Abstract
A disintegrin and metalloproteinase-17 (ADAM17, also named as tumor necrosis factor-alpha-converting enzyme) is a member of the ADAM family. Of all ADAMs, the strongest evidence for a role in malignancy exists for ADAM17. Especially, it has been demonstrated that ADAM17 expression was significantly increased in human gastric cancer. The aim of this study was to investigate the association between ADAM17 expression and the clinicopathological features of patients with gastric cancer. The expression of ADAM17 was detected by real-time quantitative RT-PCR in gastric cancer and adjacent non-cancerous tissues. In addition, ADAM17 expression was analyzed by immunohistochemistry in 220 clinicopathologically characterized gastric cancer cases. The expression levels of ADAM17 mRNA and protein in gastric cancer tissues were both significantly higher than those in non-cancerous gastric mucosa. In addition, positive expression of ADAM17 correlated with the degree of tumor differentiation, depth of invasion, lymph node metastases, distant metastases, and TNM stage (all P < 0.05). Furthermore, multivariate analysis suggested that lymph node metastases, distant metastases, TNM stage, and ADAM17 expression were independent prognostic indicators for gastric cancer. Our data suggest for the first time that the increased expression of ADAM17 in gastric cancer is associated significantly with aggressive progression and poor prognosis. ADAM17 may be an important molecular marker for predicting the carcinogenesis, progression, and prognosis of gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the second leading cause of cancer mortality and one of the most frequent digestive malignancies in the world, especially in East and Southeast Asia [1]. In China, gastric cancer constitutes approximately 33% of all worldwide gastric cancer cases [2]. Most gastric cancer patients are first diagnosed at stage III or IV, when the rate of lymph node metastasis is high. Therefore, early detection remains the most promising approach to improve the long-term survival rate. Because gastric cancer is a multistep process with a number changes of various molecules, the genetic analysis has revealed associations of certain genetic changes with pathological features and prognosis of the gastric cancer patients, which suggests that a better understanding of the molecular changes underlying gastric cancer represents a crucial step and may result in a new therapeutic target in the treatment of the disease.
In gastric cancer, cell adhesion molecules mediate the interaction of epithelial cells with basement membrane, which regulates cell growth, motility, and differentiation by integrating signals from extracellular matrix (ECM). Matrix metalloproteinases (MMPs) may play a central role in these processes. A disintegrin and metalloproteinases (ADAMs) are the new gene family of proteins with sequence similarity to the reprolysin family of snake venomases that share the metalloproteinase domain with MMPs [3, 4]. Members of this family are membrane-anchored proteins and have been implicated in a variety of biological processes involving cell–cell and cell–matrix interactions, including fertilization, muscle development, and neurogenesis [5]. Wang et al. [6] found that ADAM10 protein was upregulated in gastric cancer lesions compared with adjacent non-cancerous tissues, and positive expression of ADAM10 correlated with the advanced clinicopathological features of tumors and shorter survival of patients. ADAM17 (also named as tumor necrosis factor-alpha-converting enzyme, TACE) is a transmembrane metalloprotease and primary sheddase for multiple EGFR pro-ligands, including transforming growth factor-alpha (TGF-α), heparin-binding epidermal growth factor (HB-EGF), and amphiregulin [7]. Of all ADAM members, the strongest evidence for a role in malignancy exists for ADAM17, which has been demonstrated to be overexpressed in the breast, ovary, kidney, colon, and pancreas cancer tissues and regulates important biological phenomena in these cancers through EGFR/PI3K/AKT pathway [8–10]. In gastric cancer, Yoshimura et al. [11] detected the presence of ADAM17 overexpression . In 2007, Yasuda et al. [12] found that siRNAs-targeted ADAM17 transcripts suppress deoxycholate (DC)-induced activation of EGFR and ERK1/2, suggesting that in AGS human gastric cancer cells, DC transactivates EGFR through M-BAR- and ADAM/HB-EGF-dependent mechanisms. Furthermore, Ebi et al. [13] demonstrated that the HB-EGF-CTF nuclear translocation and EGFR transactivation from proHB-EGF shedding mediated by ADAM17 activated by TGF-β may be an important pathway of gastric cancer cell proliferation. However, there are few reports concerning the association between ADAM17 expression and clinicopathological characteristics or patient survival in gastric cancer. Therefore, the aims of this study were to quantify the expression of ADAM17 in normal and gastric cancer tissues and to investigate the association between its expression and recognized clinicopathological prognostic variables, in order to determine whether the overexpression of this protein is relevant to the malignancy of gastric cancer.
Materials and methods
Patients and tissue samples
This study was approved by the Research Ethics Committee of Nanjing Medical University, China. Informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards.
For real-time quantitative RT-PCR, 20 patients (12 men and 8 women; mean age, 60.1 years; range, 29–86) were recruited from the Department of Oncology, First Hospital of Huaian, Nanjing Medical University between January 2006 and January 2008. All had undergone total gastrectomy. Fresh samples of tumor tissue and matched normal gastric mucosa were obtained immediately after gastric resection. Resected specimens were studied pathologically according to the criteria described in the UICC pTNM classification (2002). The samples were carefully dissected from resected specimens by a pathologist and immediately snap-frozen in separate vials, using liquid nitrogen. These frozen specimens were stored at −80° in a tumor bank before use.
For immunohistochemistry analysis, gastric cancer tissues were collected from gastrectomy specimens from 220 patients (160 men and 60 women; median age, 60.0 years; range, 29–88) from the Department of Oncology, First Hospital of Huaian, Nanjing Medical University, from January 2000 to January 2006. Tissues had been formalin-fixed, paraffin-embedded, and diagnosed clinically and histopathologically at the Departments of Gastrointestinal Surgery and Pathology. All patients had follow-up records for greater than 5 years. The follow-up deadline was December 2010. The survival time was calculated from the date of surgery to the follow-up deadline or date of death, which was caused mainly by carcinoma recurrence or metastasis. Forty non-cancerous human gastric tissues were obtained from gastrectomy of adjacent gastric cancer margins of more than 5 cm.
None of these patients underwent endoscopic mucosal resection, palliative resection, or preoperative chemotherapy or had synchronous or metachronous multiple cancer in other organs.
RNA extraction and real-time quantitative RT-PCR
ADAM17 gene expression in 20 tumor tissue samples and matched normal gastric mucosa were confirmed by real-time quantitative RT-PCR. Total RNA was extracted according to the manufacturer’s instructions (TRIzol, Invitrogen, USA). Two micrograms of RNA was reverse transcribed into cDNA (Promega, Madison, WI). Quantitative ADAM17 mRNA levels were assessed by using Mastercycler® ep realplex (Eppendorf, Hamburg, Germany) with an IQTM SYBR Green Supermix Kit (BIORAD, Berkeley, CA) according to the manufacturer’s protocol. GAPDH was used as an internal control. The primers for qPCR were as follows: ADAM17 (518 bp), sense 5′-CAC CAT GAG GCG GCG TCT CCT CAT C-3′ and antisense 5′-GCA CTC TGT CTC TTT GCT GTC-3′; GAPDH (224 bp), sense 5′-TGA AGG TCG GAG TCA ACG G-3′ and antisense 5′-CTG GAA GAT GGT GAT GGG ATT-3′. Cycling conditions were as follows: 95° for 2 min, then 40 cycles of 95° for 15 s, 59° for 30 s, and 72° for 45 s, with a final extension at 72° for 5 min. Each reaction was performed in triplicate, and the mean ADAM17 mRNA level for each tumor was compared with its matched non-cancerous mucosa. The expression level of ADAM17 was expressed as 2-ΔΔCt, where ΔCt = Ct (ADAM17) − Ct (GAPDH).
Immunohistochemistry analysis
ADAM17 protein expression in 220 tumor tissue samples and 40 non-cancerous gastric tissues were confirmed by immunohistochemistry analysis, which was performed on formalin-fixed, paraffin-embedded, 3-μm-thick tissue sections using the avidin-biotin-peroxidase complex method. The sections were deparaffinized and dehydrated using a graded series of ethanol solutions. Endogenous peroxidase activity was halted through the administration of 0.3% hydrogen peroxidase and methanol for 20 min. After having been rinsed in phosphate-buffered saline (PBS), the tissue sections were processed in a 0.01 M citrate buffer (pH 6.0) inside a heat-resistant plastic container. Sections were then irradiated in a domestic microwave oven for 20 min. After microwave irradiation, the slides were allowed to cool at room temperature. The following antibody was applied as the primary antibody: rabbit antibody specific to ADAM17 (1: 15, Atlas Antibodies, Sigma-Aldrich, USA). The sections were incubated with the primary antibody overnight at 4°C followed by the secondary antibody. The results were visualized with diaminobenzidine. In each immunohistochemistry run, the negative controls were stained without primary antibody.
Following a hematoxylin counterstaining, immunostaining was scored by two independent experienced pathologists, who were blinded to the clinicopathological parameters and clinical outcomes of the patients. The scores of the two pathologists were compared, and any discrepant scores were trained through re-examining the stainings by both pathologists to achieve a consensus score. The number of positive-staining cells showing immunoreactivity on the cytoplasm for ADAM17 in ten representative microscopic fields was counted, and the percentage of positive cells was calculated. The percentage scoring of immunoreactive tumor cells was as follows: 0 (0%), 1 (1–10%), 2 (11–50%), and 3 (>50%). The staining intensity was visually scored and stratified as follows: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). A final score was obtained for each case by multiplying the percentage and the intensity score. Therefore, tumors with a multiplied score exceeding 4 (the median of the final scores for all samples) were deemed to be positive expression of ADAM17; all other scores were considered to be negative.
Statistical analysis
The software of SPSS version12.0 for Windows (SPSS Inc, IL, USA) and SAS 9.1 (SAS Institute, Cary, NC) was used for statistical analysis. Measurement data were analyzed using Student’s t test, while categorical data were studied using the Χ2 or Fisher’s exact test. Survival curves were estimated using the Kaplan–Meier method, and the log rank test was used to calculate differences between the curves. Multivariate analysis using the Cox proportional hazards regression model was performed to assess the prognostic values of protein expression. Correlation coefficients between protein expression and clinicopathological features were estimated using the Pearson correlation method. Differences were considered statistically significant when P was less than 0.05.
Results
ADAM17 gene expression in gastric cancer tissue and non-cancerous tissue
ADAM17 gene expression in gastric cancer tissues and corresponding non-cancerous tissues was analyzed using qRT-PCR. In gastric cancers, the expression levels of ADAM17 gene were significantly higher (0.88 ± 0.40) than in non-cancerous tissues (0.07 ± 0.03; P < 0.0001; Fig. 1).
ADAM17 protein expression in gastric cancer and non-cancerous gastric mucosa
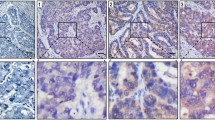
The expression of ADAM17 protein in archived gastric tissue samples and non-tumor mucosa was analyzed by immunohistochemistry. ADAM17 was predominantly expressed in the cytoplasm of epithelial cells. ADAM17 was absent or weakly expressed in normal tissue. In 82.73% (182 of 220 cases) of gastric cancer tissues, ADAM17 was overexpressed, significantly higher than 20.45% (45 of 220 cases) in nonneoplastic tissues, with a significant difference in the expression rates of ADAM17 between the cancer and the normal tissues (Χ2 = 28.99, P < 0.0001) (Fig. 2).
Immunohistochemical staining of ADAM17 in the gastric cancer and non-cancerous gastric tissues (×400). a The positive expression of ADAM17 protein was stained as yellow or brownish yellow in the cytoplasm of cancer cells. b The absent or weak expression of ADAM17 protein in non-cancerous gastric mucosa cells
Association of the ADAM17 protein expression with the clinicopathological features of gastric cancer
The association between ADAM17 expression and clinicopathological features of gastric cancer was further analyzed as shown in Table 1. The results revealed a positive association of ADAM17 expression with degree of tumor differentiation (P = 0.006). In addition, ADAM17 expression demonstrated a significantly positive correlation with the depth of tumor invasion (P < 0.0001). Moreover, ADAM17 expression in patients with lymph node metastasis and distant metastasis was significantly higher than that in patients without metastasis, respectively (both P = 0.02). According to TNM classification, stage I and II tumors showed significantly lower rates compared to stages III and IV (P = 0.03). There was no significant relationship found between ADAM17 expression and gender or age (both P > 0.05).
Influence of the ADAM17 expression on survival
In stages I and II, the 5-year survival rate of patients with positive expression of ADAM17 was both significantly higher than in patients with negative expression (both P < 0.05; Fig. 3a, b). But in stage III and IV tumors, the expression of ADAM17 did not correlate with the 5-year survival rate (P > 0.05). In terms of depth of invasion, the 5-year survival rate in patients with positive expression of ADAM17 was significantly higher than in patients with negative expression (both P < 0.05; Fig. 3c, d). In the group of lymph node-positive patients, those with ADAM17-positive gastric cancer had a better survival rate than patients with ADAM17-negative gastric cancer (P < 0.05; Fig. 3e). In the group of patients with distant metastasis, those with ADAM17-positive gastric cancer also had a better survival rate than patients with ADAM17-negative gastric cancer (P < 0.05; Fig. 3f).
Table 2 shows the multivariate analyses of factors related to patient prognosis. Factors with possible prognostic effects in gastric cancer were analyzed by Cox regression analysis. The results revealed that lymph node metastases (P = 0.02), distant metastases (P = 0.02), TNM stage (P = 0.01), and expression of ADAM17 (P = 0.008) were independent prognostic factors in patients with gastric cancer. However, age, sex, tumor differentiation, and depth of tumor invasion had no prognostic value.
Discussion
In the present study, the overexpression of ADAM17 gene and protein in gastric cancer was verified by real-time quantitative RT-PCR and immunohistochemistry analysis, respectively. Expression of ADAM17 was upregulated from early- to advanced-stage gastric cancer. Moreover, ADAM17 expression was clearly upregulated in lymph node and distant metastases. These results suggest that ADAM17 expression in gastric cancer may play an important role in the process of tumor initiation and dissemination of tumor cells to distant organs.
ADAM family has been demonstrated to be involved in the process of proteolytic ‘shedding’ of membrane-associated proteins and hence the rapid modulation of key cell signaling pathways in the tumor microenvironment [14, 15]. ADAM17, an important member of this family, is the most extensively studied ADAM molecule. It sheds a variety of important cell surface molecules, including cytokines, growth factors, and adhesion molecules [7]. For one thing, ADAM17 plays key roles in early immune defense mechanisms by releasing the soluble TNF-α, which is a proinflammatory cytokine and a key mediator in immune defense with a role in induction and amplification of inflammation, as a response to external, potential threatening stimuli [16]. For another thing, ADAM17 is involved in cell proliferation and invasion by releasing several ligands [17]. Recent studies found the upregulation of ADAM17 in tissues at different pathologic conditions including human cancers. ADAM17 is overexpressed in cancers of the breast, ovary, kidney, colon, and prostate [8–10]. In human prostate cancer cells, ectopic overexpression of ADAM17 resulted in increased cell proliferation, and ADAM17 promoted G1- to S-phase transition concomitantly with upregulation of cyclin E, CDK2, and downregulation of p21 and p27 proteins [18]. It has been shown that ADAM17-mediated EGFR ligand cleavage enhances the proliferation and survival of squamous cell carcinoma cells as well as lung cancer cells [19, 20]. In addition, ADAM17 promotes the breast cancer cell’s malignant phenotype by increased proliferation, invasion, and angiogenesis. It contributes to breast cancer progression through the activation of the EGFR-PI3 K-AKT signal pathway [21]. Moreover, the inhibition of ADAM17-mediated shedding of proTGF-α reduces the size of xenografts in nude mice suggest that ADAM17 is a target for tumorigenesis [22]. Treatment of breast cancer cell lines with anti-ADAM17 antibodies leads to a decrease in cell proliferation [23]. In the current study, the ADAM17 expression pattern was observed in gastric normal mucosa and cancers, and it was significantly higher in cancer than in normal tissue, which indicated that the expression of ADAM17 was upregulated in gastric cancer in consistent with the previous study of Yoshimura et al. [11]. Analyzing the relationship between ADAM17 expression and clinicopathological parameters of gastric cancer, we found that expression of ADAM17 was significantly correlated with the progression of stomach carcinoma, the differentiation, lymph node metastasis, depth of invasion, and TNM stage suggesting that ADAM17 may play an important role in the development and progression of stomach malignant tumor.
Furthermore, in stage I and II tumors, the 5-year survival rates of patients with high expressions of ADAM17 were significantly higher than those in patients with low expression. In stage III and IV tumors, expression of ADAM17 did not correlate with 5-year survival rate. Cox regression analysis revealed that only lymph node metastases and distant metastases, TNM stage, and expression of ADAM17 were independent prognostic factors in patients with gastric cancer suggesting that ADAM17 is an important marker for aid in the detection of cancer.
In conclusion, our data suggest for the first time that the increased expression of ADAM17 in gastric cancer is associated significantly with aggressive progression and poor prognosis. ADAM17 may be an important molecular marker for predicting the carcinogenesis, progression, and prognosis of gastric cancer. This is the first report to suggest a relationship between ADAM17 and prognosis in patients with gastric cancer, and further prospective analysis would be worth doing.
References
Zhang YZ, Zhang LH, Gao Y, et al. Discovery and validation of prognostic markers in gastric cancer by genome-wide expression profiling. World J Gastroenterol. 2011;17:1710–7.
Ye YW, Dong RZ, Zhou Y, et al. Prognostic analysis of familial gastric cancer in Chinese population. J Surg Oncol. 2011;104:76–82.
Edwards DR, Handsley MH, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29:258–89.
Murphy G. The ADAMs: signaling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929–41.
Duffy MJ, McKiernan E, O’Donovan N, et al. Role of ADAMs in cancer formation and progression. Clin Cancer Res. 2007;13:2335–43.
Wang YY, Ye ZY, Li L, Zhao ZS, Shao QS, Tao HQ. ADAM 10 is associated with gastric cancer progression and prognosis of patients. J Surg Oncol. 2011;103:116–23.
Xu P, Derynck R. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol Cell. 2010;37:551–66.
Kenny PA, Bissell MJ. Targeting TACE-dependent EGFR ligand shedding in breast cancer. J Clin Invest. 2007;117:337–45.
Bozkulak EC, Weinmaster G. Selective use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol Cell Biol. 2009;29:5679–95.
Szalad A, Katakowski M, Zheng X, et al. Transcription factor Sp1 induces ADAM17 and contributes to tumor cell invasiveness under hypoxia. J Exp Clin Cancer Res. 2009;28:129.
Yoshimura T, Tomita T, Dixon MF, et al. ADAMs (a disintegrin and metalloproteinase) messenger RNA expression in Helicobacter pylori-infected, normal, and neoplastic gastric mucosa. J Infect Dis. 2002;185:332–40.
Yasuda H, Hirata S, Inoue K, et al. Involvement of membrane-type bile acid receptor M-BAR/TGR5 in bile acid-induced activation of epidermal growth factor receptor and mitogen-activated protein kinases in gastric carcinoma cells. Biochem Biophys Res Commun. 2007;354:154–9.
Ebi M, Kataoka H, Shimura T, et al. TGFβ induces proHB-EGF shedding and EGFR transactivation through ADAM activation in gastric cancer cells. Biochem Biophys Res Commun. 2010;402:449–54.
Arribas J, Bech-Serra JJ, Santiago-Josefat B. ADAMs, cell migration and cancer. Cancer Met Rev. 2006;25:57–68.
Duffy MJ, McKiernan E, O’Donovan N, et al. The role of ADAMs in disease pathophysiology. Clin Chim Acta. 2009;403:31–6.
Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380–7.
Willems SH, Tape CJ, Stanley PL, et al. Thiol isomerases negatively regulate the cellular shedding activity of ADAM17. Biochem J. 2010;428:439–50.
Lin P, Sun X, Feng T, et al. ADAM17 regulates prostate cancer cell proliferation through mediating cell cycle progression by EGFR/PI3K/AKT pathway. 2011 In press.
Stokes A, Joutsa J, Ala-Aho R, et al. Expression profiles and clinical correlations of degradome components in the tumor microenvironment of head and neck squamous cell carcinoma. Clin Cancer Res. 2010;16:2022–35.
Baumgart A, Seidl S, Vlachou P, et al. ADAM17 regulates epidermal growth factor receptor expression through the activation of Notch1 in non-small cell lung cancer. Cancer Res. 2010;70:5368–78.
McGowan PM, Ryan BM, Hill AD, McDermott E, O’Higgins N, Duffy MJ. ADAM-17 expression in breast cancer correlates with variables of tumor progression. Clin Cancer Res. 2007;13:2335–43.
Saftig P, Reiss K. The “A Disintegrin And Metalloproteases” ADAM10 and ADAM17: novel drug targets with therapeutic potential? Eur J Cell Biol. 2011;90:527–35.
Sinnathamby G, Zerfass J, Hafner J, et al. ADAM metallopeptidase domain 17 (ADAM17) is naturally processed through major histocompatibility complex (MHC) class I molecules and is a potential immunotherapeutic target in breast, ovarian and prostate cancers. Clin Exp Immunol. 2011;163:324–32.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Tc., Zhu, Wg., Huang, Md. et al. Prognostic value of ADAM17 in human gastric cancer. Med Oncol 29, 2684–2690 (2012). https://doi.org/10.1007/s12032-011-0125-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-0125-4