Abstract
Purpose
Estrogen receptor (ER) and progesterone receptor (PR) status is prognostic and predictive in breast cancer. Because metastatic breast tumor biopsies are not routinely feasible, circulating tumor cells (CTCs) offer an alternative source of determining ER/PR tumor status.
Methods/patients
Peripheral blood was collected prospectively from 36 patients with metastatic breast cancer. CTCs were isolated using the microfluidic OncoCEE™ platform. Detection was accomplished with an expanded anti-cytokeratin (CK) cocktail mixture and anti-CD45. ER/PR protein expression was assessed by immunocytochemistry (ICC) on the CK+ cells and compared to the primary and/or metastatic tumor by immunohistochemistry (IHC).
Results
Among the 24 CK + CTC cases, a concordance of 68 % (15/22) in ER/PR status between primary breast tumor and CTCs and 83 % (10/12) between metastatic tumor and CTCs was observed. An overall concordance of 79 % (19/24) was achieved when assessing CTC and metastatic tumor (primary tumor substituted if metastatic breast biopsy not available). A test sensitivity of 72 % and specificity of 100 % was identified when comparing CTCs to tumor tissue. Of the 7 discordant cases between CTCs and primary tumor tissue, 2 were concordant with the metastatic biopsy.
Conclusions
CTC ER/PR status using the OncoCEE™ platform is feasible, with high concordance in ER/PR status between tumor tissue (IHC) and CTCs (ICC). The prognostic and predictive significance of CTC ER/PR protein expression needs further evaluation in larger trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is among the leading causes of cancer-related deaths despite advances in early detection and treatments. Hormone receptor (HR) status [the presence of the estrogen receptor (ER) and/or progesterone receptor (PR)] carries both prognostic and predictive implications in breast cancer [1]. Up to 75 % of breast tumors rely on ER signaling for growth, and targeting this pathway with anti-estrogen therapy has clear clinical benefit [2]. Various endocrine therapies have been approved for patients with HR+ breast cancer in the early-stage and advanced settings (including aromatase inhibitors, selective ER modulators, and ER down-regulators, such as fulvestrant). Based on current American Society of Clinical Oncology and College of American Pathologist (ASCO-CAP) guidelines, anti-estrogen therapy is considered for patients with ER and/or PR positivity ≥1 % by immunohistochemistry [3]. HR status may change over the course of treatment or disease progression. Several groups have reported that discordance of HR expression between primary tumor and metastases can occur in up to 40 % of matched cases [4–8]. There are a number of potential reasons that HR status may change, including selective pressure to the treatment, clonal expansion, or tumor heterogeneity. In these circumstances, a different therapeutic approach may be considered [9]. Selection of alternate therapies is dictated by a number of factors, such as HR and HER2 status, indicating the need to frequently monitor and re-test patients for such phenotypic changes to best assess for the most appropriate treatment strategy.
Circulating tumor cells (CTCs) are commonly identified in greater than 50 % of metastatic breast cancer, with enumeration demonstrating predictive implications in disease progression in patients receiving chemotherapy or endocrine therapy [10, 11]. In comparison to tumor biopsies, CTCs offer a non-invasive real-time look into the biology of a patient’s metastatic breast cancer. CTCs offer an attractive alternative source of tumor material for determining HR status and can be monitored more readily on a serial basis to enable a more effective course of treatment. A goal of CTC evaluation is to ultimately select and modify treatment decisions, such as the role of anti-estrogen therapy, based upon the CTC expression of ER/PR. Other groups have compared the rates of HR discordance between primary tumors and CTCs, ranging from 40 to 60 % [12, 13]. These have been evaluated with various technologies, such as immunomagnetic enrichment and fiber-optic array laser-scanning. Technical advances now make it possible for the detection of CTCs in whole blood [11, 14–16] and Biocept’s OncoCEE™ platform allows for detailed phenotypic and genotypic evaluation of the CTCs within a single microchannel [16]. In this study, we characterized the ER/PR status of CTCs isolated using OncoCEE-BR™ and compared the CTC expression to both the primary tumor and metastatic biopsy, when available.
Materials and methods
Laboratory information and patients collected
We prospectively enrolled 36 patients with histologically proven stage IV invasive breast cancer from January 2011 to June 2012. Patients were allowed to be receiving anti-estrogen treatment, chemotherapy, and/or biologic therapy, such as trastuzumab. Patients were recruited directly from the Columbia University Medical Center Breast Oncology Clinic. Peripheral blood was collected under appropriate third party institution review board approved protocols (Columbia University Medical Center, AdeptBio, ConversantBIO and BioOptions) and delivered to Biocept’s CLIA/CAP accredited laboratory. All patients involved in this study provided written informed consent and fully understood the different aspects of their study participation prior to signing the consent form. Two tubes of blood from each of the 36 patients were collected in CEE-Sure™ vacutainer collection tubes (Biocept Inc., San Diego, CA). Medical records were reviewed for determination of ER/PR status in the primary tumor or metastatic biopsy upon diagnosis. The primary and tumor samples were processed per ASCO-CAP guidelines [3]. This includes pre-analytic, analytic, and post-analytic standardization considerations [3]. All tumors were fixed in 10 % neutral buffered formalin for no less than 6 h and for not more than 72 h before processing. The interpretation of ER and PR included assessment of the percentage of positive tumor cell nuclei and intensity of the staining reaction. Both positive and negative controls were used with each batch of patient samples undergoing ER/PR analysis.
Cell separation, enrichment and detection
OncoCEE™ microchannel technology [17] and the process of cell separation utilizing a Percoll density gradient method to recover the peripheral blood mononuclear cell fraction (PBMC) have been described previously [16, 18]. CEE™ microchannels are manufactured at Biocept, Inc. (San Diego, CA). The cell fraction was run through the microchannel, captured using an antibody cocktail, and stained with a mixture of anti-cytokeratin antibodies labeled with AlexaFluor-488 [16, 18]. Cells were simultaneously stained with anti-CD45 labeled with AlexaFluor-594. ER/PR ICC was performed using anti-ER (Abcam, Cambridge, MA) and anti-PR (Epitomics Inc, Burlingame, CA) monoclonal rabbit antibodies and secondary anti-Rabbit antibody labeled with AlexaFluor-546. The microchannels underwent microscopic analysis for enumeration of CK+/CD45−/DAPI+ (CTC identification), CK−/CD45+/DAPI+ (background white blood cells), and all CK+ cells were assessed for ER/PR positivity. The microchannels underwent immediate manual microscopic analysis for enumeration of CTCs and assessment of ER/PR followed by taking images and X/Y coordinates recorded using Olympus Bx51 fluorescent microscopes equipped with appropriate filters and the Metasystems imaging system v5.2 (Metasystems GmbH, Germany).
Cell lines, flow cytometry, and immumocytochemistry
MDA-MB-231 [American Tissue Culture Collection (ATCC), HTB-26], ZR75-1 (ATCC, CRL-1500), MCF-7 (ATCC, HTB-22), BT474 (ATCC, HTB-20), MDA-MB-468 (ATCC, HTB-132), SKBr3 (ATCC, HTB-30), and MDA-MB-134 (ATCC, HTB-23) cells were cultured according to ATCC recommendations, verified by morphology, growth curve analysis, and tested for mycoplasma. Measurement of ER and PR antigens was performed by incubating paraformaldehyde/methanol fixed breast cancer cells (listed above) with ER or PR primary antibodies, followed by incubation with FITC-labeled anti-rabbit IgG (Sigma; 1:100), according to a standard flow cytometry protocol. After additional washes to remove excess antibody, the cells were analyzed on the Accuri C6 flow cytometer. The remaining cells were transferred to a flat bottom 96-well plate, DAPI was added and pictures were taken with the Olympus X81 microscope using a 40× objective.
Statistical analysis
To assess the analytical performance of ER/PR positivity, the sensitivity, and specificity were calculated for the patients involved in this study. Descriptive statistics were obtained in the number of CTCs detected and evaluated, as well as the ER and PR scores, as compared to the primary or metastatic sites. Cohen’s kappa statistic was conducted to assess for concordance in HR status between the primary tumor or metastatic biopsy and CTCs [19]. We had 90 % power to identify a moderate correlation coefficient in HR status between CTCs and matched tumor samples (correlation = 0.6) with 21 CTC samples (alpha = 0.05). To assure that enough samples were available for comparison, 3 additional samples were collected. Tumor tissue or CTCs were considered HR positive if ER and/or PR >1 % staining.
Results
ER and PR expression on cell lines by immunocytochemistry and FACS
Selection of the ER and PR antibodies was first performed using flow cytometric screening of numerous potential clones and the corresponding antigen expression levels for the selected clones are illustrated for several breast cancer cell lines (Fig. 1a). HR expression was assessed by ICC on the known ER and PR expressing breast cancer cell lines (Fig. 1b). The selected antibodies demonstrated specific immunoreactivity with the various breast cancer cell lines and were easily transferrable into the OncoCEE™ technology. Based on the flow cytometry and immunocytochemistry data, BT474, MCF-7 and ZR-75-1 cells were positive for both ER and PR, MDA-MB-134VI was positive for ER only, and MDA-MB-231, MDA-MB-468 and SKBR3 cells were negative for ER and PR [20].
ER and PR expression on CTCs
As required for laboratory-developed tests, HR+ cell lines (BT474 and MCF7), HR− cell lines (MDA-MB-231 and MDA-MB-468) and negative control blood from normal donors (n = 5) were initially obtained and used to demonstrate analytical sensitivity/specificity of the ER/PR antibodies. Cell lines were mixed with whole blood, captured on the OncoCEE™ microchannels, and immunostained for ER/PR. CK+/DAPI+/CD45− cells were assessed for ER/PR positivity. As illustrated in Fig. 2, BT474 stained positive as an ER/PR breast cancer cell line and MDA-MB-231 was ER/PR negative.
Concordance of ER/PR status
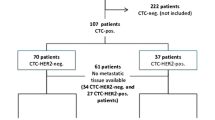
A cohort of 36 patients with defined HR tissue status signed consented for the study and had samples that were collected and processed for CTC enumeration followed by ER/PR ICC analysis (Table 1). Five patients underwent serial CTC assessment with multiple samples sent [4 patients: 2 samples (Table 2: including patient ID #16, 18, and 19); 1 patient: 3 samples (patient ID #17)]. In total, 43 samples were sent for ER/PR ICC analysis. Complete patient and clinical characteristics were available on 28 patients, as described in Table 1. The other 8 patients were not treated at Columbia University, with limited clinicopathologic information available. The mean age study was 55 years (range 30–78). Approximately two-third of patients had HR+ primary tumors (68 %) and metastatic tumors (64 %), of which the majority expressed both ER and PR (primary 89 %; metastatic 77 %). HR status on tumor tissue was assessed by IHC. All HR+ cases were ER+, except for one lung metastasis (patient ID #12). The mean number of prior treatments was 3 (range 1–11), with approximately 64 % having progressed beyond front-line treatment. In total, 39 % were being treated with only anti-estrogen treatments and had never received chemotherapy. The other patients had previously progressed on anti-estrogen therapy and/or had symptomatic disease, requiring chemotherapy. Only 3/28 (11 %) were progressing at the time of CTC collection. The most common sites of metastatic disease were bone (68 %), lymph node/chest wall (46 %), and lung/pleura (36 %).
Of the 36 patients, 19 patients (53 %) were found to have ≥1 CTC identified based on a staining pattern of CK+/CD45− cells (56 % based on CK+ staining alone). A median of 20 CK + CTCs was detected in the 24 samples (Table 2: range 1–9,005 CTCs). The median time between first CTC collection and metastatic tissue diagnostic biopsy was 16.3 months (range 0.2–44.3 months). Among the 24 CK + CTC cases, a concordance of 68 % (15/22) in HR status between primary tumor and CTCs was observed and an 83 % (10/12) concordance was identified between CTCs and the metastatic biopsy. In 2 patients, there was discordance between the primary and metastatic tumor (patient ID #11 and 12). Notably, in these 2 cases, the CTC was concordant with the metastatic site.
Overall concordance of HR positivity was assessed based upon the CTC and metastatic site. If the metastatic site information was unavailable, overall concordance was based on the CTC and the primary tumor. Overall concordance is based on the number of sample numbers. An overall high concordance of 79 % (19/24) was achieved. Of the 5 discordant cases, 3 were discordant based on primary tissue alone as shown in Table 2. All 3 were positive by IHC on the primary tumor and negative on the CTCs (patient ID #8, 9 and 19). Notably, each had relatively low numbers of CTCs detected (either 1 or 2 CTCs).
There was no difference between the numbers of CK + CTCs detected in the 18 patients with HR+ status compared to the 6 HR− cases (mean 833.89 and 524.33 CTCs, respectively, p = 0.90). An overall test sensitivity of 72 % was achieved when compared to the metastatic tumor or primary tumor when metastatic tissue was not available. Specificity was calculated to be 100 % in this cohort (Table 3). Moderate agreement was found using Cohen’s κ statistic (κ = 0.6) for concordance between HR+ status in the CTCs and tumor tissue. These results demonstrate reliable ICC detection of a validated tumor biomarker (i.e. ER/PR expression) in CTCs following enrichment and capture using the OncoCEE™ platform.
Patients with serial CTCs and clinical course
Of the 5 patients with serial CTCs, 2 patients (patient ID #16 and 18) demonstrated HR + CTCs and tumor tissue and responded to anti-estrogen therapy. Patient ID #19 was heavily pre-treated (metastatic treatment #7) at her baseline CTC (19a), having progressed on anti-estrogen therapy and receiving chemotherapy. At the repeat CTC (19b), the patient required a change in therapy due to breast cancer progression. For patient ID #17, the patient had a HR + CTC in accordance with a HR+ metastatic lesion in her bone when CTC 17a was performed and was on a clinical study of anti-estrogen therapy plus bevacizumab. For the next CTC collection (17b), the patient progressed in her bone marrow and was switched to chemotherapy. Interestingly, by the last CTC collection (17c), the patient’s CTCs were no longer expressing the hormone receptor, in accordance with her breast cancer aggressively progressing and no longer responding to anti-estrogen treatment.
Discussion
The CEE™ technology provides a sensitive platform for enhanced capture, detection and molecular characterization (ER/PR) in intact CTCs within the microchannels. Though some discordance between tumor tissue and CTCs is expected given variation in tumor heterogeneity, biopsy size, and robustness of the technical assay (especially for IHC), a blood-based CTC assay may offer more reliable testing given the advantages of serial non-invasive testing and the use of larger blood volumes, which may help to ensure informative results.
In this study, 19/36 patients (53 %) were found to have >1 CTC based on a staining pattern of CK+/CD45− cells. Of those with identifiable CTCs, we report a concordance of 68 % (15/23) in ER/PR status between primary tumor and CTCs and 83 % (10/12) between metastatic tumor and CTCs. Other groups have attempted to assess ER/PR status in CTCs by various other methods, including reverse transcription polymerase chain reaction, and have shown variable correlation to the primary tumor [12, 13, 21–23]. Differences in the time between primary tumor evaluation and CTC collection, as well as changes in pre-analytic and analytic considerations in determining HR positivity, may account for some of the variable results reported between the different studies. However, it is also possible that the OncoCEE™ microchannel and the use of the antibody capture cocktail captures a different population of cells identified by the other groups that is more representative of the primary tumor. As previously reported [16], the OncoCEE™ platform has performed better than CellSearch® in terms of identification of CTCs. In addition, a 93 % rate of concordance between CTCs and tumor tissue with HER2 status was observed with the OncoCEE™ system, higher than previous HER2 concordance rates reported in other studies [16].
In this study, we report an overall high concordance of 79 % (19/24) for ER/PR status when assessing CTC and metastatic breast tumor or primary tumor, if metastatic information not available. While others have reported cases of HR− CTCs and HR+ tumor tissue [23], it should be noted that, in our study, these discordant cases primarily occurred in patients with small numbers of identifiable CTCs (1–3 CTC). Whether this discordance between CTCs and tumor tissue is a true occurrence, identifying loss of ER function, or whether HR status will be more accurately observed with technology advancements will need to be determined in larger studies. We report a higher concordance rate with CTCs and metastatic tumor tissue. If this assessment of CTC characterization is validated in future studies, the CTC HR status can potentially be used as surrogate material for biomarker assessment, particularly if metastatic tissue is not available or biopsy is not feasible. In addition, the HR status of the CTCs can change over time. This finding is best illustrated with patient 17, whose CTC changed from HR+ to HR− with serial CTCs around the time of progression on anti-estrogen therapy. While this observation should be considered exploratory, this case highlights the potential of utilizing CTC HR status as a predictor for continued response to hormonal agents.
There are a number of strengths and weaknesses to this study. Strengths include consistency of interpretation of the CTCs, using the OncoCEETM platform to characterize ER/PR status. In addition, clinical features and primary/metastatic tissue are available for most patients accrued to this prospectively accrued cohort, with serial CTCs conducted on some patients with identifiable CTCs. Limitations include the small size of this data set. A larger cohort with uniform therapy received and longer clinical follow-up is required for validation. Because of the small sample size, this trial is not powered to determine whether it would be appropriate, for instance, to withhold endocrine therapy if the CTCs are determined to be HR−, despite HR+ tumor tissue. This proof of principle study should be viewed as hypothesis generating and justifies a next-step, prospective trial assessing this question. In addition, ER and PR CTC status was analyzed collectively, with data on each individual receptor not available.
In conclusion, heterogeneity of ER/PR protein expression is identified in CTCs, and primary tumor/metastatic biopsy material and hormonal status may change over time due to therapy. ER/PR ICC on CTCs from peripheral blood using the OncoCEE™ platform is shown to be feasible, with high concordance (79 %) in ER/PR status between primary tumor/metastatic biopsy (by IHC) and CTCs (by ICC). The significance of heterogeneity at the ER/PR protein level in CTCs related to the prognosis and predictive response to anti-estrogen therapy needs further evaluation in larger prospective clinical studies. The ultimate goal is to predict drug response with this technology, specifically anti-estrogen response, and potentially to understand resistance mechanisms.
References
Sieuwerts AM, Mostert B, Bolt-de Vries J, Peeters D, de Jongh FE, Stouthard JML. mRNA and microRNA expression profiles in circulating tumor cells and primary tumors of metastatic breast cancer patients. Clin Cancer Res. 2011;17(11):3600–18. doi:10.1158/1078-0432.ccr-11-0255.
Cleator SJ, Ahamed E, Coombes RC, Palmieri C. A 2009 update on the treatment of patients with hormone receptor-positive breast cancer. Clin Breast Cancer. 2009;9(Suppl 1):S6–17. doi:10.3816/CBC.2009.s.001 G44434VK149RM452.
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134(7):e48–72. doi:10.1043/1543-2165-134.7.e48.
Lower EE, Glass EL, Bradley DA, Blau R, Heffelfinger S. Impact of metastatic estrogen receptor and progesterone receptor status on survival. Breast Cancer Res Treat. 2005;90(1):65–70. doi:10.1007/s10549-004-2756-z.
Liedtke C, Broglio K, Moulder S, Hsu L, Kau SW, Symmans WF, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009;20(12):1953–8. doi:10.1093/annonc/mdp263 mdp263.
Aitken SJ, Thomas JS, Langdon SP, Harrison DJ, Faratian D. Quantitative analysis of changes in ER, PR and HER2 expression in primary breast cancer and paired nodal metastases. Ann Oncol. 2010;21(6):1254–61. doi:10.1093/annonc/mdp427 mdp427.
Simmons C, Miller N, Geddie W, Gianfelice D, Oldfield M, Dranitsaris G, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol. 2009;20(9):1499–504. doi:10.1093/annonc/mdp028 mdp028.
Amir E, Miller N, Geddie W, Freedman O, Kassam F, Simmons C, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;30(6):587–92. doi:10.1200/jco.2010.33.5232.
Fehm T, Muller V, Aktas B, Janni W, Schneeweiss A, Stickeler E, et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat. 2010;124(2):403–12. doi:10.1007/s10549-010-1163-x.
Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol. 2009;27(31):5153–9. doi:10.1200/jco.2008.20.6664.
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12(14 Pt 1):4218–24. doi:10.1158/1078-0432.CCR-05-2821 12/14/4218.
Aktas B, Muller V, Tewes M, Zeitz J, Kasimir-Bauer S, Loehberg CR, et al. Comparison of estrogen and progesterone receptor status of circulating tumor cells and the primary tumor in metastatic breast cancer patients. Gynecol Oncol. 2011;122(2):356–60. doi:10.1016/j.ygyno.2011.04.039 S0090-8258(11)00340-4.
Somlo G, Lau SK, Frankel P, Hsieh HB, Liu X, Yang L, et al. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary and metastatic sites in patients with locally advanced/inflammatory, and stage IV breast cancer, using a novel detection technology. Breast Cancer Res Treat. 2011;128(1):155–63. doi:10.1007/s10549-011-1508-0.
Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450(7173):1235–9. doi:10.1038/nature06385 nature06385.
Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007;253(2):180–204. doi:10.1016/j.canlet.2006.12.014 S0304-3835(06)00686-0.
Mayer JA, Pham T, Wong KL, Scoggin J, Sales EV, Clarin T, et al. FISH-based determination of HER2 status in circulating tumor cells isolated with the microfluidic CEE platform. Cancer Genet. 2011;204(11):589–95. doi:10.1016/j.cancergen.2011.10.011 S2210-7762(11)00304-8.
Dickson MN, Tsinberg P, Tang Z, Bischoff FZ, Wilson T, Leonard EF. Efficient capture of circulating tumor cells with a novel immunocytochemical microfluidic device. Biomicrofluidics. 2011;5:034119.
Pecot CV, Bischoff FZ, Mayer JA, Wong KL, Pham T, Bottsford-Miller J, et al. A novel platform for detection of CK+ and CK − CTCs. Cancer Discov. 2011;1(7):580–6. doi:10.1158/2159-8290.CD-11-0215 2159-8290.CD-11-0215.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Hollestelle A, Nagel JH, Smid M, Lam S, Elstrodt F, Wasielewski M, et al. Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast Cancer Res Treat. 2010;121(1):53–64. doi:10.1007/s10549-009-0460-8.
Fehm T, Hoffmann O, Aktas B, Becker S, Solomayer EF, Wallwiener D, et al. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res. 2009;11(4):R59. doi:10.1186/bcr2349 bcr2349.
Nadal R, Fernandez A, Sanchez-Rovira P, Salido M, Rodriguez M, Garcia-Puche JL, et al. Biomarkers characterization of circulating tumour cells in breast cancer patients. Breast Cancer Res. 2012;14(3):R71. doi:10.1186/bcr3180 bcr3180.
Babayan A, Hannemann J, Spötter J, Müller V, Pantel K, Joosse SA. Heterogeneity of estrogen receptor expression in circulating tumor cells from metastatic breast cancer patients. PLoS One. 2013;8(9):e75038. doi:10.1371/journal.pone.0075038.
Acknowledgments
The authors wish to thank all of the employees at Biocept and the following laboratory personnel for the work and dedication to this project: Mark Tong, Maryam Zomorrodi, Lisa Strauser, Valeriya Prozorovska, Ronnie Spielvogel, Leticia Flores, Christine Mitchell, Margaret Jeffrey, Andrew Nguyen, Jana Salvacion, and Ferdinand Banez. In addition, the authors would also like to thank Drs. Dawn Hershman, MD, MS and Katherine Crew, MD, MS for their support and accrual of patients to this study. This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number KL2 TR000081 and the Witten Breast Cancer Research Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
F. Z. Bischoff, J. A. Mayer, T. Pham, K. L. Wong, E. Villarin, and T. J. Pircher, are presently or were previously employees of Biocept Incorporated. None of the other contributors have any potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalinsky, K., Mayer, J.A., Xu, X. et al. Correlation of hormone receptor status between circulating tumor cells, primary tumor, and metastasis in breast cancer patients. Clin Transl Oncol 17, 539–546 (2015). https://doi.org/10.1007/s12094-015-1275-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1275-1