Abstract
Circulating tumor cells (CTCs) play a major role in the metastatic spread of breast cancer. CTC detection has proven to be an important parameter for predicting progression free and overall survival. Collection of CTCs is minimally invasive and can be performed more often than disseminated tumor cell (DTC) collection from bone marrow, thus providing a real-time “liquid biopsy”. In this review, the most important techniques for enrichment and detection of CTCs are discussed for clinical application in low and higher staged breast cancer, as well as the genetic and molecular characterization of CTCs. For CTCs, the use of immunology-based enrichment techniques with multiple antibodies is recommended in a clinical setting, as well as the use of cytometric detection techniques, combined with RT-PCR for confirmation. Special attention is given to the value of cancer stem cell (CSC) activity, which may be the main cause of ineffectiveness of the control over metastatic lesions due to intratumor heterogeneity. Accumulating information on CSCs offers new paradigms to generate effective targets for the treatment of metastatic disease. Genetic and molecular characterization of CTCs has potential to stratify patients for optimal personalized treatment regimens. CTCs can be used for monitoring patients during treatment schedules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Circulating tumor cells (CTCs) are important for the metastatic process of carcinomas [1, 2]. They may invade the bloodstream via fragile tumor vessels early in tumorigenesis. CTCs can be present in low frequencies of 1 per 108 mononuclear cells and may survive in the peripheral blood in a dormant state for several years. However, of the 106 CTCs that enter the bloodstream daily, about 85% disappear within five minutes. Only 2,5% of CTCs cause micrometastases and 0,01% form macroscopic metastases [3]. Disseminated tumor cells (DTCs) spread through the lymphatic system and can be detected in bone marrow in higher frequencies than CTCs [4]. Both CTCs and DTCs have shown their clinical importance [5–8]. Since DTCs are measured at a certain point in time at a specific location, they are thought to be less suitable than CTCs for having prognostic information [9].
Conventionally, disease staging for initial treatment is performed according to TNM classification, based on tumor size (T), lymph node positivity (N) and the presence of metastases (M) [10]. In addition to TNM staging, CTCs can be of value a. for predicting disease progression in lymph node negative patients for decision making for therapy, and b. to monitor treatment efficacy in advanced stages. Lang et al. found that 40% of lymph node negative patients are CTC positive. In their study of operable breast cancer patients, HER2 was the only primary tumor characteristic that predicted the presence of CTCs. Lang et al. hypothesized that CTCs may be prognostic of adverse outcome in early stage breast cancer, just as they are proven for metastatic breast cancer [11]. Harbeck et al. found that patients with grade 1 node-negative breast cancer have a relapse rate of almost 20%. The relapse rate in patients with grade 2 or 3 tumors was even higher [12]. Enumeration and gene expression analysis of CTCs can distinguish between high and low risk profiles for progression free survival (PFS) and overall survival (OS) [13]. After the seventh lymph node, measuring more lymph nodes does not reveal more node positivity in patients with colorectal carcinoma. This may also be true for breast carcinomas. In these patients, it might be more valuable to measure CTCs rather than more lymph nodes [14].
A higher number of DTCs can be collected from bone marrow than the number of CTCs from blood, but blood sampling is less invasive and, therefore, more convenient for the patient. It can easily be performed more frequently, which makes it the preferred method. By multiple sampling, CTCs provide a so called real-time “liquid biopsy” which gives better opportunities for selective treatment schedules than DTCs [15–19]. Cristofanilli et al. showed that CTC detection is important for estimating disease progression and survival in patients with metastatic breast carcinoma, since their prognostic value is superior to the site of metastasis, type of therapy and length of time to recurrence after primary surgery. The predictive value of the level of CTCs was found to be independent of time to metastasis, site of metastasis (visceral or non-visceral) and hormone-receptor status [5]. Nolè et al. showed that the presence of 5 or more CTCs per 7,5 ml of peripheral blood is of worse prognosis than the presence of less than 5 CTCs [20]. The relative number of obtained CTCs, however, is dependent on the processed blood volume [21]. In breast cancer, measuring CTCs can be applied after the first form of therapy to monitor the treatment efficacy in the patient. Early changes in treatment can be made if the current treatment is ineffective and a second-line therapy can be chosen [9].
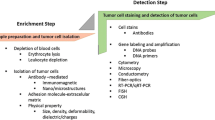
For CTC research techniques for both enrichment and detection are essential, as these can be performed separately or sequentially [2]. Enrichment techniques are either morphology or immunology-based, whereas methods for detection are cytometric or nucleic acid-based (Fig. 1) [1, 2, 9, 15]. Genetic and molecular analyses of CTCs provide insight in the metastatic process and the effectiveness of therapy [1]. Techniques to perform this genetic and molecular characterization include biomarker immunofluorescent staining, FISH, PCR-based techniques and comparative genomic hybridization [2]. Genetic and molecular analyses are specifically applied for the characterization of cancer stem cells (CSCs), being the most important cause of metastasis in breast carcinoma [1].
In this review, the most important enrichment techniques and detection methods are discussed for their clinical application, as well as the genetic and molecular characterization of CTCs, which may provide an important tool to determine the most optimal treatment (tailored treatment) for the patient.
2 Cell enrichment techniques
Since the number of CTCs in the peripheral circulation is relatively low (one CTC per 106–107 mononuclear cells), enrichment for CTCs is essential before further analysis. Available cell enrichment techniques are either morphology or immunology-based [2]. Morphology-based techniques can be subdivided into density gradient centrifugation and filtration by size of the cells. Immunology-based techniques use immunomagnetic isolation, often in conjunction with anti-epithelial cell adhesion molecule (EpCAM) or anti-cytokeratin (CK) antibodies for positive selection of CTCs, or anti-CD45 for negative depletion of mononuclear cells [1, 9, 15]. It is thought that negative depletion of mononuclear cells has the preference for collecting CTCs, since this method is independent of weak EpCAM, CK or any other antibody target expression by CTCs [22].
2.1 Morphology-based techniques
Density gradient centrifugation, such as the commercially available OncoQuick device (Greiner Bio-One GmbH, Frickenhausen, Germany) was one of the first cell enrichment approaches developed, and is based on the differential centrifugal migration of the cells according to their buoyant density using Ficoll-hypaque solution. This centrifugal migration results in the formation of separate layers in the peripheral blood sample. The epithelial tumor cells are enriched in the mononuclear lymphocyte cell fraction at the interphase between the plasma and Ficoll-hypaque. The main advantage of the OncoQuick device is the porous barrier which separates the lower phase with separation medium from the blood sample before centrifugation, making the method for aspiration of the mononuclear lymphocyte cell fraction, including the enriched epithelial tumor cells after centrifugation, easier. A disadvantage of this method, however, may be the loss of CTCs due to imperfect collection of the mononuclear lymphocyte cell fraction after centrifugation [9, 19].
Filtration by size (ISET) separates epithelial tumor cells from other cells present in peripheral blood based on their larger size, particularly if they are derived from solid tumors. An advantage of using filtration by size to enrich tumor cells is the applicability of this method to a broad range of tumors. A disadvantage is that, depending on the filter size, smaller CTCs can be lost. Other filtration by size methods, such as size-based microfilters, are still under development, but the principles behind them are promising [1, 9, 23]. Tanaka et al. studied the inertial migration of tumor cells based on the size of CTCs in the blood flow in a microchannel, and concluded that a low hematocrit is necessary for proper separation of tumor cells [24].
2.2 Immunology-based techniques
The most developed and used enrichment technique is immunomagnetic isolation. This antibody-based affinity enrichment method depends on the expression of specific antigens by the epithelial tumor cell (positive selection, e.g. EpCAM+ or CK19+) or mononuclear hematopoietic cell (negative depletion, e.g. CD45−) [9, 19]. There are a number of different devices available based on positive selection of CTCs by using anti-EpCAM antibodies, including MACS (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), CellSearch system (Veridex LLC, Raritan, NJ, USA), CTC-chip (Massachusetts General Hospital, Boston, MA, USA) and affinity-based microchips. MACS and CellSearch both use a magnetic field to separate CTCs linked to a magnetic particle-bound antibody. CTC-chip and affinity-based microchips both use an antibody-coated chip in which blood cells can flow through freely, but CTCs bind to the anti-EpCAM molecules. The drawback of this method is that not all CTCs express EpCAM on their cell membrane, or that EpCAM expression may be weak, and therefore some will be lost in the process [1, 9, 19].
Of the enrichment techniques mentioned, density gradient centrifugation is most easy to perform. Filtration by size is applicable to a broad range of tumors. Both methods, however, may result in loss of tumor cells during the process. Immunomagnetic isolation is a well-developed cell enrichment method, but there may be cell loss due to weak expression of EpCAM. Therefore, it is recommended to use immunomagnetic isolation with a combination of antibodies.
3 Cell detection methods
Since none of the enrichment methods yields a pure population of tumor cells, for all separation techniques a detection method to distinguish CTCs from other captured cells is essential. These cell detection methods can be cytometric or nucleic acid-based [2, 9]. Here, the most frequently used cell detection methods are discussed, as well as some recently developed promising techniques. The three most important cell detection systems, CellSearch system, Ariol system (Leica Biosystems, Rijswijk, Netherlands) and AdnaTest (Adnagen AG GmbH, Langenhagen, Germany) are outlined in Table 1.
3.1 Cytometric techniques
Classic immunocytochemistry (ICC) can be considered as the golden standard method to detect CTCs. A combination of immunostaining (e.g. with an anti-CK antibody) and fluorescence in situ hybridization (FISH) is the method of preference. Visual evaluation of the stained slides is typically done by trained pathologists and is time-consuming [1, 15]. Automated imaging devices can be more sensitive, reproducible and accurate than routine pathological evaluation for the detection of rare events like CTCs in peripheral blood [25].
The CellSearch system uses EpCAM-labeled iron oxide nanoparticles to enrich CTCs and anti-CK and anti-CD45 antibodies for detection. It also takes cytomorphologic characteristics (appropriate size, presence of a nucleus and appropriate nuclear to cytoplasmic ratio) into account while classifying events as tumor cells [9]. Detection of CTCs using CellSearch showed the importance of the method for prognostic patient information, both for PFS and OS. The detection of a single CTC in early stage breast cancer predicts poor disease-free, distant disease-free and overall survival after 3 years of follow-up. Elevated CTC levels at any time in the clinical course of a patient with metastatic breast cancer indicates impending progression [6, 20, 21, 26–31]. Other investigations have shown that cell detection systems, such as a combination of anti-CK and anti-EpCAM expression detection, may perform even better than CellSearch [4, 17, 32, 33]. However, in five studies no advantage was observed using one method over another [34–38]. Farace et al. compared CellSearch to ICC after enrichment by ISET. Although CellSearch finds more CTCs, ISET finds cell clusters that are being missed by CellSearch. These cell clusters might be important in metastasis, since they develop from dividing CTCs. These systems have a concordance rate of 55% in breast cancer for CTC positivity. Thus, using a combination of these methods may offer opportunities for increased performance [23].
The Ariol system is an image capture and analysis system for CTC detection. Deng et al. used the Ariol system to investigate whether enrichment using anti-CK or a combination of anti-CK and anti-EpCAM increases the sensitivity for CTC detection, because this combination is less dependent on weak EpCAM expression as presented for the CellSearch system. The Ariol system combines three fluorescent images (using FITC, TexasRed and DAPI) and one brightfield image (color of precipitate of choice) simultaneously from the same cell to detect CTCs, whereas CellSearch only uses three fluorescent images, which explaines the reduction in the number of false positive events for the Ariol system. The advantage of the brightfield image is the discrimination of cells from debris or cell fragments by identifying a smooth staining and round shape [17].
Flow cytometry uses highly specific monoclonal antibodies against CTC markers. High specificity is reached in this method by simultaneous analysis of multiple parameters, i.e. DNA content, cell size, cell viability, and intra- and extracellular markers. However, the sensitivity of flow cytometric techniques is lower compared to RT-PCR [2]. Flow-cytometric analysis of CK19-expressing CTCs in peripheral blood has been found to be a feasible and specific method to monitor breast carcinoma patients receiving chemotherapy after surgery [39].
EPISPOT (epithelial immunospot assay) detects tumor specific proteins (e.g. CK19 and MUC1), excreted only by viable tumor cells. Therefore, it differentiates between apoptotic and viable CTCs. The method is based on the enzyme-linked immunospot (ELISPOT) technology and determines protein secretion cell frequencies, allowing the assessment of protein secretion at the single cell level [1, 4, 15].
3.2 Nucleic acid-based techniques
PCR-based techniques measure the amount of DNA from CTCs, but have not been found to be specific due to the inability to differentiate between DNA from apoptotic and viable cells. RT-PCR-based techniques on the other hand, measuring mRNA, are more commonly used to estimate the number of CTCs. Since only viable CTCs produce mRNA, and mRNA from apoptotic cells is rapidly degraded, RT-PCR-based techniques may identify specifically those CTCs responsible for the metastatic process. However, the amount of mRNA that can be derived from the same cell may vary during the cell’s life cycle or as a result of dedifferentiation. This phenomenon makes it difficult to distinguish between changes in tumor cell numbers and changes in mRNA expression levels [40]. Furthermore, RT-PCR may measure nonspecific RNA from non-tumor cells, which can cause false positive results [9, 22]. Since the amount of CTCs cannot be measured exactly, RT-PCR-based techniques can be considered more useful for the characterization of CTCs than for its detection [1]. To detect most CTCs, a multi-marker approach is necessary. A number of markers that can be measured in breast cancer patients by RT-PCR are CK19, HER2/neu, MUC1, hMAM, EpCAM, EGFR, hTERT, survivin, mammaglobin, CD44 and c-Met. Although the measurement of tumor-specific mRNA/markers is more sensitive than immunocytochemistry for the detection of CTCs, if the resemblance between mRNA of CTCs and that shed by other blood cells is high, the specificity for CTCs may decline [4, 15, 33].
A promising new technique is the measurement of single RNA molecules with the RNAscope technology, which is used by CTCscope (Advanced Cell Diagnostics Inc., Hayward, CA, USA) for the detection of single CTCs from metastatic breast cancer patients, described by Payne et al. [26]. Minimal enrichment is necessary for CTCscope, and no dead or apoptotic cells are measured, since these do not produce mRNA molecules. This technique provides accurate prognostic and predictive information. Since up to now only this study by Payne et al. has been performed using CTCscope, more research on this technique should be performed to determine whether it is suitable for clinical practice [26].
AdnaTest BreastCancer is a RT-PCR-based device, measuring HER2, MUC1 and EpCAM in a multi-marker approach after immunomagnetic enrichment. It is a highly sensitive approach with a detection limit of two tumor cells. The concordance between CellSearch, AdnaTest and RT-PCR is 50–81% according to three different studies, which means that CTCs are being missed when only one technique is used. It should be investigated whether combinations of these techniques can provide a better sensitivity [1, 15, 33]. For now, Van der Auwera et al. found the combination of CK19 and mammaglobin RT-PCR (61%) to be the most sensitive method for CTC detection compared to CK19 (26%) or mammaglobin (54%) RT-PCR in single testing and to CellSearch (36%) and AdnaTest (22%) [33].
Classic immunocytochemistry remains the golden standard, but is rather time consuming. It therefore may be replaced by a fully automated cytometric-based device. Since all automated devices are dependent on the expression of tumor markers on the cell surface, it may be useful to combine the detection device with an RT-PCR based characterization device to increase its sensitivity.
4 Genetic and molecular characterization
Gerlinger et al. found evidence of intratumor heterogeneity with spatially separated heterogeneous somatic mutations and chromosomal imbalances. Of all somatic mutations found upon multi-region sequencing, 63–69% were heterogeneous and therefore not detectable in every sequenced region. They concluded that in a single tumor biopsy only a minority of genetic aberrations is found [41]. Therefore, CTCs do not always have the same genetic and molecular profile as the biopsy from the primary tumor, which makes CTCs an important tool to monitor the effectiveness of treatment schedules. Genetic and molecular characterization is, therefore, a prerequisite and can be performed by biomarker immunofluorescent staining, FISH, PCR-based techniques and comparative genomic hybridization [2].
Studies have shown the status conversion of HER2, the estrogen receptor (ER) and the progesterone receptor (PR) in CTCs. HER2 status conversion can give information about the use of HER2-targeting agents. Therefore, CTC HER2 status should be considered when choosing a therapy [1, 9, 38, 42]. CTCs in peripheral blood are often triple negative (negative for HER2, ER and PR), and these cells are usually more aggressive than non-triple negative CTCs [1, 36]. Most CTCs lack the proliferation antigen Ki-67, causing them to be resistant to regular chemotherapy [4].
Molloy et al. discovered a set of 34 genes that predicts the presence of CTCs when expressed in the primary tumor. Part of these genes encode cellular survival and proliferation factors, and others cellular migration and angiogenesis factors. These genes provided, independent from other prognostic markers, prognostic information about disease free survival [43].
Mostert et al. investigated the use of miRNA expression by CTCs as a serum tumor marker. CTC-associated miRNAs can provide important information about the subtype origin of tumor cells, which may be valuable for treatment decision making [16]. Saldova et al. investigated the changing glycosylation of CTCs. They hypothesized that a change in glycosylation may stimulate CTCs to cancer progression and to the development of metastases [44].
5 Cancer stem cells
Cancer stem cells (CSCs) constitute a subpopulation of the CTC fraction [19]. Epithelial-to-mesenchymal transition (EMT) can cause loss of EpCAM and CK expression, which can cause CTCs to change into CSCs. CSCs are often resistant to conventional therapies, such as chemotherapy and radiotherapy, therefore causing most of the metastases. Most CSCs are CD44+/CD24−/low and the most tumorigenic CSCs are also ALDH+. Overexpression of HER2 increases the ALDH-expressing CSC population. This increase can be inhibited by the use of trastuzumab [1, 2, 9, 19, 45, 46].
Breast CSC detection can be performed by four different techniques. The side population technique is based on stem cells excluding Hoechst 33342 without verapamil, but not excluding Hoechst 33342 with verapamil, whereas differentiated cells remain positive for the dye. The tumorsphere technique selects stem cells and progenitor cells, which are able to survive and proliferate in non-adherent and serum-free culture conditions, where differentiated cells undergo anoikis and die. The immunosorting technique is based on CD44+/CD24−/low surface markers, which select an important part of the CSC population, although, due to intratumor heterogeneity not all CSCs are detected by this method. The aldehyde dehydrogenase (ALDH) enzyme activity assay detects ALDH+ CSCs, which are, in contrast to ALDH− CSCs, able to generate tumors in NOD/SCID mice [47–49].
To prevent metastasis in breast cancer, CSCs should be targeted by new therapeutic approaches. One option is to direct the therapy specifically at stem cell self-renewal. Next to the WNT, NOTCH and hedgehog (HH) self-renewal pathways of normal stem cells, tumor suppressor genes such as PTEN and TP53 have been implicated in the regulation of CSC self-renewal. These pathways, that play a role in CSC self-renewal, but not in normal stem cell self-renewal, may represent potential targets for the development of new therapeutic strategies [48]. Xu et al. found that periostin protein expression was elevated in CSCs compared to control cells and that it was related to chemotherapy resistance. Therefore, periostin may be a potential target for breast cancer therapy [50].
6 Conclusions
CTCs play an important role in the metastatic spread of breast cancer. For CTC research, both enrichment and detection methods are available. The clinical application of these techniques should now be considered. Morphology-based enrichment techniques are not recommended for use in routine clinical practice, because of the relatively high number of cells lost during the preparation process. The use of immunology-based enrichment techniques is dependent on the expression and recognition of the correct antigens on CTCs. The latter techniques are very specific, but their sensitivity should be enhanced by using multiple antibodies to collect CTCs.
Of the cytometric detection techniques, CellSearch system and Ariol system are available for clinical practice, although their CTC detection rate is dependent on a combination of CK and EpCAM expression and, therefore, these systems may not detect all CTCs. To increase its sensitivity, these methods can be combined with the RT-PCR based AdnaTest. Other PCR-based techniques are more useful for the characterization of CTCs rather than its detection.
During the metastatic process, CTCs seem to acquire self-renewal capacity, similar to that of stem cells. Taking into account the intratumor heterogeneity, genetic and molecular characterization of CTCs should be considered before treatment decision. Emphasis should be put on CSC-specific enrichment and detection techniques for the further characterization of these cells to generate new targets for tailored treatment of metastatic disease.
Abbreviations
- CK:
-
Cytokeratin
- CSC:
-
Cancer stem cell
- CTC:
-
Circulating tumor cell
- DTC:
-
Disseminated tumor cell
- ELISPOT:
-
Enzyme-linked immunospot
- EMT:
-
Epithelial-to-mesenchymal transition
- EpCAM:
-
Epithelial cell adhesion molecule
- EPISPOT:
-
Epithelial immunospot assay
- FISH:
-
Fluorescence in situ hybridization
- ICC:
-
Immunocytochemistry
- ISET:
-
Isolation by size of epithelial tumor cells
- PCR:
-
Polymerase chain reaction
- PFS:
-
Progression free survival
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- OS:
-
Overall survival
References
E.S. Lianidou, A. Markou, Circulating tumor cells in breast cancer: detection systems, molecular characterization, and future challenges. Clin. Chem. 57(9), 1242–1255 (2011)
Y.F. Sun, X.R. Yang, J. Zhou, S.J. Qui, J. Fan, Y. Xu, Circulating tumor cells: advances in detection methods, biological issues, and clinical relevance. J. Cancer Res. Clin. Oncol. 137, 1151–1173 (2011)
Y. Park, T. Kitahara, T. Urita, Y. Yoshida, R. Kato, Expected clinical applications of circulating tumor cells in breast cancer. World J. Clin. Oncol. 2(8), 303–310 (2011)
T. Fehm, V. Müller, C. Alix-Panabières, K. Pantel, Micrometastatic spread in breast cancer: detection, molecular characterization and clinical relevance. Breast Cancer Res. 10, S1 (2008)
M. Cristofanilli, G.T. Budd, M.J. Ellis, A. Stopeck, J. Matera, M.C. Miller et al., Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351(8), 781–791 (2004)
M.C. Miller, G.V. Doyle, L.W.M.M. Terstappen, Significance of circulating tumor cells detected by the cell search system in patients with metastatic breast colorectal and prostate cancer. J. Oncol. 2010, 617421 (2010)
S. Braun, F.D. Vogl, B. Naume, W. Janni, M.P. Osborne, R.C. Coombes et al., A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353(8), 793–802 (2005)
S. Kasimir-Bauer, Circulating tumor cells as markers for cancer risk assessment and treatment monitoring. Mol. Diagn. Ther. 13(4), 209–215 (2009)
M. Balic, H. Lin, A. Williams, R.H. Datar, R.J. Cote, Progress in circulating tumor cell capture and analysis: implications for cancer management. Expert. Rev. Mol. Diagn. 12(3), 303–312 (2012)
S.E. Singletary, C. Allred, P. Ashley, L.W. Bassett, D. Berry, K.I. Bland et al., Staging system for breast cancer: revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg. Clin. N. Am. 83(4), 803–819 (2003)
J.E. Lang, K. Mosalpuria, M. Cristofanilli, S. Krishnamurthy, J. Reuben, B. Singh et al., HER2 status predicts the presence of circulating tumor cells in patients with operable breast cancer. Breast Cancer Res. Treat. 113, 501–507 (2009)
N. Harbeck, C. Thomssen, A new look at node-negative breast cancer. Oncologist 16, 51–60 (2011)
M.M. Reinholz, K.A. Kitzmann, K. Tenner, D. Hillman, A.C. Dueck, T.J. Hobday et al., Cytokeratin-19 and mammaglobin gene expression in circulating tumor cells from metastatic breast cancer patients enrolled in North Central cancer treatment group trials, N0234/336/436/437. Clin. Cancer Res. 17(22), 7183–7193 (2011)
H.M. Parsons, T.M. Tuttle, K.M. Kuntz, J.W. Begun, P.M. McGovern, B.A. Virnig, Association between lymph node evaluation for colon cancer and node positivity over the past 20 years. JAMA 306(10), 1089–1097 (2011)
V. Mikulová, K. Kološtová, T. Zima, Methods for detection of circulating tumour cells and their clinical value in cancer patients. Folia Biol. 57, 151–161 (2011)
B. Mostert, A.M. Sieuwerts, J.W.M. Martens, S. Sleijfer, Diagnostic applications of cell-free and tumor cell-associated miRNAs in cancer patients. Expert. Rev. Mol. Diagn. 11(3), 259–275 (2011)
G. Deng, M. Herrler, D. Burgess, E. Manna, D. Krag, J.F. Burke, Enrichment with anti-cytokeratin alone or combined with anti-EpCAM antibodies significantly the sensitivity for circulating tumor cell detection in metastatic breast cancer patients. Breast Cancer Res. 10, R69 (2008)
M. Ignatiadis, V. Georgoulias, D. Mavroudis, Circulating tumor cells in breast cancer. Curr. Opin. Obstet. Gynecol. 20, 55–60 (2008)
H. Lin, M. Balic, S. Zheng, R. Datar, R.J. Cote, Disseminated and circulating tumor cells: role in effective cancer management. Crit. Rev. Oncol. Hematol. 77, 1–11 (2011)
F. Nolé, E. Munzone, L. Zorzino, I. Minchella, M. Salvatici, E. Botteri et al., Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann. Oncol. 19, 891–897 (2008)
E. Botteri, M.T. Sandri, V. Bagnardi, E. Munzone, L. Zorzino, N. Rotmensz et al., Modeling the relationship between circulating tumour cells number and prognosis of metastatic breast cancer. Breast Cancer Res. Treat. 122, 211–217 (2010)
A. Gervasoni, R.M. Monasterio Muñoz, G.S. Wengler, A. Rizzi, A. Zaniboni, O. Parolini, Molecular signature detection of circulating tumor cells using a panel of selected genes. Cancer Lett. 263, 267–279 (2008)
F. Farace, C. Massard, N. Vimond, F. Drusch, N. Jacques, F. Billiot et al., A direct comparison of Cell Search and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br. J. Cancer 105, 847–853 (2011)
T. Tanaka, T. Ishikawa, K. Numayama-Tsuruta, Y. Imai, H. Ueno, T. Yoshimoto et al., Inertial migration of cancer cells in blood flow in microchannels. Biomed. Microdevices 14, 25–33 (2012)
W.E. Mesker, H. Vrolijk, W.C.R. Sloos, R.A.E.M. Tollenaar, H.J. Tanke, Detection of tumor cells in bone marrow, peripheral blood and lymph nodes by automated imaging devices. Cell. Oncol. 28, 141–150 (2006)
R.E. Payne, F. Wang, N. Su, J. Krell, A. Zebrowski, E. Yagüe et al., Viable circulating tumour cell detection using multiplex RNA in situ hybridisation predicts progression-free survival in metastatic breast cancer patients. Br. J. Cancer 106, 1790–1797 (2012)
J. Jacob, J. Krell, L. Castellano, L.R. Jiao, J. Stebbing, A.E. Frampton, Determination of cut-offs for circulating tumor cell measurement in metastatic cancer. Expert. Rev. Anticancer. Ther. 11(9), 1345–1350 (2011)
F.C. Bidard, C. Mathiot, S. Delaloge, E. Brain, S. Giachetti, P. De Cremoux et al., Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann. Oncol. 21, 729–733 (2010)
J.Y. Pierga, F.C. Bidard, C. Mathiot, E. Brain, S. Delaloge, S. Giachetti et al., Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin. Cancer Res. 14, 7004–7010 (2008)
H. Yagata, S. Nakamura, M. Toi, H. Bando, S. Ohno, A. Kataoka, Evaluation of circulating tumor cells in patients with breast cancer: multi-institutional clinical trial in Japan. Int. J. Clin. Oncol. 13, 252–256 (2008)
F.A.W. Coumans, S.T. Ligthart, J.W. Uhr, L.W. Terstappen, Challenges in the enumeration and phenotyping of CTC. Clin. Cancer Res. 18(20), 5711–5718 (2012)
U. De Giorgi, M. Mego, E.M. Rohren, P. Liu, B.C. Handy, J.M. Reuben et al., 18F-FDG PET/CT findings and circulating tumor cell counts in the monitoring of systemic therapies for bone metastates from breast cancer. J. Nucl. Med. 51, 1213–1218 (2010)
I. Van der Auwera, D. Peeters, I.H. Benoy, H.J. Elst, S.J. Van Laere, A. Prové et al., Circulating tumour cell detection: a direct comparison between the Cell Search system, the Adna test and CK-19/mammaglobin RT-PCR in patients with metastatic breast cancer. Br. J. Cancer 102, 276–284 (2010)
F.C. Bidard, D. Hajage, T. Bachelot, S. Delaloge, E. Brain, M. Campone et al., Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: a prospective observational study. Breast Cancer Res. 14, R29 (2012)
M.T. Sandri, L. Zorzino, M.C. Cassatella, F. Bassi, A. Luini, C. Casadio et al., Changes in circulating tumor cell detection in patients with localized breast cancer before and after surgery. Ann. Surg. Oncol. 17, 1539–1545 (2010)
J.Á. García-Sáenz, M. Martín, M.L. Maestro, M. Vidaurreta, S. Veganzones, S. Rafael et al., Circulating tumour cells in locally advanced breast cancer. Clin. Transl. Oncol. 11, 544–547 (2009)
A. Saad, A. Kanate, A. Sehbai, G. Marano, G. Hobbs, J. Abraham, Correlation among [18F]fluorodeoxyglucose positron emission tomography/computed tomography, cancer antigen 27.29, and circulating tumor cell testing in metastatic breast cancer. Clin. Breast Cancer 8(4), 357–361 (2008)
N. Hayashi, H. Yamauchi, Role of circulating tumor cells and disseminated tumor cells in primary breast cancer. Breast Cancer 19, 110–117 (2012)
L. Wang, Y. Wang, Y. Liu, M. Cheng, X. Wu, H. Wei, Flow cytometric analysis of CK19 expression in the peripheral blood of breast carcinoma patients: relevance for circulating tumor cell detection. J. Exp. Clin. Cancer Res. 28, 57 (2009)
L. Bertazza, S. Mocellin, D. Nitti, Circulating tumor cells in solid cancer: tumor marker of clinical relevance? Curr. Oncol. Rep. 10, 137–146 (2008)
M. Gerlinger, A.J. Rowan, S. Horswell, J. Larkin, D. Endesfelder, E. Gronroos et al., Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366(10), 883–892 (2012)
P. Kim, X. Liu, T. Lee, L. Liu, R. Barham, R. Kirkland et al., Highly sensitive proximity mediated immunoassay reveals HER2 status conversion in the circulating tumor cells of metastatic breast cancer patiënts. Proteome Sci. 9, 75 (2011)
T.J. Molloy, P. Roepman, B. Naume, L.J. Van’t Veer, A prognostic gene expression profile that predicts circulating tumor cell presence in breast cancer patients. PLoS ONE 7(2), 32426 (2012)
R. Saldova, J.M. Reuben, U.M. Abd Hamid, P.M. Rudd, M. Cristofanilli, Levels of specific serum N-glycans identify breast cancer patients with higher circulating tumor cell counts. Ann. Oncol. 22, 1113–1119 (2011)
M. Kakarala, M.S. Wicha, Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J. Clin. Oncol. 26(17), 2813–2820 (2008)
A. Giordano, H. Gao, S. Anfossi, E. Cohen, M. Mego, B.N. Lee et al., Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol. Cancer Ther. 11(11), 2526–2534 (2012)
J. Chen, Z.L. Chen, Technology update for the sorting and identification of breast cancer stem cells. Chin. J. Cancer. 29(3), 265–269 (2010)
E. Charafe-Jauffret, F. Monville, C. Ginestier, G. Dontu, D. Birnbaum, M.S. Wicha, Cancer stem cells in breast: current opinion and future challenges. Pathobiology 75(2), 75–84 (2008)
M.P. Ablett, J.K. Singh, R.B. Clarke, Stem cells in breast tumours: are they ready for the clinic? Eur. J. Cancer 48, 2104–2116 (2012)
Xu D, Xu H, Ren Y, Liu C, Wang X, Zhang H, et al. Cancer stem cell-related gene periostin: a novel prognostic marker for breast cancer. PLoS ONE 7(10), (2012)
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Broersen, L.H.A., van Pelt, G.W., Tollenaar, R.A.E.M. et al. Clinical application of circulating tumor cells in breast cancer. Cell Oncol. 37, 9–15 (2014). https://doi.org/10.1007/s13402-013-0160-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-013-0160-6