Abstract
Circulating tumor cells (CTCs) have a prognostic role in breast cancer (BC). The spread of hematogenic tumor cells is a crucial step in tumor progression, and blood-derived metastases are responsible for most breast cancer-related deaths. The identification and characterization of CTCs in the blood can provide prognostic information and help to identify patients who need more aggressive therapies and in monitoring treatment. Currently there are a multitude of assays for the detection of circulating tumor cells, but an extremely limited number of studies comparing the clinical relevance of the results with different test methods. Recently, breast cancer is the type of cancer in which CTC has been most studied, contributing to the clinical validity of CTC count both in early and metastatic stages. This chapter focuses on the possibility of the clinical applicability of CTCs in patients with breast cancer, in addition to demonstrating and comparing the CellSearch system (approved by the Food and Drug Administration – FDA) and ISET® Technology – Rarecells (Isolation by SizE of Tumor Cells) used for the identification and cytopathological characterization of CTCs. Therefore, we can state that the biological role of CTC as metastatic “seeds” and its corresponding clinical prognostic impact guarantee the use of CTC as an important biological and clinical tool in patients with BC.
Figures separated by Ludmilla T.D. Chinen and revised by Mauro Saieg (Cytopathologist from AC Camargo Cancer Center, São Paulo, SP – Brasil)
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Breast cancer (BC) is one of the most studied types of cancer since the last century. For this reason, numerous studies have investigated the correlation between circulating tumor cells (CTCs) and BC [1].
When we consider using CTCs as a biomarker, it becomes necessary to differentiate early BC (eBC) from metastatic BC (mBC). About 70% of patients with mBC stage IV have >1CTC in 7.5 ml of blood, using CellSearch system to isolate and quantify CTCs. However, in eBC, using this system, we rarely detect CTCs, prompting doubts about its clinical use as a biomarker.
The objective of this chapter is to evaluate the validity and clinical applicability of CTCs in early and advanced BC [2].
2.2 Micrometastasis Biomarkers in BC
Before the use of CTC as a biomarker of micrometastasis in BC, various studies tried to use bone marrow tumor cells (BMTCs) as a viable biomarker.
In 4 of 8 studies analyzed by Bidard et al., in 2016 [1], there was a correlation between BMTCs and CTCs that reached up to 94%. This same study concluded that the dissemination of tumor cells in the patients’ blood indicated an initial phase disease, while the detection of BMTCs indicated a more advanced disease [1].
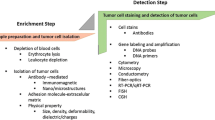
In most mBC studies, the preferred method used to identify CTCs is the CellSearch system. This system relies on a semi-automated enrichment and immunostaining device that has been, to this day, the only validated method approved by the US Food and Drug Administration (FDA) to detect CTCs and for prognostication in metastatic colorectal, prostate, and breast carcinomas. This specificity was reliably documented in normal individuals and in patients with benign tumors [8]. CTCs were defined by the CellSearch system as those co-expressing EpCAM and CKs without expressing leukocyte common antigen CD45, and positive for 4″,6-diamidino-2-phenylindole (DAPI) with a nucleus inside the cytoplasm and cell size ‘4 μm. It is important to emphasize that CTC detection using the CellSearch system does not rely on any true morphological criteria, but rather on the magnitude of antibody fluorescent signal for CK, DAPI, and CD45. The CellSearch system is an epithelium-associated marker-dependent method; therefore, it faces technical problems similar to the PCR-based molecular method; its inability to identify epithelial–mesenchymal transition (EMT)-induced CTCs can give false-negative results [3,4,5].
Another well-cited method of detecting CTCs is the ISET (isolation by size of epithelial tumor cells) method. ISET methodology is a direct method for CTC and circulating tumor microemboli (CTM) identification, in which CTCs are isolated by filtration without use of tumor-associated markers, as a consequence of their large size relative to circulating blood leukocytes. This method is easy to perform, rapid, and inexpensive and makes it possible to directly isolate and count tumor cells in patients with different types of carcinomas, by cytopathological analysis [6].
A study commanded by Farace in 2011 [7] comparing CellSearch and ISET methods, using different metastatic carcinomas, demonstrated quite considerable discrepancies between the number of CTCs enumerated by the CellSearch and the ISET systems. In total, 30% of patients were negative according to CellSearch, while only 5% were negative using ISET. Interestingly, these discrepancies depended mostly on the patients’ tumor type. Specifically, in patients with mBC, CTC counts were generally higher by CellSearch than by ISET. However, CTCs identified by CellSearch may not be true CTCs, because CTCs detected by CellSearch on the basis of the expression of an epithelial marker (EpCAM), which does not formally establish the malignant nature of circulating cells in the blood retained as CTC. Thus, the lower CTC counts obtained by ISET compared with CellSearch, most likely results from cell loss during the ISET procedure. It is important to state that this study did not compare the clinical relevance of both methods.
Although well-designed clinical trials are essential to further understand the clinical applications of ISET, this system could indeed represent a more accurate clinical tool for predicting patient’s outcome in certain tumor types, and provide a significant advantage for performing molecular analyses in the era of personalized medicine.
A review conducted by Ma in 2013 [9], confirmed these results. They concluded that, overall, more CTCs were detected by ISET than by the CellSearch system, for two reasons: (1) the CellSearch system may not detect cells if they have undergone EMT (i.e., lack expression of CK and/or EpCAM), while ISET can be much more efficient in isolating all rare cells of interest; (2) while ISET can isolate CTMs from metastatic cancer patients, the CellSearch cannot [10, 11]. Therefore, the detection of blood samples that only have CTMs will be underestimated by the CellSearch systems that use epithelial-marker-positive selection. However, the CellSearch system may overestimate CTCs in peripheral blood samples if they are contaminated with normal epidermal cells. In addition, the CTC detection efficiency varies in all relevant studies, whether by ISET or by CellSearch system. One of the main advantages of the CellSearch system is that it has the capacity to detect smaller CTCs than does ISET. On the other hand, the use of ISET for detection and identification of CTCs is more reliable than the CellSearch system and requires no expensive or special laboratory equipment. However, ISET is not sufficiently standardized in its current form to be routinely applicable in clinical practice (please see some pictures of CTCs isolated from metastatic breast cancer patients in Figs. 2.1, 2.2, 2.3, 2.4, 2.5, 2.6, 2.7, 2.8, 2.9, 2.10, 2.11, 2.12, and 2.13).
CTCs from the same patient of Fig. 2.1. CTCs were collected around 4–5 weeks after radiotherapy for brain metastatis. CTC count: 3.0 CTCs/mL
CTCs from patient of Fig. 2.3. CTCs were collected around 4–5 weeks after radiotherapy for brain metastatis. CTC count: 1.5 CTCs/mL. We can observe the presence of a hyperchromic nucleus, irregular, with irregular chromatin. Also note the abundant cytoplasm, not commonly seen in hematopoietic cells. In brown: positive staining with DAB for STGAL
Patient with 57 years old. CTCs were collected before the beginning of radiotherapy for brain metastatis. Her primary tumor was HER-2 positive. CTC count: 0.75 CTCs/mL. On the right, we can observe the presence of a hyperchromic nucleus, irregular, with irregular chromatin. Also note the abundant cytoplasm, not commonly seen in hematopoietic cells. CTC stained with HER-2
Photo from same patient Fig. 2.8 showing a cohesive group of neoplastic cells, with planetary aggregation, forming neoplastic impaction. Individually, isolated neoplastic cells are noted with alteration of the nuclear/cytoplasmic ratio and irregularity of chromatin (microscope 40×)
Same patient of Fig. 2.11 in brown : immunocytochemistry with anti-Notch antibody visualized with DAB. Here, we can see a CTC without any staining
Same patient of Fig. 2.11
2.3 Metastatic BC
2.3.1 Clinical Validity of CTCs in mBC
In contrast to that observed in eBC, there is enough evidence to utilize CTCs as a biomarker in mBC.
A study conducted by Cristofanilli in 2004 [12], utilizing the CellSearch® system to detect CTCs, analyzed the number of CTCs in patients with mBC. Before initiating a new treatment, patients underwent an evaluation of metastatic sites by means of standard imaging studies and the collection of a blood sample to be used for the enumeration of circulating tumor cells. A different blood sample was collected at the first follow-up visit, approximately 3 to 4 weeks after the initiation of the new therapy. Disease status follow-ups were made every 9 to 12 weeks, utilizing the same techniques used at baseline. This disease status was assessed without knowledge of the levels of CTCs. An alternate control group made up of 72 premenopausal healthy women and 73 postmenopausal healthy women without known illnesses and no oncologic history, 99 women with benign breast diseases, and 101 women with other nonmalignant diseases. The respective testing laboratories were aware that the samples were from a control group, but were unaware to the difference between no known illness and benign conditions.
A worse prognostic relation was established in patients with a high number of CTCs in both instances, when compared to those with a low number of CTCs pre-CT and after one cycle. Interestingly, patients with a high CTC count pre-CT, but with a low count after one cycle, had a similar prognostic value to those with a low pre-CT count. These results were corroborated by Hayes in 2006 [13].
Finally, an analysis of 1944 individuals indisputably established the superiority of using CTC count in comparison to traditional tumor markers, such as CEA and CA15, as a treatment response biomarker in patients with mBC [14].
2.4 Clinical Applicability of CTC in mBC
In a retrospective study conducted by Cristofanilli in 2018 [15], 2436 patients with mBC from 18 cohort studies were analyzed. These patients were arranged in accordance to their tumor’s biomolecular type, location, and previous treatments. A cut-off point of 5 CTCs per 7.5 ml of blood was established. Thus, a > 5CTC/7.5 mL count was determined as IV aggressive (IVa) and <5CTC/7.5 mL count as IV indolent (IVi).
Patients IVi had a higher median overall survival, when compared to those stage IVa (36.3 months vs. 16.0 months, p < 0,0001). Furthermore, patients IVi had a higher overall survival in all tumor subtypes when compared to IVa: positive hormone receptor (44 months vs. 17.3 months, P < 0.0001), HER2-positive (36.7 months vs. 20.4 months, P < 0.0001), and triple-negative (23.8 months vs. 9.0 months, P < 0.0001). Similar results were obtained independent of previous treatment or tumor location [15].
2.5 Early BC
2.5.1 CTCs as a Micrometastasis Marker in Patients with eBC Treated with Neoadjuvant Therapy
Measuring CTCs in patients, submitted to neoadjuvant chemotherapy (CT), intents on evaluating if the micrometastasis process has started and possibly evaluating its response to QT.
The IMENEO meta-analysis observed a significant association between T staging and CTCs (P < .001), using CellSearch system. Excluding tumors T4d from analysis, they observed that a positive CTC result was detached from clinical or pathological characteristics of the initial tumor. The positivity was 21.4% and 24.2% in patients with negative and positive lymph nodes, respectively. This study also showed that there was a statistically significant drop of CTC count at the end of neoadjuvant QT (p < 0.001). Furthermore, the CTC count pre-QT presented itself as a strong independent indicator of distant metastasis (hazard ratio [HR]: 3.73, 95% confidence interval [CI] = 2.82–4.90), overall survival (HR: 3.93, 95% CI = 2.81–5.45) and local relapse (HR: 3.02, 95% CI = 1.88–4.75) [16]. Curiously, the survival impact was directly related to the number of CTCs detected, suggesting the use of CTCs as a quantitative biomarker in BC (see some examples in Table 2.1).
2.5.2 CTC as a Micrometastasis Marker in Patients with eBC Treated with Adjuvant Therapy
In the context of adjuvant therapy in eBC, a multicentric randomized German study, SUCCESS-A, which tested CTCs in patients eligible to receive adjuvant CT, correlated the positivity of CTC to the lymph node status. This study confirmed that CTCs are an independent factor for disease-free survival (HR: 2.11, 95%CI = 1.49–2.99) and overall survival (HR:2.18, 95%CI = 1.32–3.59). Finally, a high CTC count was associated with worse prognosis, validating the use of CTCs as a quantitative biomarker [17]. The recently published 2-year follow-up of this study showed that those patients that had a positive CTC count after 2 years of treatment had a risk 3.9 times higher of death and 2.3 times higher of relapse in the multivariate models, when compared to those that had a negative result; all these results were true in those patients with HER2-negative BC [18].
In 2018, Sparano et al. [19] conducted a study that analyzed the recurrence of CTC detection after 4.5–7.5 years of follow-up in patients with HER2-negative BC that received primary surgical treatment, followed by adjuvant CT. In the multivariate models, a positive CTC was associated with a risk 13.1 times higher of recurrence in patients with positive hormone receptors (HR: 13.1, 95% CI = 4.7–36.3). No patients with negative hormone receptors and positive assay had a recurrence of CTC (0%, 95% CI = 0% to 37%).
The TREAT-CTC trial was the first attempt to try to demonstrate the clinical applicability of CTCs in patients with eBC. This study also tried to evaluate if the addition of a new adjuvant therapy (Trastuzumab) would help to elongate the relapse-free interval in patients with a positive CTC count. This study, therefore, concluded the following: (1) CTC-based screening is feasible in the adjuvant setting of early breast cancer. (2) CTC-positive patients do have a higher risk of relapse. (3) Trastuzumab has no effect on CTCs in HER2-negative BC [20,21,22,23].
Therefore, the use of CTCs as an evaluating tool of metastatic risk in eBC still needs further scientific comprobation. However, it is highly probable that the number of CTCs will have a significant impact as a prognostic and metastatic biomarker in eBC [1].
2.6 Conclusion
The use of CTCs as a prognostic factor in early and mBC has been shown to be quite significant. Despite the detection of CTCs in eBC being a rare event, its clinical validity as a prognosis marker has reached the highest level of scientific evidence. However, its clinical applicability is still a subject to be studied.
Focusing on adjuvant treatments such as radiotherapy, QT, and hormonal therapy, and associating these with new detecting techniques and with new biomarkers such as circulation tumor DNA, will possibly reveal new treatments and early micrometastasis diagnosis [24, 25].
And finally, when we are talking about patients with mBC, the quantitative and qualitative CTC analysis must be considered an important tool with prognostic and therapeutic implications.
References
Bidard FC, Proudhon C, Pierga JV. Circulating tumor cells in breast cancer. Mol Oncol. 2016;10:418e4 3 0.
Thery L, Meddis A, Cabel L, Proudhon C, Latouche A, Pierga JV, Bidard FC. Circulating tumor cells in early breast cancer. JNCI Cancer Spectrum. 2019;3(2):pkz026.
Sastre J, Maestro ML, Puente J, Veganzones S, Alfonso R, Rafael S, Garcia-Saenz JA, Vidaurreta M, Martin M, Arroyo M, Sanz-Casla MT, Diaz-Rubio E. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann Oncol. 2008;19:935–8. https://doi.org/10.1093/annonc/mdm583.
de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. https://doi.org/10.1158/1078-0432.CCR-08-0872.
Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Janicke F, Jackson S, Gornet T, Cristofanilli M, Pantel K. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–8. https://doi.org/10.1158/1078-0432.CCR-06-1695.
Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000;156(1):57.
Farace F, Massard C, Vimond N, et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer. 2011;105:847–53.
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904.
Ma YC, Wang L, Yu FL. Recent advances and prospects in the isolation by size of Epithelial Tumor Cells (ISET) methodology. Technol Cancer Res Treat. ISSN1533-0346. 2013;12(4):295.
Krebs MG, Hou JM, Sloane R, Lancashire L, Priest L, Nonaka D, Ward TH, Backen A, Clack G, Hughes A, Ranson M, Blackhall FH, Dive C. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7:306–15. https://doi.org/10.1097/JTO.0b013e31823c5c16.
Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, Clack G, Ranson M, Blackhall F, Dive C. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. 2011;178:989–96.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LWMM, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781e791.
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LWWM. Circulating tumor cells at eachfollow-up time point during therapy of metastatic breastcancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218e4224.
Bidard F-C, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406e414.
Cristofanilli M, Pierga J-Yves, Reuben J, et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): International expert consensus paper, critical reviews in oncology. Hematology. 2018; https://doi.org/10.1016/j.critrevonc.2018.12.004
Bidard F-C, Michiels S, Riethdorf S, et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J Natl Cancer Inst. 2018;110(6):560–7.
Rack B, Schindlbeck C, J€uckstock J, Andergassen U, Hepp P, Zwingers T, Friedl TWP, Lorenz R, Tesch H, Fasching PA, Fehm T, Schneeweiss A, Lichtenegger W, Beckmann MW, Friese K, Pantel K, Janni W, SUCCESS Study Group. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014:106, dju066.
Trapp E, Janni W, Schindlbeck C, et al. Presence of circulating tumor cells in high-risk early breast cancer during follow-up and prognosis. J Natl Cancer Inst. 2019;111(4):380–7.
Sparano J, O’Neill A, Alpaugh K, et al. Association of circulating tumor cells with late recurrence of estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(12):1700–6.
Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358(13):1409–11.
Georgoulias V, Bozionelou V, Agelaki S, et al. Trastuzumab decreases the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy-resistant CK-19mRNA-positive circulating tumor cells: results of a randomized phase II study. Ann Oncol. 2012;23(7):1744–50.
Ignatiadis M, Litie’re S, Rothe F, et al. Trastuzumab versus observation for HER2 non amplified early breast cancer with circulating tumor cells (EORTC 90091 10093, BIG 1-12, Treat CTC): a randomized phase 2 trial. Ann Oncol. 2018;29(8):1777–83.
Fehrenbacher L, Cecchini R, Geyer C, et al. Abstract GS1-02: NSABP B-47 (NRG oncology): phase III randomized trial comparing adjuvant chemotherapy with adriamycin (A) and cyclophosphamide (C) ! weekly paclitaxel (WP), or docetaxel (T) and C with or without a year of trastuzumab (H) in women with node-positive or high-risk node-negative invasive breast cancer (IBC) expressing HER2 staining intensity of IHC 1. or 2. with negative FISH (HER2- Low IBC). Cancer Res. 2018;78:GS1–G02.
Schwarzenbach H, Pantel K. Circulating DNA as biomarker in breast cancer. Breast Cancer Res. 2015;17(1):136.
Sparano JA, Henry NL. Surveillance after treatment of localized breast cancer: time for reappraisal? J Natl Cancer Inst. 2019;111(4):339–41.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
de Castro, D.G., Chen, F.K. (2021). CTCs in Solid Tumors. Clinical Applications of Circulating Tumor Cells in Breast Cancer. In: Chinen, L.T.D. (eds) Atlas of Liquid Biopsy. Springer, Cham. https://doi.org/10.1007/978-3-030-69879-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-69879-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69878-2
Online ISBN: 978-3-030-69879-9
eBook Packages: MedicineMedicine (R0)