Abstract
Paratuberculosis is one of the chronic granulomatous enteritis that predominantly affects ruminants worldwide, caused by Mycobacterium avium ssp. paratuberculosis (MAP). In ruminants, microsatellite polymorphisms of the \(3^\prime \) untranslated region (\(3^\prime \)UTR) of the solute carrier family 11 member A1 (SLC11A1) gene were associated with resistance to intracellular pathogen infections. This research was carried out to detect the polymorphisms in A and B regions of the \(3^{\prime }\)UTR of SLC11A1 gene and to evaluate the potential association between these polymorphisms and MAP infection in goats. MAP-specific antibodies were detected by ELISA and MAP infection was confirmed by IS900 PCR in 150 adult goats from different regions of Kerala, India. The polymorphism of microsatellite regions A and B at \(3^{\prime }\)UTR of the SLC11A1 gene was analysed in goats by an automated technique, fragment analysis, using fluorescent-tagged forward primers. Eight alleles with sizes ranging from 221 to 239 bp were found in region A. Region B revealed two alleles, 117 bp (\(\hbox {B}_{7}\)) and 119 bp (\(\hbox {B}_{8}\)). Animals with \(\hbox {B}_{8}\) alleles were found to have higher incidence of paratuberculosis than animals with \(\hbox {B}_{7}\) alleles (\(P< 0.01\)). There was no statistically significant association found between region A genotypes and paratuberculosis incidence. These results suggest that caprine SLC11A1 gene has significant role in paratuberculosis resistance in goats and further studies might help in development of a PCR-based genotyping test for paratuberculosis resistance and selection of superior animals for future goat breeding programmes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paratuberculosis or Johne’s disease is considered as one of the most serious, contagious, bacterial diseases of ruminants, especially in cattle, sheep and goats, caused by Mycobacterium avium ssp. paratuberculosis (MAP). It is characterized by diarrhoea, rapid weight loss, reduced milk production, reproductive failure and death in farm animals (Chiodini et al. 1984). Infections with MAP in caprine herds result in significant economic loss, through slow progressive wasting and the subsequent death of the infected animals. A study conducted by Singh et al. (2008a, b) showed that prevalence of MAP in domestic livestock in India was moderately higher and there is urgent need to control the disease at national level to improve per animal productivity in India. The disease is zoonotically important, since IS900 characterization of positive cultures in stool and biopsies from confirmed cases of Crohn’s disease in northern India, proved the association between MAP and Crohn’s disease (Singh et al. 2008a, b). Diagnostic tests include isolation of MAP, Ziehl–Nielsen’s (ZN) acid fast staining, enzyme-linked immunosorbent assay (ELISA) and IS900 PCR. Since these tests are time consuming and costly, the early detection of subclinical paratuberculosis is difficult, combined with the incapability of currently available vaccines to prevent the disease or disease shedding, necessitates the adoption of newer techniques for the prevention of MAP infection. This could be overcome by selection of disease-resistant animals by appropriate selection methods such as marker-assisted selection (MAS). Most of the recent researches regarding disease resistance suggest that limiting the spread of disease may be possible through selective breeding of animals based on genetic markers associated with resistance or susceptibility. One of the genes that have been targeted for this purpose is the solute carrier family 11 member A1 (SLC11A1) (Bellamy et al. 1998) in goats, which is located in chromosome 2 (Vacca et al. 2011).
The SLC11A1 is a potential candidate gene that confers innate resistance against various intracellular pathogens including MAP. The SLC11A1 gene, previously known as natural resistance-associated macrophage protein 1 (NRAMP1) gene is a member of large family of metal ion-transport proteins. SLC11A1 gene encodes SLC11A1 protein which is a member of large family of metal ion-transport proteins linked to infectious disease susceptibility in mouse (Vidal et al. 1993), functions as a pH-dependent transporter that prevents the acquisition of divalent cations like \(\hbox {Fe}^{2+}\) and \(\hbox {Mn}^{2+}\) towards the cytosol through the phagosome membranes and thus, it favours bacterial killing (Forbes and Gros 2003). SLC11A1 gene delivers bivalent metal cations from the cytosol into acidic endosomal and lysosomal compartments under normal physiological conditions, where the Fenton and Haber–Weiss reactions generate toxic antimicrobial radicals for direct antimicrobial activity against phagocytosed microorganisms (Goswami et al. 2001). This gene has pleiotropic effects on macrophage function that include increased keratinocyte chemoattractant (chemokine KC), tumour necrosis factor-\(\upalpha \) (TNF-\(\upalpha \)), interleukin-1\(\upbeta \) (IL-1\(\upbeta \)), inducible nitric oxide synthase and major histocompatibility (MHC) class II expression; all of them are important in resistance to intracellular pathogens (Awomoyi 2007).
In ruminants, microsatellite polymorphisms of \(3^\prime \) untranslated region (\(3^\prime \)UTR) of the SLC11A1 gene were associated with resistance to Brucella abortus, Mycobacterium bovis and Mycobacterium avium ssp. paratuberculosis (Barthel et al. 2001; Reddacliff et al. 2005; Borriello et al. 2006; Capparelli et al. 2007a; Martínez et al. 2008; Pinedo et al. 2009; Korou et al. 2010; Kadarmideen et al. 2011; Taka et al. 2013, 2015). There are two polymorphic microsatellites in the \(3^\prime \)UTR of the caprine SLC11A1 gene with a variation in the number of guanine thymine repeats \(\hbox {(GT)}_{n}\) (Vacca et al. 2011). The region A was found to be more polymorphic than the region B, where only two alleles were reported for region B in goats (Korou et al. 2010; Vacca et al. 2011). Thomas and Joseph (2012) reviewed the role of SLC11A1 gene in diseases resistance especially for intracellular pathogens and opined that disease is the most important constraint in the animal production system and the selection of animals for increased genetic resistance to diseases will lead to the production of a healthy and productive stock. The present study was designed to detect the polymorphisms in A and B regions of the \(3^\prime \)UTR of SLC11A1 gene and to evaluate the potential association between these polymorphisms and the presence or absence of MAP infection in goats of Kerala, south India.
Materials and methods
Sample collection
All experimental procedures were performed according to the guidelines of the Institutional Animal Ethics Committee of Kerala Veterinary and Animal Sciences University. Blood, serum and faecal samples were collected from 150 adult goats comprising 50 Malabari, 47 Attappady Black and 53 Malabari crossbreds from Thrissur and Malappuram districts of Kerala, India. Animals included in the present study were maintained at similar environmental conditions and not vaccinated for paratuberculosis. Blood samples were used for the isolation of genomic DNA for microsatellite genotyping of the goats under study, whereas serum and faecal samples were used for the detection of MAP antibodies by ELISA and MAP antigens by IS900 PCR.
Genomic DNA isolation and genotyping
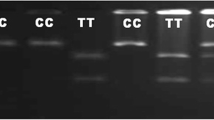
Genomic DNA was extracted from whole blood using the standard phenol–chloroform extraction procedure. DNA concentration of samples was quantified by NanoDrop (NanoDrop, ThermoScientific, USA) and stored at \(-20{^{\circ }}\hbox {C}\) until used. PCR was carried out to amplify both A and B regions at the \(3^{\prime }\)UTR of the caprine SLC11A1 gene. Primer pairs for A region (Ex15F1: \(5^\prime \)-GTCTGGACCTGTCTCATCACC-\(3^\prime \) and Ex15R1: \(5^\prime \)-ACTCCCTCTCCATCTTGCTG-\(3^\prime \)), and B region (Ex15F2: \(5^\prime \)- GGAGTTCACGGGTGGGA-\(3^\prime \) and Ex15R2: \(5^\prime \)-GGGTCTCTATGTCGTGGGGG-\(3^\prime \)), were designed on the basis of the goat genomic sequence (GenBank accession no. GU440577) using Primer3 software. To amplify PCR products of \({\sim }233\) and 117 bp. Primers Ex15F1 and Ex15F2 were \(5^\prime \) labelled with the fluorescent dye 6-FAM (6-carboxyfluorescein). PCR was carried out with 50 ng of genomic DNA in a total reaction volume of \(25\,\mu \hbox {L}\) containing \(10\times \) PCR buffer, 1.5 mM \(\hbox {MgCl}_{2}\), \(200\,\mu \hbox {M}\) dNTPs, 10 pM of forward and reverse primers and 0.5 U of Taq DNA polymerase (Sigma Aldrich). Amplification reactions were performed with an initial denaturation step of 5 min at \(94{^{\circ }}\hbox {C}\), followed by 35 cycles of 30 s at \(94{^{\circ }}\hbox {C}\), 30 s \(\hbox {X}{^{\circ }}\hbox {C}\) (X was 59 for region A and 62.2 for region B) and 25 s at \(72{^{\circ }}\hbox {C}\) with a final extension step of 5 min at \(72{^{\circ }}\hbox {C}\). PCR products were screened by 2% agarose gel electrophoresis stained with ethidium bromide and visualized in a gel documentation system (Biorad, USA).
The genotyping of microsatellite markers in the regions A and B was performed by an automated fragment analysis technique (Scigenom, Ernakulam, India). The fluorescent \(5^\prime \) end-labelled PCR products (with fluorescent dye, 6-FAM) were run on 3730 XL ABI PRISM automated genetic analyzer (Applied Biosystems, Darmstadt, Germany) and analysed. Microsatellite fragment sizing was performed by the Gene Mapper software ver. 4.0. Allele calling was performed with the software and were checked manually to avoid any false calling of alleles. Sequencing of representative samples from each pattern, obtained by genotype analysis, confirmed that the only nucleotide differences among the PCR products were in the number of GT repeats.
Detection of MAP-specific antibodies by ELISA
Blood samples were collected by jugular puncture; following centrifugation (2500 rpm for 10 min), the sera were separated and stored at \(-20{^{\circ }}\hbox {C}\) until the use. Serum samples (\(n = 150\)) were screened for detection of MAP specific antibodies by ELISA kit (ID vet innovative diagnostics, Grabels, France). Optical density (OD) values were measured at 450 nm. Positive and negative sera were included as controls. As per manufacturer’s instruction, serum samples with corrected sample / positive control ratio above 60% were considered as positive for paratuberculosis.
Detection of MAP by IS900 PCR
Faecal samples were collected by rectal pinch method. DNA was isolated from faecal sample as per Braunstein et al. (2002). IS900 PCR was performed as per the protocols of Halldorsdottir et al. (2002). The primer pair used was \(5^\prime \)-GGCCGTCGCTTAGGCTTCGA-\(3^\prime \) and \(5^\prime \)-CGTCGTTAATAACCATGCAG-\(3^\prime \) to amplify a 279-bp PCR product. The PCR mixture (\(50\,\mu \hbox {L}\) total volume) comprised \(5\,\mu \hbox {L}\) of DNA, \(10\times \) PCR Buffer, 10 pM primers, 1.5 mM for \(\hbox {MgCl}_{2}\), 0.2 mM dNTPs and 0.5 U of Taq DNA polymerase. The cycling protocol was an initial denaturation at \(94{^{\circ }}\hbox {C}\) for 3 min followed by 35 cycles of 1 min denaturation at \(94{^{\circ }}\hbox {C}\), 25 s primer annealing at \(55{^{\circ }}\hbox {C}\), and extension at \(72{^{\circ }}\hbox {C}\) for 1 min. The IS900 PCR products were separated by electrophoresis in 2% agarose gel, stained with ethidium bromide and visualized by Gel documentation system (Biorad, USA).
Statistical analysis
The allelic and genotype frequencies of A and B microsatellite loci at \(3^\prime \)UTR of SLC11A1 gene in each genetic group were calculated by direct counting method. Association of the different genotypes in A and B microsatellite regions at the \(3^\prime \)UTR of SLC11A1 gene with the presence of MAP-specific antibodies in the serum and MAP DNA in the faeces of goats were assessed by chi-square test and the Fisher’s exact test by SPSS ver. 21.
Results and discussion
The number of GT repeats found in region A ranged from 10 to 19, eight alleles being identified (\(\hbox {A}_{10}\)-GT10, \(\hbox {A}_{12}\)-GT12, \(\hbox {A}_{14}\)-GT14, \(\hbox {A}_{15}\)-GT15, \(\hbox {A}_{16}\)-GT16, \(\hbox {A}_{17}\)-GT17, \(\hbox {A}_{18}\)-GT18 and \(\hbox {A}_{19}\)-GT19) with size range of 221–239 bp and 12 genotypes were observed. The 233 bp allele was the most abundant in goat population (0.660). Only two alleles (\(\hbox {B}_{7}\)-GT7 and \(\hbox {B}_{8}\)-GT8) with three genotypes were present in region B. The direct count heterozygosity, unbiased heterozygosity and PIC value for microsatellite A region of SLC11A1 gene were 0.6833, 0.6995 and 0.5474 and for microsatellite B region were 0.4985, 0.5036 and 0.2485 in goats, respectively (table 1). Liandris et al. (2009) detected two microsatellite regions with different GT (dinucleotide) repeat numbers and different sequence motifs in native Greek goats named region A and region B at the \(3^\prime \)UTR of SLC11A1 gene and detected four alleles (GT14, GT15, GT16 or GT18) in region A and two alleles in region B (GT7 and GT8). In addition to this, four other alleles (GT11, GT12, GT17 and GT19) at region A were recognized in Sarda goats by Piras et al. (2011). Korou et al. (2010) detected six alleles (GT13, GT14, GT15, GT16, GT17 and GT18) in microsatellite region A at the \(3^\prime \)UTR of SLC11A1 gene in Greece goats, and two alleles at region B (\(\hbox {B}_{7}\) and \(\hbox {B}_{8})\). The allele frequency in region B of the \(3^\prime \)UTR of the SLC11A1 gene was slightly different to that observed by Korou et al. (2010) (53% allele \(\hbox {B}_{7}\) and 47% allele \(\hbox {B}_{8}\) in this study versus 45 and 55%; table 1); but, the percentage of goats with \(\hbox {B}_{7}/\hbox {B}_{7}\) genotype found among animals evaluated by us was similar to those reported in other goat breeds (17% in this study versus 26% and 16%) (Korou et al. 2010; Iacoboni et al. 2014). The percentage of goats with \(\hbox {B}_{7}/\hbox {B}_{8}\) and \(\hbox {B}_{8}/\hbox {B}_{8}\) genotypes, which was found to be in risk of paratuberculosis incidence was higher than those reported in other goat breeds (83% in this study versus 75% reported by Korou et al. 2010).
The incidence of paratuberculosis in Malabari, Attapady black and crossbred goat were tested by ELISA and IS900 PCR. The prevalence of paratuberculosis was between 12 and 34%. Lowest incidence was noticed in the native breeds (Attappady Black and Malabari) and highest in crossbreds. Genotype-wise results of MAP infection are presented in tables 2 and 3. The association analysis between diagnostic tests results and polymorphisms in regions A and B of the \(3^\prime \)UTR of the caprine SLC11A1 gene indicated that genotypes of A region had no significant effects on paratuberculosis resistance/incidence in goats, whereas region B showed a significant association (\(P < 0.01\)) with paratuberculosis incidence (table 4). Of the three different genotypes in the B region (117/117 (\(\hbox {B}_{7}\hbox {B}_{7}\)), 117/119 (\(\hbox {B}_{7}\hbox {B}_{8}\)) and 119/119 (\(\hbox {B}_{8}\hbox {B}_{8})\)), those genotypes with 119 bp (\(\hbox {B}_{8}\)) alleles (both in homozygous and heterozygous conditions) showed significant association with paratuberculosis incidence by both diagnostic methods (\(P < 0.01\)). The details of the association between different genotypic variants of region B and paratuberculosis incidence in goats are given in table 5. Korou et al. (2010) reported that the presence of \(\hbox {B}_{7}\) allele was significantly associated with absence of MAP specific antibodies in goats, but they did not find association between absence/presence of MAP antibodies with polymorphisms in region A, as in the present study. Similar associations of SLC11A1 gene polymorphisms with susceptibility of humans and bovines to Mycobacterium spp. and Brucella spp. were reported (Bellamy 1998; Barthel et al. 2001; Capparelli et al. 2007). The homozygous \(\hbox {B}_{7 }/\hbox {B}_{7 }\)genotype was reported to be associated with increased expression of the SLC11A1 and IL-1 \(\alpha \) genes indicating increased in vitro responsiveness and therefore, resistance of mononuclear-derived macrophages to MAP infection (Taka et al. 2013).
The results of the present study will supplement the information available for the role of SLC11A1 gene in disease resistance/susceptibility and will be useful in further studies to determine the markers for selection of paratuberculosis-resistant animals. Further investigations are necessary to unravel regulation of SLC11A1 gene expression based on the genetic variants after intracellular pathogen infection in native goats. Thus, the physiological and biochemical functions, together with the results obtained in the current research, indicate that the SLC11A1 gene might play a crucial role in disease resistance in goats. The results obtained in the research open a promising opportunity to use these markers as one of the tool in a selective breeding programme to control paratuberculosis in goats.
References
Awomoyi A. A. 2007 The human solute carrier family 11 member 1 protein (SLC11A1): linking infections, autoimmunity and cancer. FEMS Immunol. Med. Microbiol. 49, 324–329.
Barthel R., Feng J., Piedrathia J. A., Mcmurray D. N., Templeton J. W. and Adams L. G. 2001 Stable transfection of the bovine NRAMP1 gene into murine RAW 264.7 cells: effect on Brucella abortus survival. Infect. Immun. 69, 3110–3119.
Bellamy R., Ruwende C., Corrah T., McAdam K. P. W. J., Whittle H. C. and Hill A. V. S. 1998 Variation in the NRAMP1 gene is associated with susceptibility to tuberculosis in West Africans. N. Engl. J. Med. 338, 640–644.
Borriello G., Capparelli R., Bianco M., Fenizia D., Alfano F., Capuano F. et al. 2006 Genetic resistance to Brucella abortus in the water buffalo (Bubalus bubalis). Infect. Immun. 74, 2115–2120.
Braunstein M., Bardarov S. S. and Jacobs Jr W. R. 2002 Genetic methods for deciphering virulence determinants of Mycobacterium tuberculosis. Methods Enzymol. 358, 67–99.
Capparelli R., Alfano F., Amoroso M. G., Borriello G., Fenizia D., Bianco A. et al. 2007 Protective effect of the Nramp1 BB genotype against Brucelia abortus in water buffalo (Bubalus bubalis). Infect. Immun. 75, 988–996.
Chiodini R. J., Kruininjen H. J. and Merkal R. S. 1984 Ruminant paratuberculosis (Johne’s disease): the current status and future prospects. Cornell Vet. 74, 218–262.
Forbes J. R. and Gros P. 2003 Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood 102, 1884–1892.
Goswami T., Bhattacharjee A., Babal P., Searle S., Moore E., Li M. and Blackwell J. M. 2001 Natural-resistance-associated macrophage protein 1 is an H+/bivalent cation antiporter. Biochem. J. 354, 511–519.
Iacoboni P. A., Hasenauer F. C., Caffaro M. E., Gaido A., Rossetto C., Neumann R. D. et al. 2014 Polymorphisms at the 3\(^{\prime } \) untranslated region of SLC11A1 gene are associated with protection to Brucella infection in goats. Vet. Immunol. Immunopathol. 160, 230–234.
Halldorsdottir S., Englund S., Nilsen S. F. and Olsaker I. 2002 Detection of Mycobacterium avium subsp. paratuberculosis by buoyant density centrifugation, sequence capture PCR and dot blot hybridization. Vet. Microbiol. 87, 327–340.
Kadarmideen H. N., Ali A. A., Thomson P. C., Muller B. and Zinsstag J. 2011 Polymorphisms of the SLC11A1 gene and resistance to bovine tuberculosis in African Zebu cattle. Anim. Genet. 42, 656–658.
Korou L. M., Liandris E., Gazouli M. and Ikonomopoulos J. 2010 Investigation of the association of the SLC11A1 gene with resistance/sensitivity of goats (Capra hircus) to paratuberculosis. Vet. Microbiol. 144, 353–358.
Liandris E., Gazouli M. and Ikonomopoulos J. 2009 Characterization of the caprine (Capra hircus) SLC11A1 gene: innate resistance to paratuberculosis. Online J. Vet. Res. 13, 41–52.
Martínez R., Dunner S., Barrera G. and Cañon J. 2008 Novel variants within the coding regions of the Slc11a1 gene identified in Bos taurus and Bos indcus breeds. J. Anim. Breed. Genet. 125, 57–62.
Pinedo P. J., Buergelt C. D., Donovan G. A., Melendez P., Morel L., Wu R. et al. 2009 Candidate gene polymorphisms (BoIFNG, TLR4, SLC11A1) as risk factors for paratuberculosis infection in cattle. Prev. Vet. Med. 91, 189–196.
Piras G., Pazzola M., Balia F., Pira E., Dettori M. L., Carcangiu V. and Vacca G. M. 2011 Polymorphism of caprine SLC11A1 gene and relationships with hygienic characteristics of milk. Agric. Conspec. Sci. 76, 175–178.
Reddacliff L. A., Beh K., McGregor H. and Whittington R. J. 2005 A preliminary study of possible genetic influences on the susceptibility of sheep to Johne’s disease. Aust. Vet. J. 83, 435–441.
Singh S. V., Singh A. V., Singh P. K. and Sohal J. S. 2008a Prevalence of juvenile capri tuberculosis and genotyping of Mycobacterium avium subsp. paratuberculosis recovered from postnatal kids. Indian J. Anim. Sci. 79, 235–238.
Singh A. V., Singh S. V., Makharia G. K., Singh P. K. and Sohal J. S. 2008b Presence and characterization of Mycobacterium avium subspecies paratuberculosis from clinical and suspected cases of Crohn’s disease and in the healthy human population in India. Int. J. Infect. Dis. 12, 190–197.
Taka S., Liandris E., Gazouli M., Sotirakoglou K., Theodoropoulos G., Bountouri M. et al. 2013 In vitro expression of the SLC11A1 gene in goat monocyte-derived macrophages challenged with Mycobacterium avium subsp paratuberculosis. Infect. Genet. Evol. 17, 8–15.
Taka S., Gazouli M., Sotirakoglou K., Liandis E., Andreadou M., Triantaphyllopoulos K. and Ikonomopoulos J. 2015 Functional analysis of 3’UTR polymorphisms in the caprine SLC11A1 gene and its association with the Mycobacterium avium subsp. paratuberculosis infection. Vet. Immunol. Immunopathol. 167, 75–79.
Thomas N. and Joseph S. 2012 Role of SLC11A1 gene in disease resistance. Biotechnol. Anim. Husbandry 28, 99–106.
Vacca G. M., Pazzola M., Pisano C., Carcangiu V., Diaz M. L., Nieddu M. et al. 2011 Chromosomal localization and genetic variation of the SLC11A1 gene in goats (Capra hircus). Vet. J. 190, 60–65.
Vidal S. M., Malo D., Vogan K., Skamene E. and Gros P. 1993 Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 73, 469–485.
Acknowledgements
The authors acknowledge Kerala Veterinary and Animal Sciences University, Kerala, India, for providing the financial support and laboratory facilities for the successful completion of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Silvia Garagna
Rights and permissions
About this article
Cite this article
Abraham, A., Naicy, T., Raghavan, K.C. et al. Evaluation of the association of SLC11A1 gene polymorphism with incidence of paratuberculosis in goats. J Genet 96, 641–646 (2017). https://doi.org/10.1007/s12041-017-0820-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-017-0820-9