Abstract
Deinococcus radiodurans has attracted a great interest in the past decades due to its extraordinary resistance to ionizing radiation and highly efficient DNA repair system. Recent studies indicated that pprM is a putative pleiotropic gene in D. radiodurans and plays an important role in radioresistance and antioxidation, but its underlying mechanisms are poorly elucidated. In this study, pprM mutation was generated to investigate resistance to desiccation and oxidative stress. The result showed that the survival of pprM mutant under desiccation was markedly retarded compared to the wild strain from day 7–28. Furthermore, knockout of pprM increases the intercellular accumulation of ROS and the sensibility to H2O2 stress in the bacterial growth inhibition assay. The absorbance spectrum experiment for detecting the carotenoid showed that deinoxanthin, a carotenoid that peculiarly exists in Deinococcus, was reduced in the pprM mutant in the pprM mutant. Quantitative real time PCR showed decreased expression of three genes viz. CrtI (DR0861, 50%),CrtB (DR0862, 40%) and CrtO (DR0093, 50%), which are involved in deinoxanthin synthesis, and of Dps (DNA protection during starving) gene (DRB0092) relevant to ion combining and DNA protection in cells. Our results suggest that pprM may affect antioxidative ability of D. radiodurans by regulating the synthesis of deinoxanthin and the concentration of metal ions. This may provide new clues for the treatment of antioxidants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
D radiodurans is an orange-pink, non-pathogenic bacteria well-known for the robust resistance to a range of ambient stresses such as ionizing radiation, UV, oxidatives and desiccation [1]. PprM is a radiation stress response protein, which deletion increases the sensitivity of D. radiodurans to γ-rays and UV and also regulates the catalase KatE1 in D. radiodurans [2]. However, the detailed underlying molecular mechanism about pprM against envirionment stresses or attacks remains poorly understand. We previously used the overlap-PCR to knockout the pprM gene and constructed a pprM mutant strain (ΔpprM) (detailed procedures are not mentioned in this article and is in press). When exploring the functional role that pprM plays in D. radiodurans, we found that the ΔpprM present a lighter color than the wild type.
Reactive oxygen species (ROS) is a by-product of water after radiation. When facing extremely situations, more ROS would be produced in cells. The free oxygen radicals would damage DNA, RNA and proteins, thus the vitality of life would be threatened. Scavenging of ROS ability reflect the capacity of anti-oxidation in cells. D. radiodurans posseses a robust system on withstanding oxidizing material, which system includes the enzyme and non-enzyme components. Superoxide dismutase (SOD) and catalase (CAT) are two important antioxidant enzymes. The activity of SOD is 6 times and the CAT is 30 times higher than E. coli [3]. The main non-enzyme component includes carotenoid. Deinoxanthin is a carotenoid that peculiarly exists in Deinococcus and is related to the strain’s color [4]. It could protect DNA from the attacks and make tremendous contributions to the oxidation resistance [5]. Previous research discovered that CrtI (DR0861), CrtB (DR0862) and CrtO (DR0093) are three main genes that influence the biosynthesis of deinoxanthin, the deletion of these genes resulted the bacterial to be colorloss and also brings hypersensitivity to oxidative stresses [6]. The highly ratio of Mn(II)/Fe(II) in cells is another non-enzyme mechanism that facilitates the defence against oxidative damage. The abundance of Mn ions assist in removing ROS, however, the Fenton action of Fe2+ would bring huge damages to cells. Dps is a functional protection protein which can combine with Fe2+ and oxidize Fe2+ to Fe3+, and also has high affinity in binding with DNA, consequently prevent hydroxyl free radical from damaging DNA. These prominent features allow Dps detoxify Fe2+ and H2O2 simultaneously [7].
Based on our experimental results, we hypothesized that pprM may facilitate the antidesiccation ability and the decrease in antioxidation and the knockout of pprM may result in the reduction of deinoxanthin and Dps through inhibiting gene expression of no-enzyme antioxidant relevant to their synthesis.
Material and Method
Bacterial Strains and Growth Conditions
The wild type D. radiodurans R1 (CGMCC 1.633) was purchased from the China General Microbiological Culture Collection Center, and the pprM mutant were stored in laboratory and all cultivated at 30 °C in TGY broth (0.5% tryptone, 0.3% yeast extract, 0.1% glucose) or on TGY agar plates (1.5% agar) [8].
Desiccation Assay
The desiccation procedure was modified [9], as the allochroic silicagel was placed into a sterile beaker and used as desiccant to create a desiccative condition. Both the wild type and the pprM mutant were incubated for 48 h. The bacterial cultures were centrifuged at 6,000 rpm for 15 min and resuspended with 1XPBS. Four small EP tubes (1.5 ml) were added with 100 μl suspension, then subjected to 7, 14, 21 and 28 days of desiccative condition. After the desiccative time, the dehydrated cells were resuspended with sterilized water and diluted to 10−1–10−5 times, 6 μl of each diluted culture was spotted on the TGY medium and incubated at 30 °C for 3 days.
H2O2 Oxidation Assay
The detection of sensitivity to H2O2 was analyzed by using the disc inhibition assay [10]. The bacteria were cultivated in TGY broth for about 48 h 1 ml of each culture was spread-plated onto TGY agar plates by using a spreading rod, then 4 sterilized filter paper discs with 6 mm diameter were placed on the agar surface. 10 μl of H2O2 with different concentration (50, 100, 150 and 200 mM) was dropped on the paper disc respectively. The diameter of the growth inhibition zone was measured after cultivated at 30 °C for 3 days. (The wider of the sterility zone, the more sensitive to H2O2).
Intracellular ROS Accumulation Assay
ROS generation assay was performed according to previous description with some modification [11]. In brief,100 μL of exponential phase cultures were washed 3 times with 1XPBS, then resuspended with 1 ml 1XPBS containing 1 μL DCFH-DA(10 μM) and incubated at 37 °C for 20 min that avoided light with shaking. The cells were washed twice by 1XPBS and resuspended in 1 ml 1XPBS. Each cell suspension was equally dispensed into two parts, one of which was exposed to 2KGy of γ radiation and the other part was placed under room temperature as control. ROS would oxidize DCFH into DCF which is fluorescent. Thus, the fluorescence intensities that were measured using a fluorescence spectrophotometer (Shimadzu Japan) with an excitation wavelength of 485 nm and emission wavelength of 525 nm would reflect the ROS accumulation in cells.
Carotenoid Assay
The wild type and the ΔpprM were cultivated to exponential phase for about 48 h, then 1% of the cultures were dropped into 50 mL TGY fresh medium at 30 °C. Both of cultures were centrifuged 6,000 rpm,4 °C for 10 min.Cells were collected and washed by dd H2O for twice. The supernatant were discarded and the substrates were dissolved by pre-cooled acetone and methanol (V/V = 7:2), after shaking under shadow for 30 min, the solution were centrifuged under 10,000 rpm for 10 min and the supernatants were collected. The procedures were repeated twice or until the color fades. The supernatants were detected on the spectrophotometer (Shimadzu Japan), absorbance spectrum is from 350–600 nm.
RNA Isolation, Quantitative Real-Time PCR (QRT-PCR) Experiment
The wild and ΔpprM were cultivated for about 40 h in an appropriate condition until OD = 0.4, total RNA was extracted from the bacterial cultures with Trizol Reagent (Invitrogen Life Technologies) according to manufacture’s manual. Complementary DNA was synthesized in 20 μL of reaction containing 2 μg of RNA using Revert Aid First Strand cDNA Synthesis Kit (Thermo). Gene expression in RNA level was evaluated using quantitative PCR with Quant SYBR Green PCR kit (Roche). Triplicate reactions were carried out for each sample. The primers used in QRT-PCR were listed in Table 1. The 16sRNA was used as a reference for normalization. Fold change was determined using the 2ΔΔCT method.
Results and Discussion
pprM Mutant Strain is More Sensitive to Desiccation
Desiccation would occur extensive damages on DNA directly, and also increase the formation of ROS [12]. Our data (Fig. 1) illustrate that even though prolonged desiccation would bring about a side effect, the wild type still stayed viability after 7 or 14 days. However, while the cultures were diluted to different concentration, the clone of pprM mutant is scarce, presenting a significant survival retardation from the early stage (7 days) to the prolonged stage (28 days) against the wild D. radiodurans strain under desiccation.
The survival of D. radiodurans on TYG agar plate after exposed to desiccation. a wild type and b pprM mutant. The different colony spot on same row had different cell concentration with same desiccative days and the different colony spot on the same column had the same concentration with different desiccative days
pprM Mutant Strain is More Sensitive to H2O2 stress
When exposed to H2O2 stress, oxidative damage would occur to cells. We treat the bacteria under variation of H2O2 concentration, this assay demonstrated that the wild D. radiodurans shows a well-behaved resistance, however the ΔpprM appears a remarkable inhibition (Supplementary Fig. 1 shows the inhibited zone around the colony under all concentration). and the diameter was wider as the H2O2 concentration increased (measured in Table 2).
Differences in ROS Level Between the Wild Type and the ΔpprM
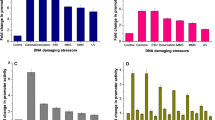
Excessive ROS would be generated after irradiation and attack cells inevitably. The detection of fluorescence intensity demonstrated that the ROS accumulation in the pprM mutant is nearly twice higher before exposed to radiation, and is almost 4 times higher after 2 k Gy irradiation against the wild type (Fig. 2). This suggested that the deletion of pprM would affect the ROS scavenging ability,and reconfirming that pprM is involved in oxidative stress response.
Deletion of pprM Leads to Decreased Carotenoid Level
Since the wild type and ΔpprM had difference in color, we attempted to detect the content of caroteniod in these two strains. Deinoxanthin is the main caroteniod in D. radiodurans and has a absorbance shoulder at around 480 nm [13]. According to the results, the wild type exhibited a higher peak than the pprM mutant (Fig. 3), which demonstrating that the deletion of pprM leading to the deficiency in deinoxanthin synthesis in D. radiodurans.
The Expression of Deinoxanthin Synthesis Related Genes and Dps Gene Decreased in the pprM Mutant
As the pprM mutant was deficient in the content of deinoxanthin, we found that CrtI (DR0861), CrtB (DR0862) and CrtO (DR0093), which are three main genes that involved in the biosynthesis of deinoxanthin, presented a lower transcriptional level in the ΔpprM (DR0861 is 0.5 fold, DR0862 is 0.4 fold, DR0093 is 0.4 fold and DRB0092 is 0.5 fold expressed) compared to the wild type through QRT-PCR assays (Fig. 4). In addition, dps-2 (DRB0092), a gene that relevant to oxidant stress and linked to the regulation of ratio of Mn/Fe [14], was also decreased (0.5 fold) in the ΔpprM, which hints that pprM may regulate the expression of the components that involved in responding ambient stresses.
Discussion
Deinococcus radiodurans exhibits extraordinary resistance to external stresses owing to collaboration of multiple regulating systems. In it’s genome, about 1/3 of the genes play significant roles in radioprotection and DNA repair. pprI is considered to be a general switch in the protection of D. radiodurans. The PprI protein was found to accelerate the expression of 210 genes after irradiation, and 21 of which are DNA damage repair genes [15]. PprM is a a homolog of a cold shock protein (CSP) and also a damage responsor that regulated by pprI [16]. Since early research states that desiccation is a significant factor that contribute D. radiodurans evolving to be such a robust organism and the resistance to oxidative stress is closely related to its survival, we investigate the pprM mutant’s resistance to stress conditions. Our results demonstrate that pprM gene knockout would affect the resistance ability to desiccation and hydrogen peroxide, indicating that pprM is involved in stress tolerance in D. radiodurans.
ROS is a reverse component generated under extremely harsh conditions. Ghosal et al. reported that 80% of the damages occurred on DNA derived from ROS [17]. The detection of ROS accumulation experiment showed that more ROS accumulated in the ΔpprM, suggesting that pprM may participate in quenching harmful ROS to protecting cells from oxidation, thus complementing the mechanism how pprM plays its role in resistance ability.
Carotenoid is well acknowledged for it’s prominent ability in antioxidation in cells. Deinoxanthin is a kind of carotenoid that only exsists in Deinococcus. It owns 13 unsaturated double bond, which made contribution to antioxidative ability and could also exhibit protection on proteins and DNA [18]. Since the ΔpprM has a lower content of deinoxanthin,and the QRT-PCR results showed that the genes DR0862, DR0861, DR0093 which were related to biosynthetic pathway of deinoxanthin were down regulated in the ΔpprM, it can be assumed that pprM may influence deinoxanthin synthesis in an indirect way.
The abundance of Mn(II) attributed a lot to antioxidantive ability in D. radiodurans. High concentration of manganese ion improved the progress of removing superoxide and facilitated the compacted of DNA [19]. Recent study found that PprI could cleave the inhibitor protein DdrO, a transcription factor that suppresses DNA damage response genes’ expression, when the Mn2+ is abundant, which also backed up the importance of Mn in D. radiodurans laterally [20]. However the excessive Fe2+ may produce cytotoxicity, thus, the highly ratio of Mn(II)/Fe(II) in cells is another non-enzyme mechanism that facilitates the defense against oxidative damage. Since the expression of dps gene is depressed in the pprM mutant, we inferred that pprM may also exert its function in regulating the intracellular ratio of Mn/Fe in D. radiodurans.
As the underlying resistance mechanism is more complex than we can anticipate, we propose that pprM itself may be a stress response protein which could exert function on oxidation resistance or prevent damages on DNA. Additionally, since the analysis of structure shows that pprM embracing RNA binding domains, and could regulate the expression of some stress response genes in a transcription level, we denounce that pprM may act as a pleiotropic regulator in D. radiodurans in an indirect way.
pprM could assist in resisting ambient stresses, which endows it to be an excellent model for research of mechanisms and applications of antioxidation and antidesiccation, and could be applied in agriculture and industry. Carotenoid had been widely applied in medical condition, it can enhance immunity and accelerate metabolism to prevent human diseases [21]. But the underlying benefits of carotenoid still need further research. Some scholars transformed deinoxanthin into cells, demonstrating that this protein can induce the apoptosis of tumor cells [22]. Our results showed that pprM knockout not only reduced the resistance to oxidative and desiccative, but also lead to the decrease of deinoxanthin and dsp through inhibiting their gene expression. This may provide a new clue for the prevention and treatment of irradiation and oxidative damages, and also for developing new antioxidant and antidesiccative agents in application.
References
Munteanu A, Uivarosi V, Andries A (2015) Recent progress in understanding the molecular mechanisms of radioresistance in Deinococcus bacteria. Extrem Life Under Extreme Cond 19:707–719. doi:10.1007/s00792-015-0759-9
Jeong SW, Seo HS, Kim MK, Choi JI, Lim HM, Lim S (2016) PprM is necessary for up-regulation of katE1, encoding the major catalase of Deinococcus radiodurans, under unstressed culture conditions. Journal of microbiology (Seoul, Korea) 54:426–431. doi:10.1007/s12275-016-6175-8
Wang P, Schellhorn HE (1995) Induction of resistance to hydrogen peroxide and radiation in Deinococcus radiodurans. Can J Microbiol 41:170–176
Lemee L, Peuchant E, Clerc M, Brunner M, Pfander H (1997) Deinoxanthin: a new carotenoid isolated from Deinococcus radiodurans. Tetrahedron 53:919–926. doi:10.1016/S0040-4020(96)01036-8
Tian B, Xu Z, Sun Z, Lin J, Hua Y (2007) Evaluation of the antioxidant effects of carotenoids from Deinococcus radiodurans through targeted mutagenesis, chemiluminescence, and DNA damage analyses. Biochem Biophys Acta 1770:902–911. doi:10.1016/j.bbagen.2007.01.016
Zhang L, Yang Q, Luo X, Fang C, Zhang Q, Tang Y (2007) Knockout of crtB or crtI gene blocks the carotenoid biosynthetic pathway in Deinococcus radiodurans R1 and influences its resistance to oxidative DNA-damaging agents due to change of free radicals scavenging ability. Arch Microbiol 188:411–419. doi:10.1007/s00203-007-0262-5
Zhao G, Ceci P, Ilari A, Giangiacomo L, Laue TM, Chiancone E, Chasteen ND (2002) Iron and hydrogen peroxide detoxification properties of DNA-binding protein from starved cells A ferritin-like DNA-binding protein of Escherichia coli. J Biol Chem 277:27689–27696. doi:10.1074/jbc.M202094200
Farci D, Slavov C, Tramontano E, Piano D (2016) The S-layer protein DR_2577 Binds deinoxanthin and under desiccation conditions protects against UV-radiation in Deinococcus radiodurans. Front Microbiol 7:155. doi:10.3389/fmicb.2016.00155
Bauermeister A, Moeller R, Reitz G, Sommer S, Rettberg P (2011) Effect of relative humidity on Deinococcus radiodurans’ resistance to prolonged desiccation, heat, ionizing, germicidal, and environmentally relevant UV radiation. Microb Ecol 61:715–722. doi:10.1007/s00248-010-9785-4
Dulermo R, Onodera T, Coste G, Passot F, Dutertre M, Porteron M, Confalonieri F, Sommer S, Pasternak C (2015) Identification of new genes contributing to the extreme radioresistance of Deinococcus radiodurans using a Tn5-based transposon mutant library. PLoS ONE 10:e0124358. doi:10.1371/journal.pone.0124358
Chen H, Xu G, Zhao Y, Tian B, Lu H, Yu X, Xu Z, Ying N, Hu S, Hua Y (2008) A novel OxyR sensor and regulator of hydrogen peroxide stress with one cysteine residue in Deinococcus radiodurans. PLoS ONE 3:e1602. doi:10.1371/journal.pone.0001602
Mattimore V, Battista JR (1996) Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J Bacteriol 178:633–637
Tian B, Hua Y (2010) Carotenoid biosynthesis in extremophilic Deinococcus-Thermus bacteria. Trends Microbiol 18:512–520. doi:10.1016/j.tim.2010.07.007
Reon BJ, Nguyen KH, Bhattacharyya G, Grove A (2012) Functional comparison of Deinococcus radiodurans Dps proteins suggests distinct in vivo roles. Biochem J 447:381–391. doi:10.1042/bj20120902
Lu H, Gao G, Xu G, Fan L, Yin L, Shen B, Hua Y (2009) Deinococcus radiodurans PprI switches on DNA damage response and cellular survival networks after radiation damage. Mol Cell Proteomics MCP 8:481–494. doi:10.1074/mcp.M800123-MCP200
Ohba H, Satoh K, Sghaier H, Yanagisawa T, Narumi I (2009) Identification of PprM: a modulator of the PprI-dependent DNA damage response in Deinococcus radiodurans. Extrem Life Under Extreme Cond 13:471–479. doi:10.1007/s00792-009-0232-8
Ghosal D, Omelchenko MV, Gaidamakova EK, Matrosova VY, Vasilenko A, Venkateswaran A, Zhai M, Kostandarithes HM, Brim H, Makarova KS, Wackett LP, Fredrickson JK, Daly MJ (2005) How radiation kills cells: survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress. FEMS Microbiol Rev 29:361–375. doi:10.1016/j.femsre.2004.12.007
Tian B, Sun Z, Shen S, Wang H, Jiao J, Wang L, Hu Y, Hua Y (2009) Effects of carotenoids from Deinococcus radiodurans on protein oxidation. Lett Appl Microbiol 49:689–694. doi:10.1111/j.1472-765X.2009.02727.x
Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, Hess M, Omelchenko MV, Kostandarithes HM, Makarova KS, Wackett LP, Fredrickson JK, Ghosal D (2004) Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science (New York, NY) 306:1025–1028. doi:10.1126/science.1103185
Wang Y, Xu Q, Lu H, Lin L, Wang L, Xu H, Cui X, Zhang H, Li T, Hua Y (2015) Protease activity of PprI facilitates DNA damage response: mn2+-dependence and substrate sequence-specificity of the proteolytic reaction. PLoS ONE 10:e0122071. doi:10.1371/journal.pone.0122071
Milani A, Basirnejad M, Shahbazi S, Bolhassani A (2016) Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol. doi:10.1111/bph.13625
Choi YJ, Hur JM, Lim S, Jo M, Kim DH, Choi JI (2014) Induction of apoptosis by deinoxanthin in human cancer cells. Anticancer Res 34:1829–1835
Acknowledgements
This work is supported by the Natural Science Foundation of China (Grant No. 81272993), and Program of Science and Technology Bureau of Hengyang city (Grant No. 2015KJ17). Graduate Student Research Innovation Project of University of South China (Grant No. 2016XCX39). The experiments were performed at the South University of China. We thank the efforts that all the colleges contributed to this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeng, Y., Ma, Y., Xiao, F. et al. Knockout of pprM Decreases Resistance to Desiccation and Oxidation in Deinococcus radiodurans . Indian J Microbiol 57, 316–321 (2017). https://doi.org/10.1007/s12088-017-0653-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-017-0653-5