Therefore, this step can be subdivided into two major classes of techniques; cell size/density-based approaches and immunomagnetic separation-based approaches (Table 1).
Physical properties
The following technologies have been developed based on differences in density, size, deformability and electrical properties [10].
Density gradient centrifugation
This is one of the best-established cell separation techniques. It is performed by cell density gradient centrifugation on a ficoll–hypaque solution [11]; OncoQuick® (GrenierBioOne, Frickenhausen, Germany) is a technology that incorporates a porous barrier for size-based separation of CTCs in conjunction with density-based centrifugation [4, 9, 10, 12]. The main advantage of this porous barrier is that makes easier the method for aspiration of the mononuclear lymphocyte cell fraction, including the enriched epithelial tumor cells after centrifugation [4]. A disadvantage of this method, however, may be the loss of CTCs due to imperfect manual collection of the mononuclear lymphocyte cell fraction after centrifugation [4].
Microfiltration
This physical separation method is based on using filters with specific pores that separate cells of interest from other cells based on their size [11]. This technique is very gentle and does not cause cell damage; therefore, cells can be further analyzed following enrichment [6].
CTC enrichment based on cell size addresses the problem of reduced EpCAM expression. However, filtration shows relatively low sensitivity and specificity because small CTCs may escape detection, whereas large leukocytes may contaminate the CTCs population [2].
Dielectrophoresis (DEP)
The DEP chip uses thin flat chambers with microelectrode arrays patterned by microfabrication [13]. Electrical properties of CTCs may be exploited to discriminate them from blood cells by applying a non-uniform electric field [10]. The main disadvantage associated with the above methods is their low throughput, as sample processing is accomplished on a non-continuous basis (i.e., stop-flow sequence) and the volume processed in each run is too small (e.g., 30–50 μl) for a typical CTC assay [5].
DEP-based isolation of CTCs is also label-free and delivers viable cells, but some factors may affect their application in practice. For instance, dielectric characteristic of cells can gradually change due to ion leakage, meaning that the isolation should be completed within a short duration after the sample processing starts [14].
Microfluidic
Microfluidic devices have been developed to achieve size and deformability-based sorting of CTCs in a more controlled fashion [10]. Higher throughput microfluidic approaches apply hydrodynamic forces to select for cells of different sizes by internal flow fractionation [10], or with an integration of microfluidic functions, based, mainly, on inertial focusing methods (CTC-iChip) [15].
Immunoaffinity
Immunomagnetic separation techniques for CTCs enrichment depend on the expression of epithelial-specific and/or tumor-associated protein markers. Enrichment approaches for CTCs can be either positive or negative. Positive selections utilize markers expressed by the targets cells (i.e., epithelial markers for tumor cell selection). Negative selection utilizes markers expressed by non-target cells (i.e., CD45 for leukocyte depletion) [6, 12, 13, 16].
Magnetic beads
A magnetic field can be used to isolate CTCs from blood if their magnetic property is selectively modified [5]. For that purpose, CTCs can be targeted using antibody-conjugated magnetic microbeads or nanoparticles that often bind to a specific surface antigen, although intracellular antigens can also be targeted [5, 9]. Immunomagnetic methods yield viable and unaltered cells as magnetic labels often do not interfere with the biological function or optical properties of the cells [5]. Unfortunately, the lack of reliable target antigens for cellular capture still represents a significant limitation for this approach [9].
Leukocyte depletion
An alternate approach to positive immunoaffinity-based CTCs selection is to use monoclonal antibodies targeting leukocyte antigens (i.e., CD45) to deplete cells of hematopoietic origin [10].
These approaches can result in false positive results due to nonspecific labeling or false negatives due to the absence of CTC antigens [13]. EpCAM-based immunomagnetic enrichment assays are perhaps the most common, however, EpCAM-independent enrichment technologies seem to be superior to detect the entire CTC population since some tumor cells have low or no EpCAM [17].
Detection methods
After successful isolation, the second challenge is to correctly identify the tumor cells from other hematopoietic cells. False results could confound clinical decision-making resulting in inappropriate treatment choices and negative impact on quality and/or life expectancy [12].
In general, there are two main methods for their detection. These are based on cytometric and nucleic acid manipulation [1, 2, 4, 12]. Both methods generally require an enrichment step to increase sensitivity of the assay [1] (Table 2).
Cytometric techniques
Cytometric detection of CTCs distinguishes rare CTCs from other cells in the blood based on differential antigen expression or other cellular characteristics, as measured by immunofluorescent labeling of cells [6].
Immunocytochemistry (ICC)
Classic ICC can be considered as the gold standard method to detect CTCs [4]. CTCs by ICC mainly refer to direct visual examination of the enriched sample to find the specifically antibody-labeled target cells. CTCs have been defined as a CK+/DAPI+/CD45− intact cells. In particular, the CD45 negativity has been used to increase the detection specificity by ruling out white blood cells (WBCs) that might be illegitimately positive for the CTCs marker(s) [5]. The nuclear dye DAPI has been used to exclude positively scored cell fragments and debris [5].
Methods that combine immunomagnetic enrichment and detection have been developed to improve CTCs isolation and detection. Examples are CellSearch®, Ariol® and Epispot® systems, and microfluidic devices such as the CTCs Chip [12]. Recently, it has been developed a new system (IsoFlux) that combine immunomagnetic beads and microfluidic devices [9, 18] (Table 2).
The limitation of the above-mentioned methods is that they are based on the detection of EpCAM and/or CK which may be down-regulated during the EMT process. These important EMT-derived CTC populations are likely missed by currently used techniques in BC patients [2].
Nucleic acid-based techniques
Molecular methods have been established as an alternative to immunocytochemistry. Various markers have been evaluated including cytokeratins, EpCAM and mammaglobin [19]. Nucleic acid-based CTCs detection techniques depend on the transcription of epithelial-specific and/or tumor-associated RNA in cancerous cells that is not present or differentially expressed in noncancerous cells. The main advantage of this approach is its sensitivity, which is considered higher than immune-mediated detection methods [16] (Table 2).
RT-PCR
Several RT-PCR methods for analysis of epithelium- or organ-specific expression may facilitate investigation of target genes relevant to metastasis [13]. Detection of mRNA of overexpressed or mutated genes in breast cancer by RT-PCR has been considered a suitable alternative in CTCs detection.
qRT-PCR
qRT-PCR allows visualization of low and high mRNA expression thus increasing discrimination of normal versus tumor cells. Unfortunately, the presence of a specific marker in breast cancer is inconsistent due to tumor heterogeneity [6, 13]. The use of internal qRT-PCR controls minimizes false positives and improves specificity of CTCs detection [6, 13].
Nucleic acid-based methods identify specific DNA o mRNA molecules (markers) in the sample to indirectly detect the presence of CTCs. To this end, specific primers are employed in PCR to target known DNA o mRNA molecules that are extracted from the enriched sample and supposedly associate with CTC-specific genes. These genes either code for tissue-, organ-, or tumor-specific proteins or polypeptides, or more specifically, contain known mutations, translocations or methylation patterns found in cancer cells [5, 20]. The nucleic acid-based approach offers the highest sensitivity for CTC detection.
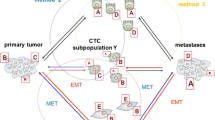
Circulating tumor cell subpopulations
Among CTCs subpopulations, there are several phenotypes: epithelial, epithelial–mesenchymal, and mesenchymal and stem like. These subpopulations are not strictly distinct as there is a continuum between their different stages [21].
Epithelial circulating tumor cells
Historically, the assumption that the CTC originated from an epithelial solid tumor, makes that, CTCs are identified based on the expression of epithelial markers such as EpCAM (Epithelial Cell Adhesion Marker), cytokeratins, and the absence of the common leukocyte marker CD45 [20].
Epithelial–mesenchymal circulating tumor cells
Higher incidence of CTCs expressing EMT-related proteins, such as vimentin and TWIST1, was found in metastatic disease. This overexpression of EMT markers on CTCs was often accompanied by the presence of stem cell markers ALDH1 and CD44 in breast carcinoma [22].
EMT markers on CTCs occur more frequently in metastatic than early-stage breast cancer and allowed more accurate prediction of worse prognosis than the expression of epithelial markers alone [22].
Mesenchymal circulating tumor cells
Differentiated epithelial carcinoma cells can be transformed into mesenchymal state and during this process, different degrees of EMT occur. Pure mesenchymal CTCs as discrete entities are difficult to demonstrate. This is due to the evolution from epithelial to mesenchymal status. The presence of CTCs bearing a mesenchymal phenotype has also been correlated with worse outcomes in breast cancer. Delving into the biology of EMT, a particularly intriguing study was able to demonstrate that a dynamic flux in breast cancer CTCs between epithelial and mesenchymal states is detectable and appears to correlate with response to therapy [23].
In 2013, Yu et al. [24] showed the presence of different populations of CTCs in breast cancer patients. They quantify tumor cells expression of seven epithelial and three mesenchymal transcripts through RNA-in situ hybridization (ISH). They found that all three major histological subtypes of invasive breast cancer contained rare tumor cells with epithelial morphology that stained with both epithelial and mesenchymal markers.
Stem-like circulating tumor cells
Numerous studies have suggested that cancer stem cells (CSCs) with tumor initiation capacity might be derived from either somatic stem cells. To understand the origin of circulating CSCs, two hypotheses can be proposed. Cancerous somatic stem cells undergoing EMT migrate from the primary tumor into the blood and can be called mesenchymal CSCs. The second possibility is that they may arise from fully differentiated cancer cells acquiring migratory properties due to the development of EMT pathways [21].
In a study of women with metastatic breast cancer, CTCs bearing the putative stem-like signature of CD44+/CD24−/Low were detectable in 80 % of women [23], also ALDH1 expression was described in metastatic breast cancer [22]. These findings have led to the position that markers of stemness on CTCs could be used as prognostic biomarkers. Keep in mind, many of these stem-like CTCs do not have EpCAM expression, but show evidence of EMT, and thus would be missed by most epithelial platforms [23].
Markers characteristic of CTCs
The idea of having a unique marker that serves to identify the population of CTCs is really complicated. As we discussed in the previous section, there are different subpopulations with variable expression of different markers making it difficult to select a single marker. Due to lack of breast cancer-specific markers, commonly used antigens are epithelial markers, such as EpCAM and cytokeratins [19].
EpCAM
Epithelial cell adhesion molecule (EpCAM, CD326) is a transmembrane glycoprotein originally discovered as a colon carcinoma-associated antigen [25]. In breast carcinoma, strong EpCAM expression is observed in less differentiated tumors and associates with larger tumors, nodal metastasis and poorer overall survival. Moreover, in breast carcinoma, EpCAM has reported to be upregulated in large metastases as compared with the matched primary tumor. Strong EpCAM expression has been shown to be a poor prognostic factor in both node-positive and node-negative disease [25, 26].
However, there are CTCs not captured by EpCAM-based positive enrichment, such as those that have undergone EMT with downregulation of EpCAM and other epithelial markers [20].
Cytokeratin (CK)
Cytokeratin belongs to a family of over 20 related polypeptides located in the cytoskeleton of various epithelial cells. Their main role is to provide cellular structure and integrity. They are markers of normal epithelial differentiation, but they can be used as diagnostic tool to detect different circulating cells of carcinoma. Epithelial cancer cells express CK8, CK18 and CK19 [21]. In 2001, Silva et al. [27] identified CK19 mRNA in plasma of breast cancer patients. On evaluation of clinicopathological parameters with molecular data, tumor size and proliferation index were associated with the presence of plasma CK19 mRNA [1]. Recently, full-length CK19 has been detected in bone marrow specimens from breast cancer patients and is associated with the presence of over metastases and poor survival rates [7].
Other markers of interest are MUC1, mammaglobin, HER2 [28], those are used basically in molecular methods.
Mucin 1 (MUC1)
MUC1 is a transmembrane mucin that was identify by its marked overexpression in human breast cancers. MUC1 is translated as a single polypeptide that undergoes auto cleavage into two subunits that form a stable non-covalent heterodimeric complex at the cell membrane [29]. A commercially available assay is the semiquantitative RT-PCR-based AdnaTest BreastCancer (AdnaGen AG, Langenhagen, Germany). CTCs are enriched by immunomagnetic beads labeled with anti-MUC1 and anti-EpCAM antibodies [19].
Mammaglobin
Human mammaglobin (MGB) belongs to the uteroglobin/Clara cell protein family of small epithelial secretory proteins. They were abundantly expressed in breast tumors relative to normal breast tissue [1, 12, 30]. Most research on the use of MGB as a potential diagnostic biomarker in cancer has focused on MGB1 [1]. Watson and Fleming reported that primary breast carcinomas overexpressed MGB1 relative to normal tissue specimens, with approximately 50 % of breast carcinoma cell lines and metastatic breast tumors analyzed exhibiting high levels of MGB1 mRNA [1, 12, 30]. This marker is widely used in molecular methods such as RT-PCR [9, 19].
HER2
The human epithelial growth factor receptor (HER) family of receptors plays a central role in the pathogenesis of several human cancers [31].
HER2 positivity is known poor prognostic factor [32]. Identification of HER2+ CTCs, as defined by expression of HER2 mRNA derived from CTCs, was also associated with shorter disease-free survival (DFS) in a cohort of early breast cancer patients, before the initiation of any adjuvant treatment, although this did not remain an independent prognostic factor in a multivariate analysis, nor was there any significant association with overall survival (OS) [1, 32].
Clinical applications
Prognostic role of CTCs in metastatic breast cancer
Cristofanilli et al. [33] demonstrated the prognostic significance of CTCs in metastatic breast cancer (MBC) using a cutoff level of 5 cells in 7.5 ml of blood. Patients with ≥5 CTCs/7.5 ml had shorter progression-free survival (PFS) (2.7 vs. 7 months, p < 0.001) and overall survival (OS) (10.1 vs. >18 months, p < 0.001) compared with patients with <5 CTCs per 7.5 ml. Further studies and a recent meta-analysis confirmed the strong prognostic value of circulating tumor cells in MBC [34–36]. Recently, the analysis of 1944 patients from 51 European centers who participated in studies between January 2003 and July 2012 has been published. 911 patients (46.9 %) had a CTCs count of 5 per 7.5 ml or higher at baseline, which was associated with decreased PFS (HR 1.92, 95 % CI 1.73–2.14, p < 0.0001) and OS (HR 2.78, 95 % CI 2.42–3.19, p < 0.0001) compared with patients with a CTCs count of less than 5 per 7.5 ml at baseline. Survival prediction was significantly improved by addition of baseline CTCs count to clinicopathological models (tumor histological subtype and histological grade, number of previous lines of chemotherapy and hormone therapy received for metastatic disease, performance status and presence of liver or visceral metastasis) [37].
However, the clinical impact of CTCs in different subtypes of breast cancer may be different. In a retrospective analysis of 517 patients, Giordano et al. [38] showed that baseline CTCs enumeration had prognostic value in all breast cancer subtypes but appeared to be more valuable in hormone receptor positive and triple negative breast cancers and less valuable in HER2+ cancer treated with targeted therapy, suggesting an interaction between CTCs and such therapies. These results were confirmed in a retrospective analysis of 235 newly diagnosed metastatic breast cancer treated with four different treatment groups (chemotherapy, endocrine therapy, chemotherapy plus bevacizumab or chemotherapy plus HER2 targeting drugs). The CTCs count was confirmed to be a prognostic marker in the general population and in patients treated with chemotherapy and endocrine therapy. Conversely, patients treated with bevacizumab or anti-HER2 therapies did not maintain the negative prognostic value of high CTCs at the baseline, suggesting a therapeutic benefit from these drugs [39].
Predictive role of CTCs in metastatic breast cancer
The level of CTCs may be useful to monitor patients for response to treatment. The enumeration of CTCs during treatment has proven to be a reliable surrogate marker of treatment response, and a potential alternative for therapy monitoring.
To address the correlation of CTCs count after first cycle of chemotherapy and survival in patients with MBC, our group determined CTCs counts at baseline, before the first cycle of chemotherapy and immediately before the second cycle of chemotherapy in 117 consecutive patients with metastatic breast cancer. Patients with <5 CTCs on day 21 had better clinical benefit rate (77 vs. 44 %, p = 0.0051), PFS (9.4 vs. 3 months, p = 0.001) and OS (38.5 vs. 8.7 months, p < 0.0001) than those with ≥5 CTCs. The OS of patients with baseline CTCs ≥ 5 that dropped to <5 on day 21 was apparently similar to those who had <5 CTCs at baseline [40]. This way, CTCs analysis can identify not only patients with poor prognosis but also those who derive little benefit from a chemotherapy regimen, defining CTCs as a strong and early predictive marker of chemotherapy response. Budd et al. compared the correlation between the presence of CTCs and objective response to therapy using radiographic studies and the correlation of these methods of evaluating response with OS [41]. Patients with non-progression by radiological assessment and ≥5 CTCs had significantly shorter OS than patients with radiological non-progression and <5 CTCs (15.3 vs. 26.9 months; p = 0.0389). Moreover, patients with radiological progression and <5 CTCs had significantly longer OS compared with patients with radiological progression and ≥5 CTCs (19.9 vs. 6.4 months; p = 0.0039). When assay and imaging reproducibility are compared, CTCs showed also a significant advantage with an interreader variability of 15 % with imaging versus 1 % with CTCs.
Early detection of progression with CTCs enumeration would decrease the exposure to toxicity from less effective therapies and allow switching to alternative regimens. SWOG S0500 is a prospective clinical trial that tested whether changing to an alternative chemotherapeutic regimen might improve the outcome of patients with metastatic breast cancer whose CTCs were not reduced after one cycle of first-line chemotherapy. Of the 595 patients who were eligible for the trial, 276 had low CTCs at baseline, and were observed on arm A and treated at the physician’s choice. The remaining 319 patients had elevated CTCs at baseline, and 286 had a CTCs result available at day 21 of the first cycle of chemotherapy. At day 21, CTCs decreased to lower levels in 163 patients, and these patients continue to receive the same chemotherapy (arm B). The 123 patients who continued to have elevated CTCs at day 21 were either randomly assigned to arm C1 and continued to receive the initial chemotherapy (64 patients) or were randomly assigned to arm C2 and had their treatment changed to a second-line chemotherapy (59 patients). There was no difference in OS between arms C1 and C2 (10.7 vs. 12.5 months, HR 1; 95 % CI 0.69–1.47, p = 0.98). Although this trial does not meet the primary objective, it confirms the prognostic role of CTCs at baseline and after the first cycle of chemotherapy. Median OS for arms A, B, and C (C1 and C2 combined) were 35, 23, and 13, respectively (p < 0.001) [42]. As the SWOG trial, the objective of the CirCe01 study is to demonstrate that patients with insufficient CTCs decrease after the first cycle of chemotherapy have to switch to another treatment. 304 patients with high CTCs count before the start of the third line of chemotherapy will be randomized between the CTCs-driven arm and the standard arm. In the CTCs-driven arm, patients without a significant reduction of CTCs count will discontinue chemotherapy and will eventually receive a further line of treatment, which will be, again, evaluated by early CTCs changes [43].
CTCs in patients with early breast cancer
Treatment and follow-up of patients potentially cured are based on clinical, pathological and recently in molecular/genomic aspects of the patient and the tumor. However, patients with the same characteristics do not have always the same evolution. Thus, CTCs could help to define better the prognosis of these patients to apply more aggressive treatments and/or surveillance for a subset of cases.
Currently, no diagnostic tools are available to monitor treatment response after the completion of adjuvant treatment. Because CTCs are the targets of adjuvant treatment, their presence after systemic therapy could be not only a potential marker permitting an individualized assessment of treatment efficacy but also this information might be useful to identify patients who might benefit from additional therapies and/or more exhaustive surveillance.
In small cohorts, CTCs were reported in 18–30 % of patients with early breast cancer and their presence predicted decreased progression-free and overall survival [44–48]. In a meta-analysis of the prognostic value of circulating tumor cells in breast cancer that included studies with early-stage breast cancer showed that the presence of CTCs significantly increase the risk of disease recurrence (HR 2.86; 95 % CI 2.19–3.75) and the risk of death (HR 2.78; 95 % CI 2.22–3.48) [36]. Recently, the translational research program of the German SUCCESS trials prospectively investigated the clinical relevance of CTCs in a large number of primary breast cancer patients. CTCs were analyzed in 2026 patients with early breast cancer before adjuvant chemotherapy and in 1492 patients after chemotherapy using the CellSearch System. Before chemotherapy, CTCs were detected in 21.5 % of node-negative and 22.5 % of node-positive breast cancer (p < 0.001). Their presence was confirmed to be an adverse prognostic marker for disease-free survival (DFS) (HR 2.11; 95 % CI 1.49–2.99, p < 0.0001) and OS (HR 2.18; 95 % CI 1.32–3.59, p = 0.002). After chemotherapy, 22.1 % of patients were CTCs positive. The presence of persisting CTCs after chemotherapy showed a negative influence on DFS (HR 1.12; 95 % CI 1.02–1.25; p = 0.02) and OS (HR 1.16; 95 % CI 0.99–1.37; p = 0.06) [49].
In the neo-adjuvant setting, pathologic complete response (pCR) is associated with good long-term clinical outcome [50]. However, not all patients achieving pCR will be cured. The presence of CTCs after neo-adjuvant chemotherapy may be a surrogate marker of response and survival. Three neo-adjuvant clinical trials (GeparQuattro, Beverly-2 and Remagus II) have evaluated the correlation between CTCs detection and pCR. In the GeparQuattro trial, there was no association between tumor response to neo-adjuvant chemotherapy and CTCs detection [45]. Recently, the BEVERLY-2 trial that evaluated the efficacy of neo-adjuvant chemotherapy with bevacizumab and trastuzumab in HER2+ inflammatory breast cancer showed that baseline CTCs detection independently predicted 3-year DFS [81 vs. 43 % for patients with <1 vs. ≥1 CTCs/7.5 ml at baseline (p = 0.01)]. Baseline CTCs count improved prognostic value of pCR after neo-adjuvant chemotherapy. Patients with pCR had a 3-year DFS of 80 %, while patients with pCR and no CTCs at baseline and pCR had a 3-year DFS of 95 % [51]. In Remagus II trial although persistence of circulating tumor cells at the end of neo-adjuvant chemotherapy was not correlated with treatment response, detection of at least 1 CTC/7.5 ml before and after adjuvant chemotherapy was an independent prognostic factor for shorter distant metastasis-free survival [52].
One possible explanation of the discordance between the presence of CTCs and pCR is that the phenotype of the primary tumor may not necessarily reflect the phenotype of metastatic disease. For example, it has been shown that CTCs in the blood are more frequently HER2-positive than the corresponding primary tumor [53]. In GeparQuattro trial, HER2 overexpressing CTCs were observed in 14 of 58 CTCs positive patients including 8 patients with HER2-negative primary tumors [45]. Thus, a potential utility of CTCs could be to select patients who need additional treatment and to evaluate therapeutic targets on these cells that will allow a more individualized therapy. Georgoulias et al. randomized 75 women with HER2-negative early breast cancer and detectable CTCs before and after adjuvant chemotherapy to receive trastuzumab versus observation. 72 % of patients (23/32) treated with trastuzumab turned CTCs-negative compared to 26 % in the observational arm (7/27). Patients treated with trastuzumab had significantly better DFS, with 11 % relapses compared to 38 % in the observational arm [54]. This strategy is also being evaluated in Treat CTCs trial for patients with HER2 negative primary breast cancer. In this phase II randomized trial, patients with >1CTC/15 ml after completion of neo-adjuvant chemotherapy and surgery will be randomized to trastuzumab versus observation. The primary endpoint is to compare CTCs detection rate at week 18 between the two arms and the secondary endpoint compares recurrence-free interval [43].
Characterization of circulating tumor cells: time to liquid biopsies?
Primary tumors and metastatic lesions can differ in molecular characteristics. Discordance rates in estrogen (ER), progesterone receptor (PR) and HER2 are in the range of 10 to 35–40 % [55]. In the metastatic setting, such predictive factors should be determined in metastatic tissue rather than in the primary tumor and treatment decisions must be made on the basis of tumor cell characteristics, not only prior to treatment initiation, but also after progression to adapt systemic therapy when necessary. However, biopsies of metastasis are invasive procedures and technical difficulties (especially for the biopsy of bone metastasis) must be considered. To overcome the limitations and risks of biopsies, the detection and characterization of CTCs in the peripheral blood of breast cancer patients could serve as a real-time tumor biopsy [56].
Although this approach is promising, several questions must be answered before CTCs can be proposed as a part of an individualized treatment.
First, CTCs may not reflect the biology of the underlying tumor. The majority of tumor cells shed into the circulation will never become a metastatic lesion. CTCs may be a marker of the presence of “metastatically” competent cells not detected with conventional assays [57]. Currently used methods to detect CTCs are based on detection of EpCAM and cytokeratins and exclude cells with epithelial to mesenchymal transition (EMT) features [58]. Given the critical role of EMT in tumor metastasis, it is necessary to optimize CTCs detection through the inclusion of EMT markers. Furthermore, the detection of cells in mesenchymal transition with EMT and stemness features may contribute to discover additional therapeutic targets.
Second, consistently with heterogeneity in primary and metastatic tumors, significant cell-to-cell variations in CTCs occur within individual patients [59]. Targeting one CTCs population might not eliminate other subpopulations so only identifying metastatic cell diversity through CTCs profiling could effectively guide drug selection.
Finally, HER2 amplification and ER/PR expression in biopsies are predictive of response to trastuzumab and hormonal therapy, respectively [60]. Although these and other biomarkers can be measured in CTCs, it is not proven that the biomarker status of a CTC predicts response to targeted therapy.
Conclusions
Nowadays, huge efforts have been made to better understand the role of CTCs in cancer patients. In the past 10 years, there has been an explosion of new techniques for CTCs detection and enumeration. However, a standard protocol for CTCs detection is still not available, due to CTCs are not completely characterized. They have often been defined as CK+/DAPI+/CD45− cells; however, not all studies have followed the same convention. Based on their underlying principle, the exiting CTC detection and isolation techniques can be broadly classified as nucleic acid-based, physical properties-based and antibody-based methods. Despite their high sensitivity and accessibility, nucleic acid-based methods can only determine whether or not a sample is positive for one or more markers and do not allow direct enumeration and cytomorphological study of CTCs. Physical properties-based methods require minimum sample preparation and can potentially isolate viable cells. Size-based approaches are the most widely used methods in this category; however, their purity rates may vary significantly. Microfluidic size-based methods have offered high recovery and purity rates when evaluated using spiked samples. However, cancer lines may not properly represent the morphologically heterogeneous nature of CTCs.
From a clinical point of view, the presence of CTCs in peripheral blood is predictor of outcome in metastatic breast cancer and comparable to imaging studies for therapeutic monitoring and assessment of metastatic progression. Despite the interest of prognostic and predictive value of CTCs enumeration, the next step is to move forward to molecular characterization.
Molecular characterization of CTCs can provide information of genomic landscape of cancer cells useful to guide treatment decisions and to understand drug-resistance mechanisms. However, the potential role of CTCs analysis to individualize treatment has to be evaluated in prospective clinical trials that assess the impact of treatment decisions based on CTCs analysis using sensitive, standardized and reproducible technology to be incorporated into clinical practice.
In conclusion, CTCs are emerging as promising tumor biomarkers in breast cancer and their clinical use as a “liquid biopsy” for stratification of patients and real-time monitoring of therapies will have a major impact in personalized medicine.
References
Friel AM, Corcoran C, Crown J, O’Driscoll L. Relevance of circulating tumor cells, extracellular nucleic acids, and exosomes in breast cancer. Breast Cancer Res Treat. 2010;123:613–25.
Liberko M, Kolostova K, Bobek V. Essentials of circulating tumor cells for clinical research and practice. Crit Rev Oncol Hematol. 2013;88:338–56.
De Wit S, van Dalum G, Terstappen LWMM. Detection of circulating tumor cells. Scientifica. 2014;2014:819362.
Broersen LHA, van Pelt GW, Tollenaar RAEM, Mesker WE. Clinical application of circulating tumor cells in breast cancer. Cell Oncol Dordr. 2014;37:9–15.
Esmaeilsabzali H, Beischlag TV, Cox ME, Parameswaran AM, Park EJ. Detection and isolation of circulating tumor cells: principles and methods. Biotechnol Adv. 2013;31:1063–84.
Lowes LE, Goodale D, Keeney M, Allan AL. Image cytometry analysis of circulating tumor cells. Methods Cell Biol. 2011;102:261–90.
Alix-Panabières C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annu Rev Med. 2012;63:199–215.
Ignatiadis M, Riethdorf S, Bidard F-C, Vaucher I, Khazour M, Rothé F, et al. International study on inter-reader variability for circulating tumor cells in breast cancer. Breast Cancer Res. 2014;16:R43.
Toss A, Mu Z, Fernandez S, Cristofanilli M. CTC enumeration and characterization: moving toward personalized medicine. Ann Transl Med. 2014;2:108.
Harouaka R, Kang Z, Zheng S-Y, Cao L. Circulating tumor cells: advances in isolation and analysis, and challenges for clinical applications. Pharmacol Ther. 2014;141:209–21.
Rodrıguez-Gonzalez FG, Mustafa DAM, Mostert B, Sieuwerts AM. The challenge of gene expression profiling in heterogeneous clinical samples. Methods San Diego Calif. 2013;59:47–58.
Ghersevich S, Ceballos MP. Mammaglobin A: review and clinical utility. Adv Clin Chem. 2014;64:241–68.
López-Muñoz E, Méndez-Montes M. Markers of circulating breast cancer cells. Adv Clin Chem. 2013;61:175–224.
Gascoyne PRC, Noshari J, Anderson TJ, Becker FF. Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis. 2009;30:1388–98.
Ozkumur E, Shah AM, Ciciliano JC, Emmink BL, Miyamoto DT, Brachtel E, et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl Med. 2013;5:179ra47.
Aurilio G, Sciandivasci A, Munzone E, Sandri MT, Zorzino L, Cassatella MC, et al. Prognostic value of circulating tumor cells in primary and metastatic breast cancer. Expert Rev Anticancer Ther. 2012;12:203–14.
Lianidou ES, Markou A. Circulating tumor cells as emerging tumor biomarkers in breast cancer. Clin Chem Lab Med CCLM FESCC. 2011;49:1579–90.
Harb W, Fan A, Tran T, Danila DC, Keys D, Schwartz M, et al. Mutational analysis of circulating tumor cells using a novel microfluidic collection device and qPCR assay. Transl Oncol. 2013;6:528–38.
Banys M, Müller V, Melcher C, Aktas B, Kasimir-Bauer S, Hagenbeck C, et al. Circulating tumor cells in breast cancer. Clin Chim Acta Int J Clin Chem. 2013;423:39–45.
Lustberg MB, Balasubramanian P, Miller B, Garcia-Villa A, Deighan C, Wu Y, et al. Heterogeneous atypical cell populations are present in blood of metastatic breast cancer patients. Breast Cancer Res. 2014;16:R23.
Barriere G, Fici P, Gallerani G, Fabbri F, Zoli W, Rigaud M. Circulating tumor cells and epithelial, mesenchymal and stemness markers: characterization of cell subpopulations. Ann Transl Med. 2014;2:109.
Grover PK, Cummins AG, Price TJ, Roberts-Thomson IC, Hardingham JE. Circulating tumour cells: the evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2014;25:1506–16.
Friedlander TW, Premasekharan G, Paris PL. Looking back, to the future of circulating tumor cells. Pharmacol Ther. 2014;142:271–80.
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–4.
Martowicz A, Spizzo G, Gastl G, Untergasser G. Phenotype-dependent effects of EpCAM expression on growth and invasion of human breast cancer cell lines. BMC Cancer. 2012;12:501.
Gostner JM, Fong D, Wrulich OA, Lehne F, Zitt M, Hermann M, et al. Effects of EpCAM overexpression on human breast cancer cell lines. BMC Cancer. 2011;11:45.
Silva JM, Dominguez G, Silva J, Garcia JM, Sanchez A, Rodriguez O, et al. Detection of epithelial messenger RNA in the plasma of breast cancer patients is associated with poor prognosis tumor characteristics. Clin Cancer Res Off J Am Assoc Cancer Res. 2001;7:2821–5.
Ignatiadis M, Kallergi G, Ntoulia M, Perraki M, Apostolaki S, Kafousi M, et al. Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14:2593–600.
Kufe DW. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene. 2013;32:1073–81.
Li G, Zhang J, Jin K, He K, Wang H, Lu H, et al. Human mammaglobin: a superior marker for reverse-transcriptase PCR in detecting circulating tumor cells in breast cancer patients. Biomark Med. 2011;5:249–60.
Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748.
Turner N, Pestrin M, Galardi F, De Luca F, Malorni L, Di Leo A. Can biomarker assessment on circulating tumor cells help direct therapy in metastatic breast cancer? Cancers. 2014;6:684–707.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91.
Nolé F, Munzone E, Zorzino L, Minchella I, Salvatici M, Botteri E, et al. Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2008;19:891–7.
Dawood S, Broglio K, Valero V, Reuben J, Handy B, Islam R, et al. Circulating tumor cells in metastatic breast cancer: from prognostic stratification to modification of the staging system? Cancer. 2008;113:2422–30.
Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18:5701–10.
Bidard F-C, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–14.
Giordano A, Giuliano M, De Laurentiis M, Arpino G, Jackson S, Handy BC, et al. Circulating tumor cells in immunohistochemical subtypes of metastatic breast cancer: lack of prediction in HER2-positive disease treated with targeted therapy. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2012;23:1144–50.
Giuliano M, Giordano A, Jackson S, Hess KR, De Giorgi U, Mego M, et al. Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast Cancer Res. 2011;13:R67.
Martín M, Custodio S, de Las Casas M-LM, García-Sáenz J-Á, de la Torre J-C, Bellón-Cano J-M, et al. Circulating tumor cells following first chemotherapy cycle: an early and strong predictor of outcome in patients with metastatic breast cancer. Oncologist. 2013;18:917–23.
Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, et al. Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12:6403–9.
Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32:3483–9.
Bidard F-C, Fehm T, Ignatiadis M, Smerage JB, Alix-Panabières C, Janni W, et al. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev. 2013;32:179–88.
Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13:688–95.
Riethdorf S, Müller V, Zhang L, Rau T, Loibl S, Komor M, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16:2634–45.
Sandri MT, Zorzino L, Cassatella MC, Bassi F, Luini A, Casadio C, et al. Changes in circulating tumor cell detection in patients with localized breast cancer before and after surgery. Ann Surg Oncol. 2010;17:1539–45.
Bidard F-C, Mathiot C, Delaloge S, Brain E, Giachetti S, de Cremoux P, et al. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2010;21:729–33.
Biggers B, Knox S, Grant M, Kuhn J, Nemunatitis J, Fisher T, et al. Circulating tumor cells in patients undergoing surgery for primary breast cancer: preliminary results of a pilot study. Ann Surg Oncol. 2009;16:969–71.
Rack B, Schindlbeck C, Jückstock J, Andergassen U, Hepp P, Zwingers T, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014;106:dju066.
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25:4414–22.
Pierga J-Y, Petit T, Lévy C, Ferrero J-M, Campone M, Gligorov J, et al. Pathological response and circulating tumor cell count identifies treated HER2+ inflammatory breast cancer patients with excellent prognosis: BEVERLY-2 survival data. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21:1298–304.
Pierga J-Y, Bidard F-C, Mathiot C, Brain E, Delaloge S, Giachetti S, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14:7004–10.
Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S, et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci USA. 2004;101:9393–8.
Georgoulias V, Bozionelou V, Agelaki S, Perraki M, Apostolaki S, Kallergi G, et al. Trastuzumab decreases the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy-resistant CK-19mRNA-positive circulating tumor cells: results of a randomized phase II study. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2012;23:1744–50.
Pusztai L, Viale G, Kelly CM, Hudis CA. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. Oncologist. 2010;15:1164–8.
Smerage JB, Hayes DF. The measurement and therapeutic implications of circulating tumour cells in breast cancer. Br J Cancer. 2006;94:8–12.
Chambers AF, Naumov GN, Vantyghem SA, Tuck AB. Molecular biology of breast cancer metastasis. Clinical implications of experimental studies on metastatic inefficiency. Breast Cancer Res. 2000;2:400–7.
Kalluri R, Weinberg RA. The basics of epithelial–mesenchymal transition. J Clin Invest. 2009;119:1420–8.
Powell AA, Talasaz AH, Zhang H, Coram MA, Reddy A, Deng G, et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS ONE. 2012;7:e33788.
Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:3997–4013.
Acknowledgments
This study was supported by funds from FEDER (RTICC-RD12/0036/0076 and RD12/0036/0006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no conflict of interests.
Additional information
R. Ramos-Medina and F. Moreno have contributed equally to this work and should be considered as first authors as equal.
Rights and permissions
About this article
Cite this article
Ramos-Medina, R., Moreno, F., Lopez-Tarruella, S. et al. Review: circulating tumor cells in the practice of breast cancer oncology. Clin Transl Oncol 18, 749–759 (2016). https://doi.org/10.1007/s12094-015-1460-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1460-2