Abstract
Patients with locally advanced/inflammatory breast cancer (LABC/IBC) face a high likelyhood of recurrence and prognosis for relapsed, or de novo stage IV metastatic breast cancer (MBC) remains poor. Estrogen (ER) and HER2 receptor expression on primary or MBC allow targeted therapies, but an estimated 10–18% of tumors do not exhibit these biomarkers and survival in these cases is even poorer. Variations in discordance rates for the expression of ER and HER2 receptors have been observed between primary and metastatic tumors and such discordances may lead to suboptimal treatment. Circulating tumor cells (CTCs) are considered the seeds of residual disease and distant metastases and their characterization could help guide treatment selection. To explore this possibility, we used multiple biomarker assessment of CTCs in comparison to primary and metastatic tumor sites. Thirty-six patients with LABC/IBC, or stage IV MBC were evaluated. Blood samples were procured prior to initiating or changing therapy. CTCs were identified based on presence of cytokeratin and nucleus staining, and the absence of CD45. A multimarker assay was developed to simultaneously quantify expression of HER2, ER, and ERCC1, a DNA excision repair protein. Novel fiber-optic array scanning technology (FAST) was used for sensitive location of CTCs. CTCs were detected in 82% of MBC and 62% LABC/IBC cases. Multiplex marker expression was successfully carried out in samples from18 patients with MBC and in 8 patients with LABC/IBC that contained CTCs. In MBC, we detected actionable discordance rates of 40 and 23%, respectively for ER and HER2 where a biomarker was negative in the primary or metastatic tumor and positive in the CTCs. In LABC/IBC, actionable discordances were 60 and 20% for ER and HER2, respectively. Pilot trials evaluating the effectiveness of treatment selections based on actionable discordances between biomarker expression patterns on CTCs and primary or metastatic tumor sites may allow for a prospective assessment of CTC-based individualized targeted therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer remains the most common and second deadliest cancer in women [1]. The disease is no longer considered a homogenous entity [2]. For locally advanced and inflammatory breast cancer (LABC, IBC), the lifetime risk of recurrence ranges between 10 to over 50% [3]. The median survival is only 2 years for all patients with stage IV metastatic breast cancer (MBC [4]), with slightly better outcome for HER2-overexpressing (HER2+) [5] and ER/PR-expressing (ER/PR+) cancers [6]. Triple negative breast cancers carry the worst prognosis [7–11].
Circulating tumor cells (CTCs) are considered seeds for distant metastases [12–14]. CTCs have been found in ~60% of MBC, and their enumeration carries prognostic implications and predicts survival [15–17]. Whether therapeutic interventions can be guided based on changes in CTC numbers as a function of therapy is currently under study (SWOG-S0500, NCT00382018) in MBC [18, 19], and molecular characterization of CTCs may facilitate treatment selection.
Discordance between primary tumors and metastases varies greatly [20–24], and multiple biopsies of metastatic sites are rarely feasible. Hence, characterization of CTCs may be of benefit. For example, HER2 status can change and HER2+ CTCs are observed in up to 50% of patients with HER2-LABC and MBC [25] and such discordance is thought to be an independent prognostic factor. EGFR, phosphorylated EGFR, PI3K, and phosphor-AKT expression may also vary between the primary and CTCs [25–28]. Subsets of CTCs with cancer stem cell-like features could be subjects for novel targeting strategies [29, 30]. The presence and numerical changes of CTCs are prognostic and predictive markers in LABC, also [31, 32]. HER2+ or ER+ expression in CTCs from patients with triple negative breast cancer could identity beneficiaries for targeted therapies. Furthermore, should the presence of markers involved in DNA damage/repair (ERCC1, PARP, γH2AX) predict the efficacy of, for example, platinum-based therapy [9, 33], CTC-based selection of chemotherapy treatments CTC-based selection of chemotherapy regimens could be incorporated into treatment planning.
The Bruce laboratory at Palo Alto Research Center (PARC) has developed a fiber-optic array laser-scanning technology (FAST), enabling rapid high-fidelity location of CTCs identified by the conventional markers cytokeratin (CK), DAPI and CD45, without the need for enrichment [34, 35]. CTCs can simultaneously be labeled for at least 3 additional tumor biomarkers. Applying FAST, we set out to compare biomarker expression of CTCs from patients with LABC and MBC to expression patterns on primary and metastatic tumor sites.
Patients and methods
Inclusion criteria
Patients with stage II/III LABC/IBC, and with newly diagnosed, recurring, or progressive MBC were enrolled sequentially between September, 2009 and May, 2010, and those with ≥1 detectable CTCs were analyzed for multiplex marker expression. All patients gave their written, voluntary, informed consent (www.clinicaltrials.gov: NCT01048918 and NCT00295893). Patients with LABC/IBC received neoadjuvant therapy with an anthracycline and taxane-based (docetaxel, doxorubicin, cyclophosphamide), or platinum-containing (doxorubicin, cyclophosphamide, followed by carboplatin and nab-paclitaxel, or carboplatin/paclitaxel) regimen with or without trastuzumab, depending on HER2 status. Patients with newly diagnosed or refractory/progressing MBC were eligible, regardless of treatment regimens.
Histopathologic and immunohistochemical (IHC) analysis
Histological parameters based on H&E staining were assessed. Expressions of ER, HER2, and ERCC1 were evaluated by IHC performed on the primary tumor from patients with LABC IBC, and MBC, and, when available, on tissue procured from metastases. Representative sections were analyzed by a member of the Department of Anatomic Pathology at the COHCC (S.K.L.).
All tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. IHC was performed using antibodies directed against the following: ER (clone 1D5, dilution 1:400, Immunotech, France), HER2 (polyclonal A0485, dilution 1:200, Dako, Carpinteria, CA), and ERCC1 (clone D-10, dilution 1:100, Santa Cruz Biotechnology, Santa Cruz, CA).
Sections were deparaffinized in xylene and rehydrated in a graded ethanol series. Slides were heated in Tris–EDTA buffer in a steamer (Black and Decker, Shelton, CT) for 20 min. Staining was performed utilizing an automated immunostainer (Dako) followed by antibody detection using the EnVisionTM+ system (Dako) and 3,3′-diaminobenzadine as chromogen. The slides were counterstained with hematoxylin and coverslipped. Appropriate positive and negative tissue controls were used. For each tumor, the percentage of immunoreactive neoplastic cells and intensity of immunoreactivity (weak, moderate, or strong) were recorded. ER positivity required that at least 1% of the cells show nuclear staining of any intensity. Tumors were classified as HER2− or + according to standard American Society of Clinical Oncology/College of American Pathologists recommendations. ERCC1 scores were reported positive if the product of the intensity of nuclei and proportion of positive nuclei was above the median score of samples tested [10].
CTC analysis
Ten and 30 ml samples of blood for MBC and LABC, respectively, were drawn into Cytochex Cell Free DNA tubes (Streck Inc., Omaha, NE), shipped overnight at room temperature and processed within 24 h. Samples were subjected to erythrocyte lysis [34, 35]. For samples from LABC/IBC 3:1 negative enrichment was done using depletion of WBC with Miltenyi CD45 microbeads (Cat. # 130-045-801, Miltenyi Biotec, Auburn, CA) in their LS column and MACS Quadro stand following manufacturer’s suggested protocol. The remaining cell pellet was washed, re-suspended in phosphate buffered saline (PBS), and plated on custom-designed adhesive glass substrates with an active area of 63.68 cm2 (Paul Marienfeld GmbH & Co., KG, Bad Mergentheim, Germany).
Following fixation, blocking and labeling were performed. Primary antibodies were mouse anti-human CD45 (MCA87, AbD Serotec, Raleigh, NC) directly conjugated with Qdot 705 (Invitrogen custom conjugation), a cocktail of mouse monoclonal anti-cytokeratin (anti-CK) antibodies for CK classes 1, 4, 5, 6, 8, 10, 13, 18, and 19 (C2562, Sigma), and mouse monoclonal anti-CK 19 antibody (RCK108, DAKO). The secondary antibody for CK was biotin-XX goat anti-mouse IgG1 (A10519, Invitrogen) and tertiary antibody was streptavidin Alexa555 conjugate (S-32355, Invitrogen). After nuclear counterstaining (0.5 μg/ml 4′,6-diamidino-2-phenylindole (DAPI), D-21490, Invitrogen), a coverslip was mounted with ProLong Gold antifade mounting medium (P-36935, Invitrogen). CTCs were identified by morphology and immuno-phenotype (CK+, CD45−, and DAPI+) [34, 35].
The multiplex assay included three antibodies in addition to CK and CD45 to measure expression levels of HER2, ER, and ERCC1. The primary antibody against HER2 (erbB2, chicken anti-human, Abcam ab14027) was followed by a Qdot655 conjugated goat anti-chicken secondary antibody (Q14421MP, Invitrogen). The primary antibody against the estrogen receptor (ER-α, rabbit anti-human, Santa Cruz Biotech Sc-543) was followed by Alexa750 tagged goat anti-rabbit secondary antibody (A-21039, Invitrogen). The primary antibody against ERCC1 (mouse anti-human IgG2b, sc-17809, Santa Cruz Biotech) was followed by Alexa647 tagged goat anti-mouse IgG2b secondary antibody (A-21242, Invitrogen). All primary antibodies were pre-mixed, incubated together for 1 h and washed away before secondary antibodies were applied. All secondary antibodies were also pre-mixed, incubated for 1 h and washed away before tertiary was applied for half an hour. Incubation steps were done in the 37°C humid chamber. Quantification of the brightness of images from an automated digital microscope was performed with a software tool, developed at PARC [34, 35]. A combination of digital analysis and visual evaluation was performed to ensure correct scoring.
The choice of the ER and HER2 antibodies was based on immunofluorescence staining quality and constraints of the multiplex assay. For our immunofluorescent assay, we chose antibodies optimized for in vitro diagnostics. The antibodies used in the multiplex assay were chosen to prevent cross reactivity in the assay and are from different species or subclasses than those used with IHC on tissue samples. The antibodies were qualified through staining on cultured cells having different biomarker expression levels. We evaluated the staining quality by measuring stain localization and contrast, and by the difference between the staining levels of moderate-expressing control cells versus leukocytes, the negative control.
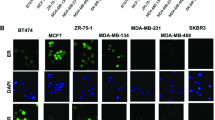
The FAST instrument (Fig. 1) scanned the glass slide containing the processed sample at 25 M cells/min, and fluorescence emission from labeled cells was collected by an array of optical fibers forming a wide collection aperture [34]. For sensitivity, samples were stained for a mixture of cytokeratin isoforms (CK) for broad coverage [36], an approach thought to be especially useful for detecting normal and basal-like types which have reduced EpCAM expression [37]. Cell morphology was well preserved during preparation (Fig. 2).
Images of tumor cells labeled for biomarkers. Cell 1 (top row) is labeled for HER2, Cell 2 (middle row) is labeled for ER and cell 3 (bottom row) is labeled for ERCC1. The first column a is a composite image of CK (red), nucleus (blue), and biomarker (green). Subsequent columns show images of individually labeled targets: CK (b), biomarker (c), and nucleus (d), respectively
Scoring methodology was adapted from tissue analysis, combining expression level and the percentage of expressing cells [15, 23, 27, 34]. Only intact CTCs with the nucleus surrounded by cytokeratin and without apoptosis were used. Each cell was scored as either 0, 1, 2 or 3, depending on whether it was negative (equal to leukocyte controls: score = 0), in the bottom third of the intensity distribution from the positive control cell line (score = 1), the middle third of the positive control (score = 2), or the top third (score = 3, see Fig. 3 as an example for HER2 assessment). If less than 10% of the cells scored (either 1,2 or 3), the final sample score was 0. We took the average score of the cells scoring 1, 2, or 3, and multiplied it by a factor determined by the percent of cells with a positive score. We chose a sample score ≥3 as positive for HER2+, and for ER, any score greater than zero was considered ER+, to emulate the current standard for tissue IHC scoring. As an example, 10 CTCs with scores 1, 2, 3, and seven 0’s would be scored a 6, corresponding to the average of the positive cells of 2, and the factor of 3 due to the 30% positive cells. Positive controls used for individual CTC scoring were MDA-Mb-453 for HER2 [38, 39], T-47D for ER [40], and A-549 for ERCC1 [10, 41, 42]. ERCC1 scoring was performed by adapting tissue-based scoring [10]. The protocol for CTC identification and biomarker analysis was established and refined through independent assessments and consensus between two research personnel with inputs from two pathologists. For data in this article, one of the research personnel (RHB) assessed all the CTC counts and biomarker expression using this established analysis, with feedback from the others.
Statistical methods
Summary statistics were used to describe the number of CTCs detected/evaluated, and HER2 and ER scores were compared to the primary/metastatic site IHC. ERCC1 analysis used IHC and CTC scoring as a continuous variable, and did not include a discussion of discordance as no validated cut-point has been established from IHC.
We defined “actionable discordance” on a specific marker (ER or HER2) as situations where the primary and/or metastatic tumor was negative for a marker, while the CTC score was positive. Non-actionable discordance refers to situations where the tissues express a marker, which is not detectable in CTCs.
Results
The disease and treatment characteristics of the 26 patients whose samples were analyzed for ER, HER2, and ERCC1, were not significantly different from those of the entire patient population tested for CTCs (data not shown), and are depicted in Table 1.
From September 2009 through May 2010, blood samples were procured from13 patients with LABC/IBC for CTC analysis. ≥1 CTCs were detected in 9 of these patients prior to treatment, but multiplex marker analysis could be interpreted in 8. In samples procured from 23 patients with newly diagnosed (1st line) or recurring, or progressing MBC, morphology from 1 sample was not interpretable, and ≥1 CTCs were observed and multiplex marker analysis was carried out in 18 of 22 patients (Table 2).
MBC cases
We detected ≥1 CTCs in 82% (18/22), and ≥5 CTCs were observed in 55% (12/22) among those with stage IV MBC. In the samples with detectable CTCs, the median number observed was 6 (range: 2–400) (Table 2). Among the patients with stage IV MBC, 14 patients were enrolled with HER2- primary tumors, of whom 13 had ≥1 detectable CTCs, and 3 of these 13 CTC samples were characterized as HER2+. Hence, 23% (3/13) of the HER2- cases with CTCs revealed a potentially actionable discordance (primary tumor: HER2−, CTC: HER2+). In 10 patients with biopsy specimens from both the primary and a metastatic site, we found no discordances between the tissues in their HER2 status or ER status based on tumor IHC.
We attempted to measure ER expression on CTC samples from 18 patients having ≥1 CTCs. The assay was successful in 14 of 18 samples with CTCs. Of the 5 stage IV cases whose primary was ER− and in whom CTCs were detected, CTCs were ER+ in 2 (40% potentially actionable discordance). The overall actionable discordance rate considering HER2 and ER biomarkers, illustrated in Table 3, is 28% (5/18). CTCs were both ER+ and HER2+ in two cases, one was concordant with the primary site and another had a primary tumor which was HER2−/ER+. The discordance rates for non-actionable discordance were as follows: 3 of 9 patients with ER+ primaries had no ER signal on CTCs, and 3 of 5 patients with HER2+ tumors had no HER2 signal on CTCs (Table 3).
We measured primary ERCC1 expression on 11 patients having ≥ 1 CTCs. We found poor correlation between scores on CTCs and the primary tumor (r = −0.16). There was also poor correlation (r = 0.15) of ERCC1 between the primary and biopsied metastatic sites (N = 8).
Sequential samples for CTCs were assessed for persistence in marker expression profile, but due to the limited number of patients (4) with multiple evaluable samples available without change in treatment, meaningful evaluation was not feasible (data not shown). Analysis for trends between response to therapy and biomarker expression on CTCs did not yield any significant results in this heterogenous population treated with a variety of different regimens (data not shown).
LABC/IBC
For this cohort, ≥1 CTC was detected for 9/13 (69%) patients, and all of the samples with CTCs were evaluated for marker expression. Five or more CTCs were detected in 5/13 patients (38%). In samples from LABC/IBC patients with detectable and evaluable CTCs, the median number of CTCs observed was 5.5 (range: 1–40). For the entire cohort of 13 patients, the median was 3 CTCs (range 0–40).
Of the 9 patients with CTCs, 8 were evaluable for protein expression; one sample could not be assessed due to a technical problem. Of 5 patients with ER− primaries and ≥1 CTCs, CTCs were ER+ in 3, resulting in a 60% potentially actionable ER discordance rate for ER− patients with CTCs and biomarker evaluation. Of 5 patients with HER2− primaries and ≥1 CTCs, CTCs were HER2+ in one case, resulting in a 20% potentially actionable HER2 discordance rate in this subset. One patient with triple negative primary LABC was found to have both ER+ and HER2+ CTCs. The overall actionable discordance for any markers tested in the 8 patients with CTCs assessable is 3/8 (~38%).
The discordance rates for non-actionable discordance were as follows: 2 of 3 patients with ER+ primaries had no ER signal on CTCs, and 3 patients with HER2+ primaries had no HER2 signal on the CTCs detected. Table 3 illustrates overall actionable and non-actionable discordances.
Discussion
Several detection methods using immunocytochemistry (ICC) for CTC identification have been explored. Incorporating enrichment technologies, such as magnet-activated cell sorting (MACS), into stable platforms with automated sample preparation (CellSearch) has enabled consistent enumeration of CTCs [14, 17, 31]. More recently, a microfluidic approach has been reported where anti-EpCAM is coated on posts instead of beads, and the CTCs are separated by flowing whole blood past these coated posts [43–45], however, obtaining high quality images of CTCs is challenging.
The above techniques lose sensitivity at low levels of EpCAM expression [36, 37]. Tumors and CTCs with normal/basal genotype (triple negative phenotype), have very low levels of EpCAM expression [37]. A recent comparison of the CellSearch technology and two of the microfluidic approaches reported sensitivities that were lower for cell line models with low EpCAM expression [30]. As an alternative, physical properties of CTCs for enrichment such as size [46] and dielectric constant can be exploited, but the heterogeneity of CTC phenotypes remains a problem.
PCR amplification of DNA or mRNA in blood serum for tumor-specific markers offers another approach for biomarker detection and monitoring of disease and treatment outcomes [47]. However, detection of EGFR or p53 mutations in DNA from serum has been shown to be less accurate than the detection of the same marker in CTCs [43, 48].
Applying FAST technology, we were able to detect at least 1 CTC in 69% of cases with LABC/IBC, and ≥5 CTCs in 38% of cases. We detected at least 1 CTC in 82% of patients with stage IV MBC, with ≥5 CTCs observed in 54.5%. Taking into account the limited sample size, CTC detection is comparable to what has been reported with CellSearch technology [14, 17, 31, 49]. While both approaches count only intact CTCs (i.e., CTCs with the nucleus surrounded by cytoplasm), the fidelity of our images is considerably higher than images generated with CellSearch (Fig. 2). We are currently comparing CTC detection between FAST and CellSearch approaches for two patient cohorts: in triple negative breast cancer in which EpCAM expression is expected to be low and in patients with HER2+ disease, with the EpCAM expression expected to be high. We will test whether a non-EpCAM targeting method is more sensitive for detecting CTCs with normal and basal/triple negative subtypes of breast cancers [37].
The literature is controversial regarding discordance between primary and metastatic sites assessed for ER, PR, and HER2 expression. Liedtke et al. [24] describe high levels of combined actionable and non-actionable discordance for ER, PR, and HER2 (18.4, 40.3, and 13.6%). However, Tapia et al. [23] reported 7.6% discordance between primary and metastatic sites for HER2 overexpression by FISH, but only 4.7% were unequivocal for discordance on further review. Carlsson et al. [20] found no discordances between the primary and metastases in regional lymph nodes in 45 patients who were also identified as having simultaneous distant metastases (not assessed in their study). A more recent study by Thompson et al. [50] reported low 0.7% non-actionable and 2.2% actionable discordance for HER2 between the primary and distant sites (by FISH), and ER and PR actionable discordance rates of 8 and 11% and non-actionable discordance rates of 2.2 and 8.8%.
There are many possible explanations for the differences across studies. The inconsistency in time between primary diagnosis and relapse and sites of metastatases are likely variables. The type and speed of procurement of tissue, variability in preservation/time of processing, inconsistency among the reading pathologists, staining techniques and cut-off for defining ER positivity, and the methods of HER2 testing, may also differ, as do the sample sizes.
The reasons for our finding of discordance between CTC expressions and primary/metastatic sites but not between primaries and metastases are likely multifactorial. Biopsies from the metastatic sites always preceded (occasionally by years) the procurement of CTCs, since, in order to be part of our studies, patients had to have evidence of MBC. We hypothesize, that the discordances observed between CTCs and primary and metastatic sites are partly due to the CTCs acquiring more stem cell-like (EMT-like) features, enabling them to further metastasize [29]. Also, with more time lapsed since diagnosis, CTCs can undergo further clonal evolution. Finally, the methodology of characterizing CTCs is still undergoing evolution. Regardless, assessment of CTC characteristics does carry the potential of modifying our therapies.
We were able to assess tumor biomarkers for ER, HER2, in the majority of patients with detectable CTCs, and ERCC1 was assessed in 70% of patients with detectable CTCs. Non-actionable discordance, especially with regard to ER, while reported, should be interpreted with caution, as a low frequency of positive CTCs would result in a high probability of a false negative signal due to the limited number of CTCs sampled. While the actionable discordance rate increases with the number of CTCs, we could not detect statistically significant differences when comparing discordances among samples with ≥5 versus <5 CTCs. The rates observed for actionable discordance, based on observing a positive CTC signal, were 28% in MBC and 38% in LABC/IBC. While these findings are in line with discordance rates reported in the literature, previous studies have typically relied on RT-PCR-based technology [39–44], which does not provide the benefit of morphological identification of marker expression within individually visualized CTCs.
Assessment of ERCC1 represents a novel paradigm for predictive testing of efficacy. We found no correlation between tumor tissue IHC and CTC immunofluorescence (IF) levels for ERCC1. Further testing in larger sample size and validation of the scoring system both in tissue and in CTCs is needed in addition to studying other DNA-repair associated markers as well.
Our ultimate goal is to prospectively evaluate the potential of CTC-enhanced selection of systemic therapy for patients with LABC/IBC/MBC based on actionable discordance with biomarker expression on CTCs. Work is ongoing to enable initiation of a clinical trial following development of replicable FAST product prototype/s that can be deployed to research groups and meet CLIA certification requirements. We plan to assess whether CTC-directed targeted therapy results in a decrease in CTCs as a surrogate marker for efficacy in a pilot prospective clinical trial designed to guide treatment based on actionable discordances for ER, HER2, or both. A positive outcome would then lead to prospective randomized testing of the concept of CTC-guided targeted therapies based on actionable discordances for current and new therapeutic targets.
References
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5):277–300. doi:10.3322/caac.20073
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. doi:10.1038/35021093
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13(15 Pt 1):4429–4434. doi:10.1158/1078-0432.CCR-06-3045
Chia SK, Speers CH, D’Yachkova Y, Kang A, Malfair-Taylor S, Barnett J, Coldman A, Gelmon KA, O’Reilly SE, Olivotto IA (2007) The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 110(5):973–979. doi:10.1002/cncr.22867
Robert N, Leyland-Jones B, Asmar L, Belt R, Ilegbodu D, Loesch D, Raju R, Valentine E, Sayre R, Cobleigh M, Albain K, McCullough C, Fuchs L, Slamon D (2006) Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol 24(18):2786–2792. doi:10.1200/JCO.2005.04.1764
Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Goldstein LJ, Gradishar WJ, Hayes DF, Hudis CA, Jahanzeb M, Kiel K, Ljung BM, Marcom PK, Mayer IA, McCormick B, Nabell LM, Pierce LJ, Reed EC, Smith ML, Somlo G, Theriault RL, Topham NS, Ward JH, Winer EP, Wolff AC (2009) Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Cancer Netw 7(2):122–192
Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, Sledge GW, Carey LA (2008) Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res 14(24):8010–8018. doi:10.1158/1078-0432.CCR-08-1208
Sirohi B, Arnedos M, Popat S, Ashley S, Nerurkar A, Walsh G, Johnston S, Smith IE (2008) Platinum-based chemotherapy in triple-negative breast cancer. Ann Oncol 19(11):1847–1852. doi:10.1093/annonc/mdn395
Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, Fatima A, Gelman RS, Ryan PD, Tung NM, De Nicolo A, Ganesan S, Miron A, Colin C, Sgroi DC, Ellisen LW, Winer EP, Garber JE (2010) Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 28(7):1145–1153. doi:10.1200/JCO.2009.22.4725
Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH, Stahel R, Sabatier L, Pignon JP, Tursz T, Le Chevalier T, Soria JC, Investigators IB (2006) DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 355(10):983–991. doi:10.1056/NEJMoa060570
Sidoni A, Cartaginese F, Colozza M, Gori S, Crino L (2008) ERCC1 expression in triple negative breast carcinoma: the paradox revisited. Breast Cancer Res Treat 111(3):569–570. doi:10.1007/s10549-007-9804-4
Riethdorf S, Wikman H, Pantel K (2008) Review: biological relevance of disseminated tumor cells in cancer patients. Int J Cancer 123(9):1991–2006. doi:10.1002/ijc.23825
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10(20):6897–6904. doi:10.1158/1078-0432.CCR-04-0378
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8):781–791. doi:10.1056/NEJMoa040766
Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen LW, Hayes DF (2006) Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res 12(21):6403–6409. doi:10.1158/1078-0432.CCR-05-1769
Nole F, Munzone E, Zorzino L, Minchella I, Salvatici M, Botteri E, Medici M, Verri E, Adamoli L, Rotmensz N, Goldhirsch A, Sandri MT (2008) Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol 19(5):891–897. doi:10.1093/annonc/mdm558
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12(14 Pt 1):4218–4224. doi:10.1158/1078-0432.CCR-05-2821
Hayes DF, Smerage J (2008) Is there a role for circulating tumor cells in the management of breast cancer? Clin Cancer Res 14(12):3646–3650. doi:10.1158/1078-0432.CCR-07-4481
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25(33):5287–5312. doi:10.1200/JCO.2007.14.2364
Carlsson J, Nordgren H, Sjostrom J, Wester K, Villman K, Bengtsson NO, Ostenstad B, Lundqvist H, Blomqvist C (2004) HER2 expression in breast cancer primary tumours and corresponding metastases. Original data and literature review. Br J Cancer 90(12):2344–2348. doi:10.1038/sj.bjc.6601881
Koo JS, Jung W, Jeong J (2010) Metastatic breast cancer shows different immunohistochemical phenotype according to metastatic site. Tumori 96(3):424–432
Stoecklein NH, Klein CA (2010) Genetic disparity between primary tumours, disseminated tumour cells, and manifest metastasis. Int J Cancer 126(3):589–598. doi:10.1002/ijc.24916
Tapia C, Savic S, Wagner U, Schonegg R, Novotny H, Grilli B, Herzog M, Barascud AD, Zlobec I, Cathomas G, Terracciano L, Feichter G, Bubendorf L (2007) HER2 gene status in primary breast cancers and matched distant metastases. Breast Cancer Res 9(3):R31. doi:10.1186/bcr1676
Liedtke C, Broglio K, Moulder S, Hsu L, Kau SW, Symmans WF, Albarracin C, Meric-Bernstam F, Woodward W, Theriault RL, Kiesel L, Hortobagyi GN, Pusztai L, Gonzalez-Angulo AM (2009) Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol 20(12):1953–1958. doi:10.1093/annonc/mdp263
Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S, Beitsch P, Khan A, Euhus D, Osborne C, Frenkel E, Hoover S, Leitch M, Clifford E, Vitetta E, Morrison L, Herlyn D, Terstappen LW, Fleming T, Fehm T, Tucker T, Lane N, Wang J, Uhr J (2004) HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci USA 101(25):9393–9398. doi:10.1073/pnas.0402993101
Payne RE, Yague E, Slade MJ, Apostolopoulos C, Jiao LR, Ward B, Coombes RC, Stebbing J (2009) Measurements of EGFR expression on circulating tumor cells are reproducible over time in metastatic breast cancer patients. Pharmacogenomics 10(1):51–57. doi:10.2217/14622416.10.1.51
Wulfing P, Borchard J, Buerger H, Heidl S, Zanker KS, Kiesel L, Brandt B (2006) HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res 12(6):1715–1720. doi:10.1158/1078-0432.CCR-05-2087
Kallergi G, Agelaki S, Kalykaki A, Stournaras C, Mavroudis D, Georgoulias V (2008) Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients. Breast Cancer Res 10(5):80. doi:10.1186/bcr2149
Reuben JM, Lee BN, Li C, Gao H, Broglio KR, Valero V, Jackson SA, Ueno NT, Krishnamurthy S, Hortobagyi GN, Cristofanilli M (2010) Circulating tumor cells and biomarkers: implications for personalized targeted treatments for metastatic breast cancer. Breast J 16(3):327–330
Punnoose EA, Atwal SK, Spoerke JM, Savage H, Pandita A, Yeh RF, Pirzkall A, Fine BM, Amler LC, Chen DS, Lackner MR (2010) Molecular biomarker analyses using circulating tumor cells. PLoS One 5(9):e12517. doi:10.1371/journal.pone.0012517
Pierga JY, Bidard FC, Mathiot C, Brain E, Delaloge S, Giachetti S, de Cremoux P, Salmon R, Vincent-Salomon A, Marty M (2008) Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res 14(21):7004–7010. doi:10.1158/1078-0432.CCR-08-0030
Pachmann K, Camara O, Kavallaris A, Krauspe S, Malarski N, Gajda M, Kroll T, Jorke C, Hammer U, Altendorf-Hofmann A, Rabenstein C, Pachmann U, Runnebaum I, Hoffken K (2008) Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J Clin Oncol 26(8):1208–1215. doi:10.1200/JCO.2007.13.6523
Wang LH, Pfister TD, Parchment RE, Kummar S, Rubinstein L, Evrard YA, Gutierrez ME, Murgo AJ, Tomaszewski JE, Doroshow JH, Kinders RJ (2010) Monitoring drug-induced gammaH2AX as a pharmacodynamic biomarker in individual circulating tumor cells. Clin Cancer Res 16(3):1073–1084. doi:10.1158/1078-0432.CCR-09-2799
Krivacic RT, Ladanyi A, Curry DN, Hsieh HB, Kuhn P, Bergsrud DE, Kepros JF, Barbera T, Ho MY, Chen LB, Lerner RA, Bruce RH (2004) A rare-cell detector for cancer. Proc Natl Acad Sci USA 101(29):10501–10504. doi:10.1073/pnas.0404036101
Hsieh HB, Marrinucci D, Bethel K, Curry DN, Humphrey M, Krivacic RT, Kroener J, Kroener L, Ladanyi A, Lazarus N, Kuhn P, Bruce RH, Nieva J (2006) High speed detection of circulating tumor cells. Biosens Bioelectron 21(10):1893–1899. doi:10.1016/j.bios.2005.12.024
Deng G, Herrler M, Burgess D, Manna E, Krag D, Burke JF (2008) Enrichment with anti-cytokeratin alone or combined with anti-EpCAM antibodies significantly increases the sensitivity for circulating tumor cell detection in metastatic breast cancer patients. Breast Cancer Res 10(4):R69. doi:10.1186/bcr2131
Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, Martens JW, Gratama JW, Sleijfer S, Foekens JA (2009) Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst 101(1):61–66. doi:10.1093/jnci/djn419
Rhodes A, Borthwick D, Sykes R, Al-Sam S, Paradiso A (2004) The use of cell line standards to reduce HER-2/neu assay variation in multiple European cancer centers and the potential of automated image analysis to provide for more accurate cut points for predicting clinical response to trastuzumab. Am J Clin Pathol 122(1):51–60. doi:10.1309/61AN-J1XV-3MW8-78YP
Szollosi J, Balazs M, Feuerstein BG, Benz CC, Waldman FM (1995) ERBB-2 (HER2/neu) gene copy number, p185HER-2 overexpression, and intratumor heterogeneity in human breast cancer. Cancer Res 55(22):5400–5407
Thompson EW, Paik S, Brunner N, Sommers CL, Zugmaier G, Clarke R, Shima TB, Torri J, Donahue S, Lippman ME, Martin GR, Dickson RB (1992) Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol 150(3):534–544. doi:10.1002/jcp.1041500314
Sommers CL, Byers SW, Thompson EW, Torri JA, Gelmann EP (1994) Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res Treat 31(2–3):325–335
Tabibzadeh SS, Kong QF, Kapur S (1994) Passive acquisition of leukocyte proteins is associated with changes in phosphorylation of cellular proteins and cell-cell adhesion properties. Am J Pathol 145(4):930–940
Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ, Bell DW, Digumarthy S, Muzikansky A, Irimia D, Settleman J, Tompkins RG, Lynch TJ, Toner M, Haber DA (2008) Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 359(4):366–377. doi:10.1056/NEJMoa0800668
Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK, Floyd FP Jr, Gilman AJ, Lord JB, Winokur D, Springer S, Irimia D, Nagrath S, Sequist LV, Lee RJ, Isselbacher KJ, Maheswaran S, Haber DA, Toner M (2010) Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci USA 107(43):18392–18397. doi:10.1073/pnas.1012539107
Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M (2007) Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450(7173):1235–1239. doi:10.1038/nature06385
Lin HK, Zheng S, Williams AJ, Balic M, Groshen S, Scher HI, Fleisher M, Stadler W, Datar RH, Tai YC, Cote RJ (2010) Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res 16(20):5011–5018. doi:10.1158/1078-0432.CCR-10-1105
O’Driscoll L, Kenny E, Mehta JP, Doolan P, Joyce H, Gammell P, Hill A, O’Daly B, O’Gorman D, Clynes M (2008) Feasibility and relevance of global expression profiling of gene transcripts in serum from breast cancer patients using whole genome microarrays and quantitative RT-PCR. Cancer Genomics Proteomics 5(2):94–104
Chen Z, Feng J, Buzin CH, Liu Q, Weiss L, Kernstine K, Somlo G, Sommer SS (2009) Analysis of cancer mutation signatures in blood by a novel ultra-sensitive assay: monitoring of therapy or recurrence in non-metastatic breast cancer. PLoS One 4(9):e7220. doi:10.1371/journal.pone.0007220
Hayes DF, Walker TM, Singh B, Vitetta ES, Uhr JW, Gross S, Rao C, Doyle GV, Terstappen LW (2002) Monitoring expression of HER-2 on circulating epithelial cells in patients with advanced breast cancer. Int J Oncol 21(5):1111–1117
Thompson AM, Jordan LB, Quinlan P, Anderson E, Skene A, Dewar JA, Purdie C, The Breast Recurrence Tissue Study Group (2010) Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence in Tissue Study (BRITS). Breast Cancer Res 12:R92
Acknowledgments
The authors thank Norma Baker, Sue Swain-Cabriales, Kim Robinson, and Michele Kirschenbaum for their enthusiastic and professional assistance, Janey Ly, Robin Bennis, and Laurie Kara for developing the assay and using it to analyze patient samples, Erich Schwartz MD for assistance in CTC identification and biomarker analysis, and Carol Wuenschell for editorial assistance [reported in part in J Clin Oncol 2009 (suppl; abstr 1092)]. This work was supported by NIH CA 111359-03 and NIH ARRA 11359-03-04S1, and by NCI CA 33572, and NIH M01RR00043.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Somlo, G., Lau, S.K., Frankel, P. et al. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary and metastatic sites in patients with locally advanced/inflammatory, and stage IV breast cancer, using a novel detection technology. Breast Cancer Res Treat 128, 155–163 (2011). https://doi.org/10.1007/s10549-011-1508-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1508-0