Abstract
Backgrounds
A disintegrin and metalloproteinase (ADAM) 17 has been indicated to be an indispensable regulator of cellular events from proliferation to migration. Although prognostic importance of ADAM17 expression has been investigated in several tumours, its clinical utility as a useful prognostic molecular marker remains unclear in gastric cancer. In the current study, we evaluated the expression of ADAM17 and its prognostic significance in gastric cancer patients after curative gastrectomy.
Methods
The prognostic significance of ADAM17 expression was analysed immunohistochemically in 156 patients with gastric cancer who had undergone curative gastrectomy, and the relationship between its expression and clinicopathological factors was also evaluated.
Results
High ADAM17 expression was detected in 79 patients (51 %), whereas low expression was found in 77 cases (49 %). There was significant correlation between gender, histology, lymph node metastasis, vascular invasion, the presence of recurrence and high ADAM17 expression. Recurrence in patients with high ADAM17 expression was significantly higher than that for patients with low ADAM17 expression (p = 0.032). The median disease-free survival (DFS) time for patients with tumours with high ADAM17 expression was worse than that of patients with tumours with low ADAM17 expression (16.6 vs. 44.2 months, p = 0.004). In addition, patients with low ADAM17 expression had a higher median overall survival (OS) (49.6 vs. 26.9 months, p = 0.019) compared to those with high ADAM17 expression. Multivariate analysis indicated that the rate of ADAM17 expression was an independent prognostic factor for DFS, in addition to the already known important clinicopathological prognostic indicator. But the prognostic importance of ADAM17 expression could not be proved by multivariate analysis for OS.
Conclusions
The potential value of ADAM17 expression as a useful molecular marker in gastric cancer progression should be evaluated comprehensively; it may predict recurrence and poor prognosis in patients with gastric cancer after curative resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer remains one of the most common cancers and causes of cancer-related deaths globally [1, 2]. Although the prevalence has decreased in recent years, the expected recovery in the disease’s prognosis has not been improved. The most important causes of mortality in gastric cancer patients include tumour invasion and metastases after gastrectomy. This process is a multi-stepped complex process, including adhesion molecules, proteolytic enzymes, angiogenesis and growth factors [3, 4]. Another factor that plays a role in this process is ADAM17, also known as a tumour necrosis-converting enzyme (TACE), which is a member of the ADAM (a disintegrin and metalloproteinase) protein family [3–5]. From proliferation to migration, it is the irreplaceable regulator of almost every cellular activity [3–5].
The central role of ADAM17 in cell regulation is due to its various substrates. From these substrates, cytokines, growth factors and their receptors and adhesion molecules are all activated or inactivated by ADAM17 cleavage [3–5]. All of these factors exist in a natural balance. Tissue homeostasis, i.e. keeping anabolism and catabolism at a balance, is due to a healthy continuation of the apoptosis/proliferation balance. Losing this balance plays a role in carcinogenesis. A high expression of ADAMs, including ADAM17, is mostly related with cancer [6, 7].
In most cancer types, the high expression of ADAM17 is a poor prognostic factor [5–7]. As with other cancer types, a few trials have previously analysed the relation between the expression of ADAM17 and gastric cancer in the literature. Shou et al. [8] showed that in gastric cancer patients who had undergone curative gastrectomy, high ADAM17 expression was related with poor prognosis, and there was a significant association between the expression levels of ADAM17 and disease-free survival (DFS) and overall survival (OS). Zhang et al. [9] showed similar results in their study.
In the present study, we aimed to identify the prognostic importance of the expression of ADAM17 in patients with gastric cancer who underwent radical gastrectomy. It was also investigated if there was any relation between ADAM-17 expression and clinicopathological factors and the effect of this expression on survivals.
Methods
A total of 156 patients with gastric cancer who had undergone a R 0 curative gastrectomy and who were followed up and treated at the Department of Medical Oncology, Dr. Lütfi Kirdar Education and Research Hospital, between September 2007 and July 2013. Details concerning age, gender, resection type, tumour location, histopathology, pT stage, tumour size, histological grade, lymph node involvement, lymphatic vessel invasion, blood vessel invasion and perineural invasion, resection margins, adjuvant chemotherapy and radiation therapy, responses to treatment and survival were obtained from patients’ charts after written informed consent had been obtained from patients or their relatives. Furthermore, the Local Ethics Committee of our hospital approved the study.
Primary tumours were staged according to the seventh edition of American Joint Committee on Cancer (AJCC) TNM staging classification for gastric cancer [10]. Moreover, clinicopathological findings were determined according to the Japanese Classification of Gastric Carcinoma (JCGC) [11]. D1 dissection was defined as the dissection of the perigastric nodes directly attached along the lesser curvature and greater curvatures of the stomach (stations 1–6, N1 level). An incomplete N1 dissection was defined as D0 lymphadenectomy. D2 dissection (N2 level) was defined as the dissection of nodes along the left gastric artery (station 7), common hepatic artery (station 8), celiac trunk (station 9), splenic hilus and splenic artery (station 10 and 11). D3 dissection included the dissection of lymph nodes at stations 12 through 14, along the hepatoduodenal ligament and the root of the mesentery (N3 level).
Follow-up schedule
Medical histories and physical examinations were carried out every 3 months in the first post-operative year, every 6 months in the second post-operative year and annually thereafter for at least 5 years during follow-up. Complete blood counts and biochemistry panels, as well as tumour markers were examined every 3 months in the first and second years, and annually thereafter. Chest X-rays and abdominal CT scans were also performed every 3 months in the first year, every 6 months in the second post-operative year and annually thereafter for 5 years.
Evaluation of ADAM17 expression
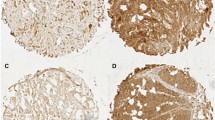
Histological tumour specimens were re-evaluated by two pathologists, who were experts in matters of gastric cancer and who had no knowledge of pathological findings. Firstly, paraffin blocks of gastric tumour tissue were cut into sections of 3 μm and deparaffinised. Hydrogen peroxide was applied after antigen retrieval solution to block the endogen peroxide activity. Next, sections were incubated with monoclonal mouse anti-human ADAM17 (Novus-Biologicals-NBP1-74061) antibody in 1/200 dilution. After being applied to Bond primary and Bond polymer, the sections were kept in Bond DAB mix solution, and haematoxylin was applied as contrast stain. The intensity of cellular staining and the proportion of stained tumour cells were scored separately. The scoring was designed according to the staining intensity of the tumour cells in sections: score 0, no stain; score 1, weak stain; score 2, medium stain and score 3, strong stain. The scoring was designed according to the proportion of stained tumour cells in the sections: score 0, less than 5 % stained; score 1, between 6–25 % stained, score 2, between 26–50 % stained and score 3, more than 51 % stained. The product of the scores for intensity and proportion was used to indicate the level of protein expression. The expression of protein was considered low if the product was 3 or less, and high if the product was 4 or more. Figure 1 shows the immunohistochemical staining intensity of ADAM17 in gastric cancer specimens.
Adjuvant treatment
More than half of the patients (n = 102, 65 %) were treated with adjuvant chemotherapy. The majority received fluoropyrimidine-based regimens, but only 78 patients (77 %) were able to complete their adjuvant treatment. Seventy-nine (51 %) of the patients received adjuvant radiotherapy, but only 70 (89 %) patients were able to complete the adjuvant treatment. During the follow-up, 69 (44 %) patients developed relapse. Regarding the localization of the relapse, 38 % of the patients had relapse in the liver, others in the peritoneum, para-aortic lymph nodes, lung, stomach, bone or ovaries. Only 38 % of the patients with metastases were received palliative chemotherapy.
Statistical methods
All of the statistical analyses were made with SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Descriptive analyses were presented using means and standard deviations for normally distributed variables. The significance of the differences among the means were determined by the Mann–Whitney U test. The chi-squared test and Fisher’s exact test were used to analyse the relationships between clinicopathological factors and ADAM17 expression. Survival analysis and curve were compared using the log-rank test by the Kaplan–Meier method. Disease-free survival (DFS) was defined as the time from curative surgery to disease progression or recurrence, or to the date of death or lost in follow-up. Overall survival (OS) was described as the time interval from diagnosis to the date of the patient’s death or lost in follow-up. Univariate analyses were carried out to evaluate the significance of ADAM17 expression and other clinicopathological features as prognostic factors, then the multivariate analysis with the Cox proportional hazards model was performed in order to further analyse the ADAM17 expression and all of the significant prognostic factors which were found in the univariate analysis. The confidence intervals (CI) of 95 % were defined to indicate the relationship between overall survival and each independent factor. All p values are two sided, and the values less than 0.05 were accepted to be statistically significant.
Results
One hundred patients (64 %) were male and 56 (36 %) were female, with a median age of 61 years (range 26–82 years). Seventy-one patients were older than 60 years (51 %). Fifty-five percent of the patients underwent subtotal gastrectomy and 45 % underwent total gastrectomy. The majority of the patients (54 %) had tumours located in the lower part, 22 % in the middle part and 21 % in the upper part of the stomach. Most of the patients (n = 136, 87 %) had Eastern Cooperative Oncology Group (ECOG) Performance Status between 0–1. Histopathological analysis revealed that 54 % had pure adenocarcinoma histology, and more than half (n = 95, 61 %) had poorly or undifferentiated tumours. According to the type of lymph node dissection, the majority of patients underwent D1 and D2 lymphadenectomy (46.2 and 37.2 %, respectively), while D0 dissection was performed in 11 patients (7.1 %) and D3 in 15 patients (9.6 %). Based on the presence of lymph node metastasis, 70 % of the patients were classified as node-positive (16 % had N1, 20 % had N2 and 34 % had N3 lymph node involvement), the remaining 30 % of the patients were node-negative. Seventeen patients (11 %) were classified as stage I, 56 (36 %) as stage II and 83 (53 %) as stage III according to the TNM staging. After IHC analysis was carried out to evaluate the frequency of ADAM17 expression in 156 gastric tumour samples, low ADAM17 expression was detected in 77 (49 %) patients, whereas 79 patients (51 %) had high ADAM17 expression. Tumour diameter, histopathology, lymph node involvement, blood vessel invasion and the presence of recurrence were found to be significantly related to ADAM17 expression levels. The rate of high ADAM17 expression was significantly higher in patients with tumour diameters >5 cm (p = 0.04) and vascular invasion (p = 0.015). High ADAM17 expression was closely correlated with lymph node metastasis (p = 0.003) compared with low ADAM17 expression. However, tumour with pure adenocarcinoma histology highly expressed ADAM17 more than other types (p = 0.029). The presence of recurrence for patients with high ADAM17 expression was significantly higher than that for patients with low ADAM17 expression (p = 0.032). The relationships between clinicopathological factors and ADAM17 expression levels are listed in Table 1.
At the median follow-up of 23 months (range 3–80 months), the median DFS time of patients with low ADAM17 expression was significantly better than that of patients with high ADAM17 expression (44.2 vs. 16.6 months, p = 0.004, Fig. 2). On the other hand, patients with low ADAM17 expression had the higher OS interval compared to patients with high ADAM17 expression (49.6 vs. 26.9 months, p = 0.019). Figure 3 shows the OS curves according to the ADAM17 expression.
In the univariate analysis for DFS in the entire cohort of patients, ADAM17 expression, tumour localization, vascular and perineural invasion, lymph node involvement, pT stage and TNM stage were found to be statistically significant prognostic factors. Furthermore, the univariate analysis showed that ADAM17 expression, vascular and perineural invasion, lymph node involvement, pT stage, TNM stage were important prognostic indicators for OS. The results of univariate analysis according to DFS and OS are summarized in Tables 2 and 3.
A multivariate analysis with the Cox proportional hazards model was performed in order to further evaluate all of the significant prognostic factors that were found in the univariate analysis for survival. For DFS, it was found that the rate of ADAM-17 expression [p = 0.038, HR 1.61 (0.93–2.79)] was an independent prognostic factor, as were TNM stage [p = 0.04, HR 2.12 (1.02–4.42)] and vascular invasion [p = 0.016, HR 3.53 (1.26–9.82)] However, in the multivariate analysis, lymph node involvement and vascular invasion were independent prognostic indicators for OS [p = 0.023, HR 1.95 (0.96–3.15) and p = 0.03, HR 2.69 (1.10–6.61), respectively]. Table 4 shows the results of multivariate analysis of DFS and OS.
Discussion
A member of the ADAM enzyme family, ADAM17, is an essential regulator of almost every cellular incident, from proliferation to migration [3–5]. Because the enzyme processes growth factors required for tumour development and multiplication, ADAM17 is related to carcinogenesis; it also plays a role in inflammation, which is frequently seen in tumours. It has been proven that the increase in tissue shedding of the epidermal growth factor ligands has a role in showing the malignant phenotype [12], and high expression of ADAMs, including ADAM17, is generally related with malignant disease [6, 12]. Although ADAM17 has a role in many cancer types, its role has currently been observed in greatest detail in breast cancer [13, 14]. In breast cancer, overexpression of ADAM17 has shown to be related with TGFα expression [14, 15], tumour progression and metastasis [14–16]. Moreover, increased ADAM17 expression has found to be an indicator of shorter survival [16, 17]. Moreover, in many cancer types, a high expression of ADAM17 has found to be important prognostic factor for malignancy [7].
In the literature, only few clinical trials have addressed the relation between gastric cancer and ADAM17 expression levels. In those studies, it was shown that in gastric cancer patients who had undergone curative gastrectomy, high expression levels of ADAM17 was related with poor prognosis of malignancy, and levels of ADAM17 expression were significantly related to DFS and OS [8]. In our study, we detected that ADAM17 expression levels were significantly associated with tumour diameter, histopathology, lymph node involvement, vascular invasion and the presence of recurrence. Patients with tumours greater than 5-cm diameter, pure adenocarcinoma histology, lymph node involvement, showing vascular invasion and who developed recurrence during follow-up, had higher ADAM17 expression levels. A retrospective study performed by Shou et al. [8], in 436 patients, who underwent curative gastrectomy and lymph node dissection with gastric cancer, showed that patients with advanced pT stage (pT3, pT4) and TNM stage were found to be significantly related to high ADAM17 levels. Thus, our results were compatible with their findings [8]. Likewise, Zhang et al. retrospectively analysed 220 gastric cancer patients who underwent radical gastrectomy in their study. The authors found that tumours with malignant differentiation, advanced depth of invasion (pT3, pT4), lymph node involvement, advanced TNM stage and development of metastases during follow-up had significantly higher ADAM17 expression levels [9]. In the univariate analysis, DFS was found to be significantly longer in patients with gastric tumour localized in the upper and lower parts of the stomach, without blood vessel and perineural invasion, without lymph node involvement, with pT1, pT2, with stages I–II and low ADAM17 expression. In our study, the median DFS time was found to be significantly higher for patients with low ADAM17 expression compared to patients with high ADAM17 levels (44.2 vs. 16.6 months, p = 0.004). In addition, the multivariate analysis for DFS demonstrated that TNM stage, blood vessel invasion and ADAM17 expression were independent prognostic factors. Similarly, Shou et al. and Zhang et al. reported that the median DFS interval was significantly longer for patients with low ADAM17 expression, and ADAM17 expression was identified to be an independent prognostic indicator. Hence, our results were compatible with the literature [8, 9].
When the univariate analysis was carried out for OS, it showed that the median OS interval was significantly shorter for patients with blood vessel and perineural invasion, lymph node involvement, advanced pT and TNM stages and high ADAM17 expression. The patients with low ADAM17 expression had the median OS time of 49.6 months, whereas in those with high ADAM17 expression, OS was found to be 26.9 months (p = 0.019). In the multivariate analysis for OS, it indicated that the only independent risk factors were lymph node involvement and vascular invasion. In other words, the prognostic significance of ADAM17 expression could not be showed by the multivariate analysis. In the study by Shou et al. [8], similar to our study, patients with low ADAM17 expression had significantly longer OS (51.7 vs. 27.4 months, p < 0.001). In contrast to our results, both Shou et al. [8] and Zhang et al. [9] identified ADAM17 expression as an independent prognostic factor of both DFS and OS. This might be related to the relatively small sample size and short follow-up interval of the study.
The present study has several limitations. The retrospective nature of our study and short follow-up interval are major limitations, which might have influenced our results. The other important limitation of this study is the relatively small sample size. Although our results should be confirmed using prospective studies with larger sample sizes, we believe that our study is noteworthy and our results contribute to the knowledge of tumour invasion and metastasis for gastric cancer.
In conclusion, our study indicates that high ADAM17 expression was related to a poor prognosis for patients with gastric cancer who underwent curative surgery. ADAM17 may be an important molecular marker for carcinogenesis, prognosis and progression in gastric cancer. To validate the findings of this study, further research is needed, preferably by prospective studies that analyse the correlation between ADAM17 expression and treatment response including more patients. Thus, it would be possible to develop treatments targeting ADAM17 and advance new treatment strategies for gastric cancer.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50.
Blobel CP. ADAMs: key components in EGFR signaling and development. Nat Rev Mol Cell Biol. 2005;6:32–43.
Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Asp Med. 2008;29:258–89.
Gooz M. ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol. 2010;45(2):146–69.
Duffy MJ, McKiernan E, O’Donovan N, McGowan PM. Role of ADAMs in cancer formation and progression. Clin Cancer Res. 2009;15:1140–4.
Rose-John S. ADAM17, shedding, TACE as therapeutic targets. Pharmacol Res. 2013;71:19–22.
Shou ZX, Jin X, Zhau ZS. Upregulated expression of ADAM17 is a prognostic marker for patients with gastric cancer. Ann Surg. 2012;256(6):1014–22.
Zhang TC, Zhu WG, Huang MD, Fan RH, Chen XF. Prognostic value of ADAM17 in human gastric cancer. Med Oncol. 2012;29(4):2684–90.
Edge SB, Byrd DR, Compton CC, et al., editors. American Joint Committee on Cancer Staging Manual. 7th ed. Springer, New York; 2010. p. 117.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 2nd English ed. Gastric Cancer. 1998;1:10–24.
Katakowski M, Jiang F, Zheng X, Gutierrez JA, Szalad A, Chopp M. Tumorigenicity of cortical astrocyte cell line induced by the protease ADAM17. Cancer Sci. 2009;100:1597–604.
Arribas J, Bech-Serra JJ, Santiago-Josefat B. ADAMs, cell migration and cancer. Cancer Metastasis Rev. 2006;25:57–68.
Zheng X, Jiang F, Katakowski M, Zhang ZG, Lu QE, Chopp M. ADAM17 Promotes breast cancer cell malignant phenotype through EGFR-PI3K-AKT activation. Cancer Biol Therapy. 2009;8(11):1045–54.
Borrell-Pages M, Rojo F, Albanell J, Baselga J, Arribas J. TACE is required for activation of the EGFR by TGF-alpha in tumors. EMBO J. 2003;22:1114–24.
McGowan PM, Ryan BM, Hill AD, McDermott E, O’Higgins N, Duffy MJ. ADAM-17 expression in breast cancer correlates with variables of tumor progression. Clin Cancer Res. 2007;13:2335–43.
McGowan PM, McKiernan E, Bolster F, Ryan BM, Hill AD, McDermott EW, et al. ADAM-17 predicts adverse outcome in patients with breast cancer. Ann Oncol. 2008;19:1075–81.
Conflict of interest
This manuscript was not supported by any financial or other relationships.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was presented in ASCO, 2015, Gastrointestinal Cancer Symposium, in a poster session. January 15–17, 2015, San Francisco, California.
Rights and permissions
About this article
Cite this article
Aydin, D., Bilici, A., Yavuzer, D. et al. Prognostic significance of ADAM17 expression in patients with gastric cancer who underwent curative gastrectomy. Clin Transl Oncol 17, 604–611 (2015). https://doi.org/10.1007/s12094-015-1283-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1283-1