Abstract
Early-life challenges, particularly infections and stress, are related to neuropsychiatric disorders such as autism and schizophrenia. Here, we conducted a wide range of behavioral tests in periadolescent (postnatal day (PN) 35) and adult (PN70) Swiss mice neonatally challenged with LPS on PN5 and -7, to unveil behavioral alterations triggered by LPS exposure. Immune and neurotrophic (brain-derived neurotrophic factor—BDNF) alterations were determined in the prefrontal cortex (PFC), hippocampus (HC), and hypothalamus (HT). Since the incidence and clinical manifestations of neurodevelopmental disorders present significant sex-related differences, we sought to distinctly evaluate male and female mice. While on PN35, LPS-challenged male mice presented depressive, anxiety-like, repetitive behavior, and working memory deficits; on PN70, only depressive- and anxiety-like behaviors were observed. Conversely, females presented prepulse inhibition (PPI) deficits in both ages studied. Behavioral changes in periadolescence and adulthood were accompanied, in both sexes, by increased levels of interleukin (IL-4) (PFC, HC, and HT) and decreased levels of IL-6 (PFC, HC, and HT). BDNF levels increased in both sexes on PN70. LPS-challenged male mice presented, in both ages evaluated, increased HC myeloperoxidase activity (MPO); while when adult increased levels of interferon gamma (IFNγ), nitrite and decreased parvalbumin were observed. Alterations in innate immunity and parvalbumin were the main LPS-induced remarks between males and females in our study. We concluded that neonatal LPS challenge triggers sex-specific behavioral and neurochemical alterations that resemble autism spectrum disorder, constituting in a relevant model for the mechanistic investigation of sex bias associated with the development of this disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is largely acknowledged that early-life adversities may trigger the onset of neuropsychiatric disorders [1]. The early and long-term consequences of these adversities have been studied in animal models based, for example, on the neonatal exposure to stress [2] or through the intracerebral [3] or systemic administration of endotoxin lipopolysaccharide (LPS) [4]. Since LPS is the major virulent constituent of the outer membrane of Gram-negative bacteria, neonatal LPS exposition seemingly mimics physiological and behavioral alterations triggered by a Gram-negative bacterial infection [5].
Crucially, infections caused by Gram-negative bacteria are of high prevalence during prenatal and neonatal periods. Furthermore, LPS neonatal challenge to rodents may mimic early-life imbalances of the gut microbiota also referred as gut microbiota dysbiosis. Indeed, it has been observed that drug-naive HIV-infected children presented with significant gut microbiota translocation, a major event for immune activation [6], with plasma LPS constituting in a relevant marker of immune activation [7].
The already described long-term behavioral consequences induced by pre- and postnatal LPS exposure to rodents include (i) autistic-like behaviors (induced by prenatal challenge on embryonic day (E) 9.5 [8] or neonatal challenge on postnatal day (PN) 3 [4]), (ii) schizophrenia-like alterations (induced by LPS challenge on E15 and 16) [9]), and (iii) anxiety-like behaviors (induced by LPS challenge on PN3 and -5 [10]). Regarding LPS-induced anxiety-like alterations, it seems to be part of a complex syndrome such as autism spectrum disorders (ASD) or schizophrenia. Indeed, anxiety can occur in up to 65% of patients with either schizophrenia [11] or ASD [12]. Therefore, we consider that the aforementioned preclinical findings related to long-term behavioral consequences of early-life exposure to LPS may in fact reflect overlapping symptoms between ASD and schizophrenia [13] with anxiety as comorbid. In contrast, LPS exposure during adulthood triggers depressive-like behavior characterized by increased immobility time in the forced swimming test and anhedonic behavior in animals [14, 15] and humans [16].
The present study was designed to evaluate a broad spectrum of behavioral tests as an attempt to better address the long-term behavioral alterations induced by neonatal LPS. Since PN5–7 in rodents correspond to a 36–40-week gestation (term infant), we chose this period for LPS exposure in order to provide neurodevelopment insights for an equivalent challenge at the end of the third trimester of human pregnancy [17,18,19,20]. It is important to highlight that most previous studies investigating LPS neonatal exposure were conducted in animals challenged on PN3 and -5, which actually relate to 23–32-week gestation (preterm infant) [4, 10].
To date, few preclinical studies have associated early postnatal exposure to LPS with ASD-like behavioral alterations. In this context, a recent study demonstrated that LPS challenge on PN3 resulted in impaired communicative (evaluated on PN10) and cognitive functions (evaluated on PN40) [4]. These authors associated the neurobehavioral abnormalities triggered by LPS neonatal challenge with ASD [4]. Nonetheless, the fact that early-life immune challenges can induce symptoms overlapping both ASD and schizophrenia, the onset time of symptoms is a distinguishing core feature between these disorders, with ASD occurring earlier during childhood and adolescence [4], while schizophrenia takes place at the end of adolescence or during young adulthood [21]. In line with these observations, the results of animal models based on immune challenge revealed that neonatal LPS (PN3) causes communicative alterations on PN10 (equivalent to human infant), which seems to be related to ASD [4]. On the other hand, neonatal challenge with the virus-mimetic poly (I:C) on PN5 and -7 relates to the development of schizophrenia-like alterations only on PN70 (equivalent to human adulthood) [22]. For this reason, the present study sought to assess alterations induced by neonatal LPS on PN35, which correlates to the age of 12 years old in human (early adolescence) [19], and on PN70, which represents adulthood. PN35 would clearly represent a period of intense physical, cognitive, emotional, and social development.
Autism is a neurodevelopmental disorder characterized by deficits in social interaction and cognition, difficulties associated with communication, repetitive/restricted behavior, and being also negatively associated with both the experience of social pleasure as well as general pleasure [23]. This disorder is 4–5.1 times more prevalent in males. Males and females also display distinct symptoms [24]. This makes ASD diagnosis more challenging to assess and contributes to its underreporting among females [25]. To our knowledge, only few preclinical studies evaluated sex-specific neurobiological alterations and their influence towards the development of ASD.
The neurobiological alterations underpinning ASD seems to be dependent upon the severity of the disorder. In this respect, serum levels of the neurotrophin brain-derived neurotrophic factor (BDNF) were found to be significantly higher in atypical autistic subjects (clinically milder phenotype where behavior pattern does fit all the inclusion criteria for typical autism) than in controls, but not in relation to typical ASD cases (clinically severe phenotype) [26]. Immune deregulation characterized by atypical levels of cytokines, such as interleukin (IL)-6, IL-4, and IFN-γ, and high production of nitric oxide (NO) has been observed in ASD [27]. High plasma levels of NO are probably a result of increased interferon gamma (IFN-γ) activity [28]. Furthermore, parvalbumin-immunoreactive neurons (putatively, GABAergic) were associated with cognitive deficits and symptoms of both schizophrenia and autism. Hence, based on the evidence above, we sought to include the investigation of neurotrophic (BDNF) and immune-inflammatory (MPO, cytokines, and nitrite) parameters, as well as parvalbumin expression to ascertain neonatal LPS as a study model of ASD.
The present study aimed to test the hypothesis that male and female mice exposed to neonatal challenge with LPS from Escherichia coli would present behavioral and neurochemical alterations during adolescence (PN35) and adulthood (PN70) that would resemble ASD in humans. To do this, the following behavioral parameters were evaluated: prepulse inhibition (PPI) of the startle reflex (a measure of sensorimotor gating and attentional processes) [29], immobility time in the forced swimming test (a measure of depressive-like alteration [30]), sucrose preference (a measure of anhedonic behavior [31]), social preference, working memory in the Y maze task [32]), acute psychological stress response using the cat odor test [33]), and locomotor activity. As a secondary outcome, we determined immune alterations in brain areas related to ASD, namely, prefrontal cortex (PFC), hippocampus (HC), and hypothalamus (HT), by measuring myeloperoxidase (MPO) activity and levels of IL-4 and IL-6. Furthermore, we assessed the hippocampal levels of IFNγ, nitrite (as an indirect measure of nitric oxide—NO), parvalbumin, and BDNF of adult mice.
Methods
Animals and Treatment
Swiss mice were maintained at a controlled temperature (23 ± 1 °C) with 12-h dark/light cycle (lights on at 6:00 AM) and free access to water and food. Procedures were conducted in accordance with the Brazilian College of Animal Experimentation (COBEA) guidelines for the care and use of laboratory animals, and the Guide for the Care and Use of Laboratory Animals National Institutes of Health, USA [34]. The Ethical and Animal Research Committee of the Universidade Federal do Ceará (UFC) approved the study.
Pregnant females were monitored for the parturition day, which was counted as postnatal day 0 (PN0). All animals were obtained from the UFC Animal Facility. In the present study, 40 couples were mated to obtain 320 mice that were used for the experimental procedures (approximately 160 males and 160 females). The offspring were maintained with their respective dams until weaning. On PN5 and -7, the pups received a single daily intraperitoneal (i.p.) injection of 50 μg/kg LPS from E. coli (serotype 055:B5 purified by phenol extraction from CDC 1644-70 strain, Sigma-Aldrich, USA) or sterile saline (control group) in a volume of 20-μl/5 g body weight. Injections were given between 10:00 AM and 12:00 PM for 2 days (PN5 and -7). During injections, dams were removed from the home cage and returned once all pups had received the injection. LPS injections caused no obvious behavioral alterations or mortality in the pups. Maternal separation did not exceed more than 5 min.
To avoid “litter effect”, only one offspring from each dam was used for the composition of each experimental group [35]. On PN21, after weaning, male or female offspring from different dams of the same neonatal treatment conditions were allocated to either saline or LPS group. For the behavioral and neurochemical determinations, the animals were evaluated on PN35 (equivalent to human periadolescence) and PN70 (equivalent to human adulthood), except for IFNγ, nitrite, parvalbumin, and BDNF, whose levels were evaluated only on PN70.
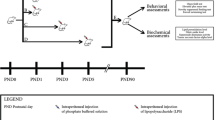
The correlation of the neurodevelopmental periods from animals to humans followed a previous report [18]. For the behavioral determinations, distinct groups of mice on PN35 and -70 were randomized in five subgroups. Subgroup 1 was submitted to PPI (n = 7–10 animals/group/sex/age), subgroup 2 to Y maze task (n = 6–10 animals/group/sex/age), subgroup 3 to social interaction and forced swimming (n = 7–10 animals/group/sex/age), subgroup 4 to open field test and cat odor test (n = 6–8 animals/group/sex/age), and subgroup 5 to sucrose consumption (n = 6–9 animals/group/sex/age). Figure 1 shows a representation of the experimental design.
Behavioral Determinations
Prepulse Inhibition of the Startle Reflex
PPI levels may indicate the current integrity of sensorimotor gating mechanisms, providing an operational measure of sensorimotor gating, i.e., the ability of a sensory event to suppress a motor response [36]. The determinations of startle amplitudes (a measurement of a constellation of reflexes elicited by sudden, relatively intense stimuli) and PPI levels (a measurement of sensorimotor performance) were conducted in a startle response system (Insight, São Paulo, Brazil), as previously described [37]. Firstly, a subject was placed in the stabilimeter cage for a 5-min exposure to the background noise (65 dB). After this acclimatization period, the animal was presented with a series of ten stimuli (pulse alone—120 dB, 50-ms duration), with an inter-trial interval of 15 s to allow within-session habituation to the startle stimulus. The PPI modulation of the acoustic startle was tested in 74 trials pseudo-randomly divided into seven different categories presented with an inter-trial interval of 15 s; 20 presentations of pulse alone (120 dB, 50-ms duration), eight presentations of prepulse intensity alone (PP 70, 75, or 80 dB, 3000-Hz frequency, 20-ms duration), and ten presentations of each prepulse intensity + pulse (PP—with 50-ms interval). Mean amplitude of startle response to pulse-alone (P) and prepulse-pulse (PP + P) trials was calculated for each subject. The PPI level of each mouse was determined by expressing the prepulse + pulse startle amplitude as a percentage decrease from pulse-alone startle amplitude, according to the following formula: % PPI = 100 − [100 × (PP / P)]. Mean % PPI was calculated using the results obtained in the three PP intensities.
Forced Swimming Test (FST)
For this test, also termed behavioral despair, mice were individually forced to swim in an open cylindrical container (diameter, 22 cm; height, 40 cm) with 20 cm of water held at 25 ± 1 °C. The total time during which the mouse remained immobile during a 5-min period was recorded. Immobility was defined as the animal floating in the water without struggling and making only very minimal movements necessary to keep its head above the water. An increase in the duration of immobility is an indicative of depressive-like behavior [30] or depression associated with negative schizophrenia-like symptom [38].
Sucrose Preference Test (SPT)
This test evaluates the decreased ability to experience pleasure being used as an indicator of anhedonia. Anhedonia represents one of the core symptoms of depression. The test was performed as described previously [39]. Briefly, 72 h before the test, mice were trained to adapt 1% sucrose solution (w/v): two bottles of 1% sucrose solution were placed in each cage, and 24 h later, 1% sucrose in one bottle was replaced with tap water for 24 h. After the adaptation, mice were deprived of water and food for 24 h. Sucrose preference test was conducted at 9:00 A.M. in which mice were housed in individual cages and were free to access two bottles containing 100 ml of sucrose solution (1% w/v) and 100 ml of water. After 1 h, the volumes of consumed sucrose solution and water were recorded and the sucrose preference was calculated by the following formula:
Social Interaction Test (SIT)
This test allows the evaluation of social affiliation/motivation. It was conducted in a Plexiglas box divided into three chambers. An iron cage in one of the two side chambers contained the probe mice. Mice were allowed 5 min of exploration time in the box; after which, an unfamiliar, same-sex probe mouse from the same experimental group was placed in one of two restraining cages [40]. The time spent in each of the three chambers was measured, and social preference was defined as follows: (% time spent in the social chamber) − (% time spent in the opposite chamber).
Spontaneous Alternation in the Y Maze
This protocol enables to test short-term spatial working memory by using a Y maze made of black acrylic [32]. This behavioral test measures the willingness of rodents to explore new environments. For this reason, to keep the task novel, it was conducted in different animals on PN35 and -70. The testing procedure was described elsewhere [41]. For the test session, each mouse was placed in one of the arm compartments being allowed to move freely until its tail completely enters another arm. The sequence of arm entries was manually recorded, the arms being labeled A, B, or C. An alternation was defined as entry into all three arms consecutively. For instance, if one animal makes the following arm entries: ACB,CAB,C,A,CAB,C,A, the animal made 13 arm entries nine, of which were considered correct alternations. The number of maximum spontaneous alternations was considered as the total number of arms entered minus two, and the percentage alternation was calculated as {(actual alternations/maximum alternations) × 100}. Each mouse was allowed to move freely through the maze for an 8-min test session. In the present study, mice that entered arms less than eight times during the test were excluded, because the data obtained from these mice were not considered to reflect precise alternation.
Cat Odor Test (COT)
This test was used to assess fear-related behavioral response [42]. Cat odor was obtained by rubbing a damp cloth (18 × 22 cm) vigorously against the fur of a domestic female adult cat for 5 min. This procedure was carried out 1 h prior to the experimental session. The cat odor cloth was kept in a sealed plastic bag. Each cloth was used for four exposures only. Damp pieces from the original cloth, not rubbed on the cat, were used for the neutral odor. All odor exposures took place in a separate, small, dimly lit room, and neutral odor exposures always preceded the cat odor exposures in order to prevent any traces of cat odor influencing the neutral odor group. Prior to the first cat odor exposure, the cloth with cat odor was left in the test room for 10 min. Each mouse was carried to the exposure room in a home cage that was placed next to the odor cloth wedged between the cage tops. The cloth was placed at the opposite end from the food and water containers. The duration of the test was 5 min, and the mice were videotaped for later scoring. The time (s) of cloth contact was defined as direct contact or sniffing ≤5 cm from the cloth [43].
Open Field Test (OFT)
This test was used to evaluate exploratory activity and anxiety during 5 min [44]. The arena was divided into nine squares of equal areas. In this apparatus, we determined the number of squares crossed by the animal (crossings), grooming behavior, and time spent in the center of the field (an indicative of anxiety-like behavior).
Neurochemical Determinations
Immediately after the behavioral determinations, the animals were decapitated and the brain areas PFC, HC, and HT dissected under ice, randomized in groups according to age, challenge, and sex, and stored at −80 °C. For MPO activity, homogenates prepared from fresh tissues were used. The levels of IFNγ, nitrite, parvalbumin, and BDNF were determined only in the HC.
Analysis of MPO Activity
Extracellular MPO activity gives an estimate of the oxidative stress in inflammatory diseases [45]. Peroxidase activity with 3,3′,5,5′-tetramethylbenzidine (TMB) was measured as described elsewhere [46]. Absorbance was measured at 450 nm and background corrected to estimate MPO activity (U MPO/min/mg tissue).
Determination of IL-4, IL-6, IFNγ, and BDNF Levels
Cytokines and BDNF were determined in each sample by enzyme immunoassays according to the manufacturers’ instructions (R&D systems, Minneapolis, MN, USA). The results were expressed as pg/g wet tissue.
Determination of Nitrite Levels
Nitrite levels were determined based on Griess reaction (Green and Goldman 1981; Radenovic and Selakovic 2005) and expressed as nM/g wet tissue.
Immunoblot Analysis
Hippocampi were homogenized in RIPA lysis buffer (25 mM Tris-HCl, pH 7.6; 150 mM NaCl; 5 mM EDTA; 1% NP40; 1% Triton X-100; 1% sodium deoxycholate; 0.1% SDS) with protease inhibitor (1 μL inhibitor: 100 μL RIPA). For protein extraction, HC samples were centrifuged at 13,000 rpm for 17 min under refrigeration (4 °C) and supernatant collected. Protein concentrations were determined by the method of Bradford according to the manufacturer’s protocol. SDS polyacrylamide gel electrophoresis (10%) was performed using 50 μg of protein (previously prepared with Laemmli sample buffer and heated at 95 °C for 5 min). The proteins were transferred to PVDF membrane, blocked with BSA 5% for 1 h, and incubated overnight with rabbit anti-parvalbumin IgG primary antibody (1:10,000; Abcam, USA) or mouse anti-α-tubulin IgG primary antibody (1:4000; Sigma, USA). After washing, the blots were incubated with horseradish peroxidase conjugated goat anti-rabbit IgG secondary antibody (1:1000; Thermo Scientific, USA) or goat anti-mouse IgG secondary antibody (1:1000; Thermo Scientific, USA) for 90 min at room temperature. Signal was detected using the ECL system (Bio-Rad, USA) according to the manufacturer’s instructions, and then the bands were captured with a CCD camera using the ChemiDoc system (Bio-Rad, USA). Densitometry quantification of bands was performed with NIH ImageJ software.
Statistical Analysis
For PPI analysis, repeated measures three-way ANOVA followed by Bonferroni post hoc test was used with “PP intensities” (70, 75, and 80) as within-subjects and LPS challenge (saline and LPS), “age” (PN35 and -70), and “sex” (male and female) as between-subjects factors. The other analyses were performed by univariate three-way analysis of variance (ANOVA) with Bonferroni post hoc test considering the same between-subjects factors described above. The levels of IFNγ, nitrite, relative protein expression of parvalbumin, and BDNF were analyzed by two-way ANOVA considering sex and LPS challenge as between-subjects factors with Bonferroni as post hoc test. Significance was set at 95% with alpha error at 5% (P ≤ 0.05). Effect size was estimated by partial eta squared (partial η2). In the description of post hoc comparisons (Results section) mean differences (MD) ± standard errors are shown in brackets together with respective P values. All analyses were performed using SPSS Software v.23 (IBM Analytics).
Results
LPS Neonatal Challenge Causes Age- and Sex-related Behavioral Alterations
A significant interaction between sex versus LPS challenge was observed in PPI analysis (ANOVA: (F(1,58) = 5.344, P = 0.02, partial η 2 = 0.084)). Bonferroni test revealed that LPS-challenged female mice on PN35 (Fig. 2a) presented significant deficit in % PPI at PP80 (MD = −20.6 ± 9.9, P = 0.04); while on PN70 (Fig. 2b), this deficit in female animals was observed in all three PP intensities evaluated (PP70: MD = −32.4 ± 9.1, P = 0.0007; PP75: MD = −30.9 ± 8.7, P = 0.0007; PP80: MD = −28 ± 9.8, P = 0.006). In both ages, the significance was observed when compared to saline-treated female mice.
Effect of LPS neonatal challenge on % PPI on postnatal day (PN) 35 (a) and PN70 (b). Male or female mice were intraperitoneally injected with LPS or saline on PN5 and -7. Bars are means ± SEM (n = 7–10 animals/group/sex/age). Data were analyzed using three-way ANOVA followed by Bonferroni’s post hoc test considering PP intensities (70, 75, and 80) as within-subjects factor (repeated measure). *P < 0.05 vs. saline-treated female mice. Abbreviations: M male, F female
We next evaluated depressive-like behavior (Fig. 3a) and anhedonia (Fig. 3b). A significant interaction between sex versus LPS challenge was observed in the determination of immobility time in the FST (Fig. 3a) (ANOVA: F(1,54) = 16.375, P = 0.0002, partial η 2 = 0.233). Post hoc test revealed that LPS challenge caused a significant increase in the immobility time in male mice on PN35 (MD = 50.6 ± 11.9, P < 0.0001) and PN70 (MD = 36.8 ± 11.5, P = 0.002) compared to age- and sex-matched controls. Additionally, we observed that saline-treated male mice on PN35 showed increased immobility time when compared to saline male animals on PN70 (MD = 24.4 ± 11.9, P = 0.05). The same increase in immobility time was observed in male LPS-treated mice on PN35 in relation to male LPS-treated mice on PN70 (MD = 38.2 ± 11.5, P < 0.002). No significant alterations were observed in the sucrose preference test (Fig. 3b).
Effect of LPS neonatal challenge in immobility time (a) and sucrose consumption (%) (b) on postnatal day (PN) 35 and PN70. Male or female mice were intraperitoneally injected LPS or saline on PN5 and -7. Bars are means ± SEM (n immobility time = 7–10; n sucrose consumption = 6–9 animals/group/sex/age). Data were analyzed using three-way ANOVA followed by Bonferroni’s post hoc test. *P < 0.05, **P < 0.01, ****P < 0.0001. Abbreviations: M male, F female
In the analysis of social preference (Fig. 4a), no significant alterations were observed. For the evaluation of working memory in the Y maze, despite the absence of significant interaction between factors, post hoc test revealed significant deficits in working memory in LPS-challenged male animals on PN35 when compared to age- and sex-matched controls (MD = −10.5 ± 4.9, P = 0.04) (Fig. 4b). In the cat odor test (Fig. 4c), a significant three-way interaction, age versus sex versus LPS challenge (F(1,45) = 16.869, P = 0.0002, partial η 2 = 0.273) was observed. Post hoc test showed that LPS-challenged male mice on PN35 presented significant increase in the time of cloth contact compared to age- and sex-matched control (MD = 44.86 ± 6.6, P < 0.0001).
Effect of LPS neonatal challenge in % social preference (a), % correct alternations (b), and time of cloth contact (c) on postnatal day (PN) 35 and PN70. Male or female mice were intraperitoneally injected LPS or saline on PN5 and -7. Bars are means ± SEM (n social preference = 7–10, n correct alternations = 6–10, n cat odor test = 6–8 animals/group/sex/age). Data were analyzed using three-way ANOVA followed by Bonferroni’s post hoc test. *P < 0.05 vs. saline-treated age- and sex-matched mice, ****P < 0.0001. Abbreviations: M male; F female
No significant alteration in the locomotor activity was registered (Fig. 5a). On the other hand, a significant interaction between sex versus LPS challenge (F(1,56) = 7.06, P = 0.01, partial η 2 = 0.086) and sex versus age (F(3,56) = 5.23, P = 0.002, partial η 2 = 0.173) was observed in the analysis of grooming behavior. Bonferroni test showed increased grooming behavior in LPS-challenged male mice evaluated on PN35 when compared to saline-treated animals at the same age (MD = 2.3 ± 0.68, P = 0.001). We also observed that LPS-challenged male mice presented a significant decrease in the time spent in the center of the open field on PN35 (MD = −18.7 ± 5.1, P = 0.001) and PN70 (MD = −16.2 ± 5, P = 0.002) when compared to age- and sex-matched controls. Additionally, on PN35, an increase in the time spent in the center of the field was observed in saline-treated male animals when compared to saline-treated female animals (MD = 14.4 ± 5.6, P = 0.01) (ANOVA of time spent in the center of the field: sex versus LPS challenge interaction (F(1,56) = 10.647, P = 0.002, partial η 2 = 0.153)).
Effect of LPS neonatal challenge in the number of crossings (a), grooming (b), and time spent in the center of the field (c) on postnatal day (PN) 35 and PN70. Male or female mice were intraperitoneally injected LPS or saline on PN5 and -7. Bars are means ± SEM (n = 6–8 animals/group/sex/age). Data were analyzed using three-way ANOVA followed by Bonferroni’s post hoc test. *P < 0.05, ***P < 0.001. Abbreviations: M male, F female
LPS Neonatal Challenge Causes Sex- and Age-related Brain Immune Alterations
Based on LPS immunomodulating properties, we next attempted to determine if the activity of MPO (a marker of innate immune activation) and the cytokines IL-4 and IL-6 were altered in brain areas of periadolescent and adult mice neonatally challenged with LPS. The activity of MPO (Fig. 6a–c) was significantly increased in the PFC (MD = 0.58 ± 0.28, P = 0.04), HC (MD = 0.685 ± 0.23, P = 0.005), and HT (MD = 1.38 ± 0.25, P < 0.0001) of LPS-challenged male mice on PN35 and in the HC on PN70 (MD = 0.58 ± 0.24, P < 0.05) compared to sex- and age-matched controls. In contrast, female animals presented no alteration in MPO activity. In the analysis of MPO activity, we observed significant interactions only in the HT (age versus LPS challenge (F(1,42) = 6.7, P = 0.01, partial η 2 = 0.138) and sex versus LPS challenge (F(1,42) = 12.415, P = 0.013, partial η 2 = 0.228)).
Effect of LPS neonatal challenge in myeloperoxidase activity (MPO) (prefrontal cortex (a), hippocampus (b), and hypothalamus (c)), IL-4 (prefrontal cortex (d), hippocampus (e), and hypothalamus (f)) and IL-6 (prefrontal cortex (g), hippocampus (h), and hypothalamus (i)) on postnatal day (PN) 35 and PN70. Male or female mice were intraperitoneally injected LPS or saline on PN5 and -7. Bars are means ± SEM (n = 6–8 animals/group/sex/age). Data were analyzed using three-way ANOVA followed by Bonferroni’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 ****P < 0.0001
Regarding the levels of IL-4 (Fig. 6d–f), we observed a significant three-way interaction in the PFC (ANOVA: age versus sex versus LPS challenge (F(1,48) = 8.278, P = 0.006, partial η 2 = 0.147). Bonferroni post hoc test showed that in the PFC, HC, and HT of LPS-challenged male animals, there were significant increases in the levels of IL-4 in both ages studied compared to saline-treated mice. In females, IL-4 significantly increased in the PFC and HC of adolescent animals and in the HC and HT of adult mice. Interestingly, in adult male mice, the LPS-induced significant increases of IL-4 levels in all brain areas studied in relation to LPS-challenged females. Analysis of IL-6 (Fig. 6g–i) showed a significant interaction between sex versus LPS challenge in the PFC (ANOVA: (F(1,45) = 7.876, P = 0.007, partial η 2 = 0.149)): while in the HC, significant interaction of sex versus LPS challenge (F(1,44) = 6.86, P = 0.011, partial η 2 = 0.135) and sex versus age were observed (F(2,44) = 3.98, P = 0.03, partial η 2 = 0.153). In the HT, there was a significant sex versus age versus LPS challenge interaction (ANOVA: (F(1,42) = 5.69, P = 0.022, partial η 2 = 0.103)). Post hoc test showed that the levels of IL-6 were significantly decreased in the PFC, HC, and HT of both LPS-challenged male and female mice when compared to age- and sex-matched saline-treated animals. We also detected that in the HT, the levels of IL-6 were increased in saline-treated female mice on PN70 when compared to the same experimental group on PN35.
LPS Neonatal Challenge Induces Sex-related Immune, Parvalbumin, and Neurotrophic Alterations in the HC of Adult Mice
Since we observed important long-lasting alterations (during adult life) in the MPO activity, IL-4 and IL-6 especially in the HC of LPS-challenged mice, we pursued evaluation of additional parameters, including IFNγ, nitrite, parvalbumin, and BDNF in the HC of adult mice (Fig. 7).
Effect of LPS neonatal challenge in the levels of IFN-γ (a), nitrite (b), parvalbumin (c), and BDNF (d) in the hippocampus of adult mice (PN70). Male or female mice were intraperitoneally injected LPS or saline on PN5 and -7. Bars are means ± SEM (n = 6–8 animals/group/sex/age). Data were analyzed using three-way ANOVA followed by Bonferroni’s post hoc test. **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. saline-treated age- and sex-matched mice
Two-way ANOVA of IFNγ results revealed no significant interaction between the factors studied, although the levels of IFNγ in the HC of both LPS-challenged male (MD = 257.7 ± 38.9, P < 0.0001) and female (MD = 231.3 ± 37.6, P < 0.0001) adult animals were increased when compared to their respective saline-treated counterparts (Fig. 7a).
Nitrite levels were increased by 18-fold in the HC of LPS-challenged male mice on PN70 when compared to control mice (MD = 1161.8 ± 126, P < 0.0001) (ANOVA: sex versus LPS challenge: (F(1,21) = 46.32, P < 0.0001, partial η 2 = 0.688)) (Fig. 7b). Parvalbumin expression was decreased in the HC of LPS-challenged male mice in relation to their controls (MD = −0.291 ± 0.137, P = 0.05), without any significant interactions between factors (Fig. 7c). In LPS-challenged females, nitrite and parvalbumin seemed to be unaltered.
Both LPS-challenged male and female adult mice presented increased levels of BDNF in their HC when compared to sex-matched controls (male: MD = 4042.5 ± 606.7, P < 0.0001; female: MD = 4603.6 ± 609.7, P < 0.0001), again without any significant interactions between factors (Fig. 7d).
Discussion
The key findings of this study are the sex-specific and age-dependent influence induced by neonatal LPS challenge on long-term behavior. Indeed, male mice presented depressive-like, risk-taking, anxiety-like, and repetitive behaviors with working memory deficits at periadolescence; while at adulthood, only depressive- and anxiety-like behaviors were observed. In contrast, females only presented PPI deficits in both ages studied.
The neurobiological alterations exclusive to LPS-challenged males were related to their increased hippocampal nitrite levels (PN70), MPO activity (PN35 and -70), and decreased parvalbumin (PN70). Both sexes, in turn, presented at periadolescence and adulthood with increased levels of IL-4 (PFC, HC, and HT) and a decrease of IL-6 (PFC, HC, and HT), while an elevation of IFNγ and BDNF was observed in adults only.
LPS Challenge Induces Sex-biased Behavioral Alterations
Owing a strong relationship between prenatal/neonatal LPS challenges and the manifestation of neuropsychiatric disorders, such as autism [4, 8], schizophrenia [47], and anxiety [10], in rodents, we sought to assess behavioral alterations and pinpoint neuropsychiatric consequences arising from early phase exposure to LPS in periadolescent and adult mice.
The neonatal day of LPS exposure may be of substantial relevance for the determination of the behavioral patterns observed later in life. Hence, we carefully selected the exposure PNs for the design of our study to correspond to an expressive homologous period in humans. PN1–3 correspond to 23–32 weeks of gestation in humans (preterm infant), which is characterized by an intense development of the immune system and establishment of the blood-brain barrier. Conversely, during PN5–9, the immune system is consolidating, while axonal and dendritic densities both increase and gliogenesis peaks [19]. We chose this particular window period, as it would offer the best opportunity to evaluate impact on behavior.
Furthermore, our selection of behavioral tasks had a purpose to capture meaningful behavioral information, such as PPI, to enable measurement of features of ASD, a disorder characterized by deficits in the gating of motor, sensory, and/or cognitive information [48].
Here, we observed that only female mice challenged with LPS presented impaired PPI function in both ages studied. Due to the heterogeneity within ASD, the results of PPI in patients with this disorder are conflicting. In this regard, deficits in PPI were observed in male adults with ASD being these deficits associated with restricted and repetitive behaviors as well as poor verbal fluency test performance [49]. On the other hand, no PPI alterations were observed in high functioning autism [50]. Contrary to our results, a preclinical study showed that male rats challenged with bilateral infusion of LPS into the ventral hippocampus on PN7 and -8 presented deficits in PPI when evaluated during adulthood [51]. It is important to note that, to date, the translational value of studies based on the intracerebral (i.c.) infusion of LPS remains a controversial matter, since they do not model the systemic or maternal origin of perinatal infections [4]. Furthermore, local injection of an inflammatory stimulus might only activate immune cells locally, which could lead to unexpected differences when compared to a systemic activation of the immune system.
In line with our findings of PPI deficit in LPS-challenged female mice and a previous report linking PPI deficits with verbal fluency in ASD [49], a study from the UK investigated the potential diagnosis gap of ASD between men and women and showed that females who fell short of the threshold for ASD diagnosis actually presented with increased communication difficulties though with reduced social impairments compared to matched males [52]. Overall, the lack of clinical studies investigating the influence of sex on PPI function in ASD compromises the discussion of our preclinical findings.
In our study, only periadolescent male animals presented increased immobility time in the FST (corresponding to depressive-like behavior), increments in the time of cloth contact in the COT (risk-taking behavior), increased grooming behavior (corresponding to repetitive behavior), decreased time spent in the center of the open field (anxiety-like behavior), and deficits in the correct alternations in the Y maze task (working memory impairment). When the animals were evaluated during adulthood, only depressive- and anxiety-like alterations remained. It is worth mentioning that a clinical study, based on a large community sample of 241 adolescents and adults with ASD, revealed that older sample members (31 years and older) had fewer maladaptive behaviors and experienced improvement of repetitive behaviors over time [53]. In terms of working memory, specific problems on spatial working memory are often seen in high-functioning adolescents with ASD [54]. Thus, comparing our preclinical behavioral data with the symptoms of ASD in humans, we can find some similarities showing that this model presents face validity, although further studies are needed to strengthen this conclusion.
Supporting our observations, Walker et al. [55] found that the neonatal administration (PN3 and -5) of LPS from Salmonella enteritidis caused anxiety-like alterations in animals when evaluated during adulthood. It is important to highlight that in this study, the authors evaluated only anxiety-like alterations by using the elevated plus maze test. Early-life challenge with LPS was also related to long-lasting depressive-like behavior in rodents [56].
All in all, our investigation suggests that LPS challenge may in fact have a more profound implication than anticipated. Rather than simply predicting punctual anxiety-like or depressive-like alterations, more complex age-dependent and sex-specific features may crucially determine phenotype and behavioral alterations. Therefore, the design of robust experimental protocols for LPS-challenging (and as a result for preterm and neonatal Gram-negative infections) to evaluate long-term consequences and disentangle a wide range of behavioral patterns (rather than focusing on simple traces) will be of great relevance and translational value.
Influence of Sex in Brain Inflammatory, Parvalbumin, and Neurotrophic Alterations in Adult Mice Neonatally Exposed to LPS
Immunological imbalances (including autoimmunity) have been proposed as a major etiological component in ASD. In line with the immunological imbalances, individuals with ASD show alterations in the proportion and activity of various immune cells, including natural killer (NK) cells and macrophages. With regard to innate immunity, MPO is a heme enzyme involved in the production of oxidizing species by phagocytic cells, such as macrophages. This enzyme catalyzes oxidative reactions during inflammation being up-regulated in several acute and chronic inflammatory conditions, including schizophrenia [57] and autism [58].
Our data indicate that the activity of MPO was increased in all brain areas studied of LPS-challenged male periadolescent mice: while in adult animals, this increase was observed only in the HC. A recent study using the model of autism induced by prenatal exposure of rats to valproic acid showed that on PN50, the animals presented augmentation of blood-brain barrier (BBB) permeability, increased brain MPO activity, and oxidative imbalance (decreased reduced glutathione (GSH) and increased lipid peroxidation) [59]. Similarly, decreased levels of GSH and increased oxidative imbalance were related to chronic inflammatory response, increased mitochondrial superoxide production, protein oxidation, and DNA damage in brain areas of ASD patients [58].
Dysregulation in cytokine’s levels are important findings in patients with ASD [27] as well as may be related to sex-specific alterations observed in Asperger’s syndrome [60]. In the present study, we observed that the brain areas of both periadolescent and adult male and female mice neonatally exposed to LPS presented increased levels of the T-helper 2 (Th2) cytokine, IL-4. Similarly, the serum levels of IL-4 were increased in individuals with ASD, being this increase associated, in a previous study, with greater impairments in non-verbal communication [61]. Despite the increase in IL-4 levels observed in ASD, the role this cytokine in this disorder, i.e., pathogenic versus protective, is still elusive [62]. Another important observation related to our results is that adult male LPS-challenged mice presented higher levels of IL-4 when compared to their female counterparts. The higher levels of IL-4 observed in the brain of LPS-challenged male animals are partially in line with the findings of Schwarz et al. [60]. These authors found that one core biological difference between males and females with Asperger’s syndrome was the increased levels of IL-4 in male subjects.
IL-6, in turn, is a neuropoietic cytokine involved in neurodevelopment and brain function. This cytokine can cross the BBB influencing processes in the adult brain [63]. Alterations in behavior, cognition, gamma-amino butyric acid (GABA) neurotransmission, and immune function are among the functions compromised by hippocampal IL-6 dysfunction during neurodevelopment [64]. Increased levels of IL-6 are associated with ASD [65]. On the contrary, in our results, we observed that male and female mice in both ages evaluated presented decreased brain levels of IL-6. Of note, IL-6 deficits are related to higher aggression and emotionality [66]. Furthermore, IL-6KO mice showed impaired memory processes accompanied by a higher susceptibility for stress and reduced exploratory behavior [67].
In our results, the decreased brain levels of IL-6 and increased IL-4 observed in both male and female animals might be related to a modulation of hypothalamic-pituitary-adrenal (HPA) axis. Elevation of glucocorticoids results in suppression of proinflammatory cytokines, such as IL-6 and TNF-α, and up-regulation of anti-inflammatory cytokines, such as IL-4 and IL-10, and cytokine receptors [68]. Of note, children with ASD present significantly higher peak cortisol levels and prolonged duration and recovery of cortisol elevation following stress [69]. Thus, further studies are needed to test the hypothesis of increased activity of HPA axis in neonatal animals challenged with LPS on PN5 and -7.
Since the main neurobiological alterations observed in the present study took place in the HC and knowing the importance of this brain area to the performance of the behavioral tasks conducted here, we decided to evaluate the other neurochemical parameters only in this brain area.
Regarding IFN-γ, we observed increased levels of this Th1 cytokine in both male and female LPS-challenged adult mice. IFN-γ was related to NO overproduction in ASD patients [28]. In line with this evidence, we also observed high levels of nitrite in the HC of LPS-challenged male adult mice. LPS-induced nitrite levels may reflect an overexpression of inducible nitric oxide (iNOS) in microglial cells of male animals. Therefore, together with MPO results, we can suggest the occurrence of LPS-induced sex-specific alterations in innate immune markers, in our protocol.
Since we observed a proinflammatory profile in the HC of LPS-challenged adult animals, we decided to determine parvalbumin expression (i.e., a marker of GABAergic interneurons). In the HC, parvalbumin-expressing interneurons coordinate hippocampal network dynamics being required for spatial working memory [70]. In our results, parvalbumin protein expression was decreased in male animals exposed to LPS. Importantly, parvalbumin neurons were decreased in the medial PFC of autistic subjects [71] reflecting a disruption in the balance of excitation/inhibition being BDNF, a regulator of the synchronized network activity through parvalbumin interneurons [72]. Despite the studies showing BDNF regulation of parvalbumin neurons, the relationship between BDNF and parvalbumin neurons in typical and atypical autism, as far as we know, was not studied yet. Furthermore, redox dysregulation was associated with impairment of parvalbumin neurons in the ventral HC [73].
Here, we observed that both male and female animals challenged with LPS presented a mean increase of twofold in hippocampal BDNF levels. It was previously determined in a clinical study that BDNF may be an important biomarker for the differentiation of typical and atypical autism. Indeed, serum BDNF levels were found significantly higher in atypical autistic subjects (clinically milder phenotype) as compared to controls, but not in typical ASD cases (clinically severe phenotype) [26]. Lower BDNF levels may indicate impairment in neuroprotective mechanism, while higher levels may imply a manifested protective response. Despite this, the increase in these neurotrophin levels, as observed in our results, could be also an indicative of impairment in synaptic pruning, which has been previously related to some ASD endophenotyphes, such as social behavior impairment [74].
Our results, as far as we know, provide the first evidences for a distinct behavioral profile in LPS-challenged male mice accompanied by increases of innate immunity-related parameters (MPO activity and nitrite levels), higher levels of IL-4, as well as parvalbumin alterations, in relation to female counterparts. In fact, one of the most consistent findings in ASD research is a higher rate of ASD diagnosis in males than females [75]. Despite this evidence, little is known about the influence of sex on the ASD. Therefore, this study provides initial evidence with respect to molecular changes related to sex influences in the ASD that need to be better evaluated in future studies.
This study presents some limitations since we did not evaluate HPA axis activity in periadolescent and adult mice and synaptic pruning during neonatal life. Another important limitation is that the cytokines and BDNF were not tested in plasma samples to perform a correlation between plasma and brain parameters in order to guide future studies evaluating biomarkers of sex differences in ASD.
Conclusions
Systemic neonatal challenging with LPS on PN5 and -7 triggers distinct behavioral patterns in periadolescent and adult mice, crucially with some of them apparently sex-specific. Our results reflect the role of a set of cytokines, rather than an individual one, for the development and course of LPS-induced behavioral alterations. Our results support the contribution of neonatal infections towards the manifestation of long-lasting ASD-like alterations in both male and female animals, adding further evidence for the relevance of investigating sex differences in ASD using LPS-challenged animal models. Finally, our findings warrant the design of studies addressing novel strategies, either preventive or therapeutic in nature, to guide sex-specific interventions for ASD. Therefore, the design of experimental protocols evaluating the long-term consequences of LPS challenge in a wide range of behavioral determinations and not only single determinations might be of great importance and translational value.
References
Kessler RC, Davis CG, Kendler KS (1997) Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med 27:1101–1119. doi:10.1017/S0033291797005588
Roth TL, Lubin FD, Funk AJ, Sweatt JD (2009) Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry 65:760–769. doi:10.1016/j.biopsych.2008.11.028
Pang Y, Cai Z, Rhodes PG (2003) Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res 140:205–214
Pang Y, Dai X, Roller A et al (2016) Early postnatal lipopolysaccharide exposure leads to enhanced neurogenesis and impaired communicative functions in rats. PLoS One 11:e0164403. doi:10.1371/journal.pone.0164403
Alexander C, Rietschel ET (2001) Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res 7:167–202. doi:10.1177/09680519010070030101
Pilakka-Kanthikeel S, Kris A, Selvaraj A et al (2014) Immune activation is associated with increased gut microbial translocation in treatment-naive, HIV-infected children in a resource-limited setting. J Acquir Immune Defic Syndr 66:16–24. doi:10.1097/QAI.0000000000000096
Vassallo M, Mercie P, Cottalorda J et al (2012) The role of lipopolysaccharide as a marker of immune activation in HIV-1 infected patients: a systematic literature review. Virol J 9:174. doi:10.1186/1743-422X-9-174
Kirsten TB, Lippi LL, Bevilacqua E, Bernardi MM (2013) LPS exposure increases maternal corticosterone levels, causes placental injury and increases IL-1B levels in adult rat offspring: relevance to autism. PLoS One. doi:10.1371/journal.pone.0082244
Wischhof L, Irrsack E, Osorio C, Koch M (2015) Prenatal LPS-exposure—a neurodevelopmental rat model of schizophrenia—differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Prog Neuro-Psychopharmacol Biol Psychiatry 57:17–30. doi:10.1016/j.pnpbp.2014.10.004
Walker AK, Nakamura T, Byrne RJ et al (2009) Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis. Psychoneuroendocrinology 34:1515–1525. doi:10.1016/j.psyneuen.2009.05.010
Temmingh H, Stein DJ (2015) Anxiety in patients with schizophrenia: epidemiology and management. CNS Drugs 29:819–832. doi:10.1007/s40263-015-0282-7
Chalfant AM, Rapee R, Carroll L (2007) Treating anxiety disorders in children with high functioning autism spectrum disorders: a controlled trial. J Autism Dev Disord 37:1842–1857. doi:10.1007/s10803-006-0318-4
Stone WS, Iguchi L (2011) Do apparent overlaps between schizophrenia and autistic spectrum disorders reflect superficial similarities or etiological commonalities? N Am J Med Sci (Boston) 4:124–133. doi:10.7156/v4i3p124
Custódio CS, Mello BSF, Cordeiro RC et al (2013) Time course of the effects of lipopolysaccharide on prepulse inhibition and brain nitrite content in mice. Eur J Pharmacol 713:31–38. doi:10.1016/j.ejphar.2013.04.040
Tomaz VS, Cordeiro RC, Costa AMN et al (2014) Antidepressant-like effect of nitric oxide synthase inhibitors and sildenafil against lipopolysaccharide-induced depressive-like behavior in mice. Neuroscience 268:236–246. doi:10.1016/j.neuroscience.2014.03.025
DellaGioia N, Devine L, Pittman B, Hannestad J (2013) Bupropion pre-treatment of endotoxin-induced depressive symptoms. Brain Behav Immun 31:197–204. doi:10.1016/j.bbi.2012.10.008
Andersen SL, Navalta CP (2004) Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs. Int J Dev Neurosci 22:423–440. doi:10.1016/j.ijdevneu.2004.06.002
Andersen SL (2003) Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27:3–18. doi:10.1016/S0149-7634(03)00005-8
Semple BD, Blomgren K, Gimlin K et al (2013) Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106–107:1–16. doi:10.1016/j.pneurobio.2013.04.001
Clancy B, Finlay BL, Darlington RB, Anand KJS (2007) Extrapolating brain development from experimental species to humans. Neurotoxicology 28:931–937. doi:10.1016/j.neuro.2007.01.014
Piontkewitz Y, Arad M, Weiner I (2011) Risperidone administered during asymptomatic period of adolescence prevents the emergence of brain structural pathology and behavioral abnormalities in an animal model of schizophrenia. Schizophr Bull 37:1257–1269. doi:10.1093/schbul/sbq040
Ribeiro BMM, MRS d C, Freire RS et al (2013) Evidences for a progressive microglial activation and increase in iNOS expression in rats submitted to a neurodevelopmental model of schizophrenia: reversal by clozapine. Schizophr Res 151:12–19. doi:10.1016/j.schres.2013.10.040
Novacek DM, Gooding DC, Pflum MJ (2016) Hedonic capacity in the broader autism phenotype: should social anhedonia be considered a characteristic feature? Front Psychol. doi:10.3389/fpsyg.2016.00666
Lai M-C, Lombardo MV, Auyeung B et al (2015) Sex/gender differences and autism: setting the scene for future research. J Am Acad Child Adolesc Psychiatry 54:11–24. doi:10.1016/j.jaac.2014.10.003
Beggiato A, Peyre H, Maruani A et al (2016) Gender differences in autism spectrum disorders: divergence among specific core symptoms. Autism Res. doi:10.1002/aur.1715
Kasarpalkar NJ, Kothari ST, Dave UP (2014) Brain-derived neurotrophic factor in children with autism spectrum disorder. Ann Neurosci 21:129–133. doi:10.5214/ans.0972.7531.210403
Goines PE, Ashwood P (2013) Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol Teratol 36:67–81. doi:10.1016/j.ntt.2012.07.006
Sweeten TL, Posey DJ, Shankar S, McDougle CJ (2004) High nitric oxide production in autistic disorder: a possible role for interferon-γ. Biol Psychiatry 55:434–437. doi:10.1016/j.biopsych.2003.09.001
Scholes KE, Martin-Iverson MT (2009) Relationships between prepulse inhibition and cognition are mediated by attentional processes. Behav Brain Res 205:456–467. doi:10.1016/j.bbr.2009.07.031
Porsolt RD, Bertin A, Jalfre M (1978) “Behavioural despair” in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol 51:291–294. doi:10.1016/0014-2999(78)90414-4
Papp M, Willner P, Muscat R (1991) An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology 104:255–259. doi:10.1007/BF02244188
Li B, Arime Y, Hall FS et al (2010) Impaired spatial working memory and decreased frontal cortex BDNF protein level in dopamine transporter knockout mice. Eur J Pharmacol 628:104–107. doi:10.1016/j.ejphar.2009.11.036
Staples LG (2010) Predator odor avoidance as a rodent model of anxiety: learning-mediated consequences beyond the initial exposure. Neurobiol Learn Mem 94:435–445. doi:10.1016/j.nlm.2010.09.009
NIH (1996) Guide for the care and use of laboratory animals—Institute of Laboratory Animal Research—National Research Council. Natl. Acad. Press, Washington, D.C.
Lazic SE, Essioux L (2013) Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci 14:37. doi:10.1186/1471-2202-14-37
Powell SB, Weber M, Geyer MA (2012) Genetic models of sensorimotor gating: relevance to neuropsychiatric disorders. Curr Top Behav Neurosci 12:251–318. doi:10.1007/7854_2011_195
Levin R, Calzavara MB, Santos CM et al (2011) Spontaneously hypertensive rats (SHR) present deficits in prepulse inhibition of startle specifically reverted by clozapine. Prog Neuro-Psychopharmacol Biol Psychiatry 35:1748–1752. doi:10.1016/j.pnpbp.2011.06.003
Noda Y, Yamada K, Furukawa H, Nabeshima T (1995) Enhancement of immobility in a forced swimming test by subacute or repeated treatment with phencyclidine: a new model of schizophrenia. Br J Pharmacol 116:2531–2537
Mao Q-Q, Huang Z, Zhong X-M et al (2014) Brain-derived neurotrophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav Brain Res 261:140–145. doi:10.1016/j.bbr.2013.12.020
Radyushkin K, Hammerschmidt K, Boretius S et al (2009) Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav 8:416–425. doi:10.1111/j.1601-183X.2009.00487.x
Sarter M, Bodewitz G, Stephens DN (1988) Attenuation of scopolamine-induced impairment of spontaneous alteration behaviour by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology 94:491–495
Zangrossi H, File SE (1992) Behavioral consequences in animal tests of anxiety and exploration of exposure to cat odor. Brain Res Bull 29:381–388. doi:10.1016/0361-9230(92)90072-6
Kirsten TB, Chaves GP, Taricano M et al (2011) Prenatal LPS exposure reduces olfactory perception in neonatal and adult rats. Physiol Behav 104:417–422. doi:10.1016/j.physbeh.2011.04.049
Archer J (1973) Tests for emotionality in rats and mice: a review. Anim Behav 21:205–235
Pulli B, Ali M, Forghani R et al (2013) Measuring myeloperoxidase activity in biological samples. PLoS One 8:e67976. doi:10.1371/journal.pone.0067976
Suzuki K, Ota H, Sasagawa S et al (1983) Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem 132:345–352. doi:10.1016/0003-2697(83)90019-2
Zhu F, Zhang L, Ding Y, Qiang et al (2014) Neonatal intrahippocampal injection of lipopolysaccharide induces deficits in social behavior and prepulse inhibition and microglial activation in rats: implication for a new schizophrenia animal model. Brain Behav Immun 38:166–174. doi:10.1016/j.bbi.2014.01.017
Braff DL, Geyer MA, Swerdlow NR (2001) Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology 156:234–258
Perry W, Minassian A, Lopez B et al (2007) Sensorimotor gating deficits in adults with autism. Biol Psychiatry 61:482–486. doi:10.1016/j.biopsych.2005.09.025
Kohl S, Wolters C, Gruendler TOJ et al (2014) Prepulse inhibition of the acoustic startle reflex in high functioning autism. PLoS One 9:e92372. doi:10.1371/journal.pone.0092372
Feleder C, Tseng KY, Calhoon GG, O’Donnell P (2010) Neonatal intrahippocampal immune challenge alters dopamine modulation of prefrontal cortical interneurons in adult rats. Biol Psychiatry 67:386–392. doi:10.1016/j.biopsych.2009.09.028
Dworzynski K, Ronald A, Bolton P, Happé F (2012) How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J Am Acad Child Adolesc Psychiatry 51:788–797. doi:10.1016/j.jaac.2012.05.018
Shattuck PT, Seltzer MM, Greenberg JS et al (2007) Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 37:1735–1747. doi:10.1007/s10803-006-0307-7
Barendse EM, Hendriks MPH, Jansen JFA et al (2013) Working memory deficits in high-functioning adolescents with autism spectrum disorders: neuropsychological and neuroimaging correlates. J Neurodev Disord 5:14. doi:10.1186/1866-1955-5-14
Walker FR, March J, Hodgson DM (2004) Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav Brain Res 154:63–69. doi:10.1016/j.bbr.2004.01.019
Dinel A-L, Joffre C, Trifilieff P et al (2014) Inflammation early in life is a vulnerability factor for emotional behavior at adolescence and for lipopolysaccharide-induced spatial memory and neurogenesis alteration at adulthood. J Neuroinflammation 11:155. doi:10.1186/s12974-014-0155-x
Al-Asmari AK, Khan MW (2014) Inflammation and schizophrenia: alterations in cytokine levels and perturbation in antioxidative defense systems. Hum Exp Toxicol 33:115–122. doi:10.1177/0960327113493305
Rose S, Melnyk S, Pavliv O et al (2012) Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry 2:e134. doi:10.1038/tp.2012.61
Kumar H, Sharma B (2016) Memantine ameliorates autistic behavior, biochemistry & blood brain barrier impairments in rats. Brain Res Bull 124:27–39. doi:10.1016/j.brainresbull.2016.03.013
Schwarz E, Guest PC, Rahmoune H et al (2011) Sex-specific serum biomarker patterns in adults with Asperger’s syndrome. Mol Psychiatry 16:1213–1220. doi:10.1038/mp.2010.102
Ashwood P, Krakowiak P, Hertz-Picciotto I et al (2011) Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 25:40–45. doi:10.1016/j.bbi.2010.08.003
Gadani SP, Cronk JC, Norris GT, Kipnis J (2012) IL-4 in the brain: A cytokine to remember. J Immunol 189:4213–4219. doi:10.4049/jimmunol.1202246
Stolp HB (2013) Neuropoietic cytokines in normal brain development and neurodevelopmental disorders. Mol Cell Neurosci 53:63–68. doi:10.1016/j.mcn.2012.08.009
Samuelsson A-M, Jennische E, Hansson H-A, Holmäng A (2006) Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol 290:R1345–R1356. doi:10.1152/ajpregu.00268.2005
Wei H, Zou H, Sheikh AM et al (2011) IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation 8:52. doi:10.1186/1742-2094-8-52
Alleva E, Cirulli F, Bianchi M et al (1998) Behavioural characterization of interleukin-6 overexpressing or deficient mice during agonistic encounters. Eur J Neurosci 10:3664–3672. doi:10.1046/j.1460-9568.1998.00377.x
Baier PC, May U, Scheller J et al (2009) Impaired hippocampus-dependent and -independent learning in IL-6 deficient mice. Behav Brain Res 200:192–196. doi:10.1016/j.bbr.2009.01.013
Chrousos GP (1995) The hypothalamic–pituitary–adrenal axis and immune-mediated inflammation. N Engl J Med 332:1351–1363. doi:10.1056/NEJM199505183322008
Spratt EG, Nicholas JS, Brady KT et al (2012) Enhanced cortisol response to stress in children in autism. J Autism Dev Disord 42:75–81. doi:10.1007/s10803-011-1214-0
Murray AJ, Sauer J-F, Riedel G et al (2011) Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci 14:297–299. doi:10.1038/nn.2751
Hashemi E, Ariza J, Rogers H et al (2016) The number of parvalbumin-expressing interneurons is decreased in the medial prefrontal cortex in autism. Cereb Cortex:bhw021. doi:10.1093/cercor/bhw021
Zheng K, An JJ, Yang F et al (2011) TrkB signaling in parvalbumin-positive interneurons is critical for gamma-band network synchronization in hippocampus. Proc Natl Acad Sci 108:17201–17206. doi:10.1073/pnas.1114241108
Steullet P, Cabungcal J-H, Kulak A, et al (2010) Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci 30:2547–2558. doi:10.1523/JNEUROSCI.3857-09.2010
Zhan Y, Paolicelli RC, Sforazzini F et al (2014) Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17:400–406. doi:10.1038/nn.3641
Halladay AK, Bishop S, Constantino JN et al (2015) Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol Autism 6:36. doi:10.1186/s13229-015-0019-y
Acknowledgements
The authors acknowledge the Brazilian Governmental Institutions CAPES, CNPq, and FUNCAP for the financial support of this study (scholarships and research grant).
Contributors
Authors DSM, CSC, and DFL contributed to the design of the study. CSC, BSFM, AJMCF, and RCC performed the behavioral experiments. Authors DFL, ACO, and DSM analyzed behavioral data. Authors DSM, TB, JQ, GZR, and FM analyzed the biochemical data and wrote the first draft of the paper. Authors CNCL, RCC, SMMV, and CSC performed neurochemical analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• LPS neonatal challenge on PN5 and -7 triggers sex-related behavioral alterations.
• LPS challenge in males increases brain innate immunity and decreases parvalbumin.
• Both sexes showed similar alterations in IL-4 and IL-6 when adolescent and adult.
• Both sexes showed increased hippocampal BDNF when adult.
• Behavioral and immune/neurotrophic alterations resemble autism spectrum disorders.
Rights and permissions
About this article
Cite this article
Custódio, C.S., Mello, B.S.F., Filho, A.J.M.C. et al. Neonatal Immune Challenge with Lipopolysaccharide Triggers Long-lasting Sex- and Age-related Behavioral and Immune/Neurotrophic Alterations in Mice: Relevance to Autism Spectrum Disorders. Mol Neurobiol 55, 3775–3788 (2018). https://doi.org/10.1007/s12035-017-0616-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0616-1