Abstract
Rodent model systems using lipopolysaccharide (LPS) administration have been developed to examine the effect of postnatal inflammation on neuropsychiatric behaviors. In this study, we investigated the effect of 250 μg/kg of LPS administration in male and female Wistar rats at three different postnatal periods. Rats were divided into four groups: a control group which received an intraperitoneal (IP) injection of phosphate buffer saline (PBS) on postnatal day 1 and three experimental groups which received an IP injection of LPS (250 μg/kg) at three different postnatal periods: day 1, day 3 and day 5. Each group consisted of 12 rats and had an equal gender distribution. At three months, rats were subjected to neurobehavioral assessments and biochemical oxidative and inflammatory assays. As a trend, LPS administration generates uniform anxiogenic behaviors, a depressive response, and increased central oxidative and inflammatory stress across all groups as compared to controls. Males receiving an LPS injection at postnatal days 1 and 3 and females receiving it at days 3 and 5 displayed more anxiogenic and depressive responses than their gender counterparts. Females had higher biomarker levels, with results being more pronounced in the group receiving injection on day 3. Postnatal LPS induces emotional disturbances along with oxidative stress and inflammation in brain tissues. Response to LPS injection seems to be gender-dependent and modulated by several variables, including its time of administration. Further investigations that take into consideration these variables, among others, are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The postnatal period is critical for the developing brain, as the central nervous system (CNS) undergoes substantial development via numerous key processes, including neurogenesis, synaptogenesis, myelination, pruning, and programmed cell death [14]. Any disturbance of these physiological processes might profoundly impact neurodevelopment. Indeed, there is now substantial evidence in both preclinical [43] and clinical models [49] suggesting that early-life stress can influence postnatal growth, contributing to the increased risk of late-life psychopathologies, such as the occurrence of psychiatric disorders [4].
One method to induce stress during early-life is by the activation of the immune system: an exaggerated stimulation of immune cells postnatally has been associated with long-lasting neuropsychiatric sequelae, such as anxiety, affective disorders, schizophrenia, and autism [10, 31]. Reciprocally, patients suffering from a wide range of psychiatric problems have increased peripheral and central inflammatory markers, while concomitantly displaying diminished efficacy of immune elements crucial for defense against pathogens [42].
As the interplay between early-life stress, inflammation, and late-life psychiatric disorders remains intricate in nature, several rodent model systems have been deployed to assess the impact of inflammatory activation on neuropsychiatric behavior. These models offer a particularly coherent picture of the influence of inflammation on perception, emotion, cognition and behavior. When exposed to a stimulus, be it an infectious agent, isolated molecules like bacterial lipopolysaccharide (LPS), or the body's own cytokines generated in response to infection, the innate immune inflammatory response triggers a distinctive, predetermined behavioral and physiological state [45]. It features a constellation of symptoms characterized by lethargy, fatigue, anhedonia, psychomotor retardation, cognitive dulling, social withdrawal, and alterations in sleep and appetite patterns, which reflect the symptoms observed in humans with major depressive disturbances [20].
LPS is a component of the cell wall of gram-negative bacteria that stimulates the immune system similar to a bacterial infection [15]. It activates the immune response and then initiates an intracellular signaling cascade, ultimately resulting in the generation and liberation of central and peripheral pro-inflammatory cytokines [37]. Furthermore, a large body of data indicates that peripheral LPS treatment stimulates the innate immune system and causes significant central monoaminergic changes in mice [20]. These inflammatory and biomolecular alterations may contribute to the spectrum of behavioral disturbances, anxious and depressive in nature, seen after administration of LPS in humans [5] and in animal models [21].
The majority of experiments examining the effects of LPS administration in rodents during the postnatal period have been carried out in males only [25]. Those assessing for sex-related differences have thus far shown inconsistent results [25, 40]. The knowledge about the supposed effect of sex on behavioral responsiveness and the underlying neurochemical mechanisms of this inflammatory model of early stress remains restricted. Previous studies have employed various dosages of LPS, obtained from different strains of bacteria, and injected at diverse postnatal periods, in order to stimulate early stress. However, to our knowledge, none assessed the effects of administrating a single dose of LPS at different postnatal periods on late-life outcomes. Generally, the hypothalamic-pituitary-adrenal (HPA) axis in rats is known to undergo a “stress hyporesponsive period” (SHRP) from postnatal day (PND) 4 to 14 (References), where behavioral responses to the LPS test are blunted [3]. Perhaps the LPS challenge, administered at PND 3 and 5, fell within a similar developmental “hyporesponsive period. In addition, our group has shown previously that rats exposed to LPS at PND 1 exhibit increased LPS-induced behavioral responses during adulthood (PND 90) [3].
Therefore, to address the gap in knowledge related to the effect of gender and period of LPS administration in determining future adult behavioral and biochemical responses, we studied the impact of injecting LPS (250 μg/kg) in male and female Wistar rats at three postnatal periods. Rodents were then subjected, during adulthood, to tests screening for anxious and depressive-like behaviors followed by biochemical assays measuring peripheral and central oxidation and inflammation.
Material and methods
Handling animal model
At the outset, a group of pregnant female Wistar rats (n=8) obtained from Ibn Tofail University's laboratory of Genetics, Neuroendocrinology, and Biotechnology were individually accommodated in conventional plexiglass cages measuring 430×290×210 mm. Maintaining a consistent temperature of 22 °C ± 2, a 12-hour light-dark cycle was upheld, with lights activated from 7:00 am to 7:00 pm. Unrestricted access to water and food was granted. The pregnant females produced a combined total of 89 offspring (42 males and 47 females). The day following parturition, the newborns were randomly assigned to four experimental groups. Accounting for the mortality rate observed during the study (2 males and 6 females, who either died at birth or after LPS injection), each group was adjusted to include a total of 12 healthy pups, ensuring an equal distribution of males and females. The remaining 33 pups were donated for other research experiments.
All protocols adhered to the guidelines outlined in the National Institutes of Health for the care and utilization of laboratory animals, as well as the scientific procedures involving living animals as detailed in the European Council Directive. Every possible measure was taken to reduce animal suffering. Approval for the study was granted by the Council Committee of Research Laboratories at Ibn Tofail University's Faculty of Sciences.
Experimental groupings
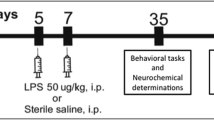
Wistar rat pups were randomly distributed into four groups, with each group comprising 12 animals, evenly divided between males and females (6 males and 6 females). Pups in the control group received an intraperitoneal (IP) injection of phosphate buffer solution at postnatal day 1 (PND 1). The three experimental groups received an IP injection of LPS [Escherichia coli, serotype 026: B6; L-3755 (Sigma, St. Louis MO); 250 μg/kg] at three different postnatal periods: day 1, day 3, and day 5 (referred to as LPS P1, LPS P3, and LPS P5 groups respectively). The LPS dose used here was based on earlier research [3]. Each randomized rat had its tail marked based on its assigned group. Pups of the same gender and group were then housed together in plexiglass cages, totaling 8 cages and kept with mother until PND 21 when they were weaned and separated. At PND 90, we tested for anxiety and depressive-like behaviors in the different experimental groups using the following behavioral tests: open field test, elevated plus maze test, novelty suppressed feeding test, and forced swimming test. Additionally, we conducted biochemical analyses on both peripheral and central tissues to assess levels of lipid peroxidation (MDA), nitric oxide (NO), superoxide dismutase (SOD), and tumor necrosis factor-alpha (TNF-α). Figure 1 provides a schematic representation of the experiment design.
Neurobehavioral tests
All pups underwent the four aforementioned tests, and prior to each assessment, the rats were positioned in the testing apparatus with their behaviors recorded for subsequent analysis.
Anxiety-Like measurement
Open field test
The OFT serves as a tool for assessing anxiety-like behavior in rodents [29]. During the test, the rodent is placed in an arena, granted 10 minutes of unrestricted movement, and recorded by an overhead camera for later analysis. The evaluated parameters include the time spent in the center area (TCA), the number of returns to the center (NRC), and the total number of squares covered (NTS). The entries in the central area and the time spent in this area by the rats are inversely proportional to the anxiety level. A lower anxiety level corresponds to a higher time spent in the central area. Conversely, a higher anxiety level results in less time spent there. Additionally, the total number of squares covered serves as a dependable indicator of overall locomotor activity. The apparatus underwent cleaning between each examination, utilizing a 7% ethanol solution [24].
Elevated Plus Maze (EPM)
The Elevated Plus Maze (EPM) is employed to evaluate anxiety-related behavior in rodent [12]. Rats are positioned in the central area of the maze, facing an open arm, and allowed to explore the EPM for 5 minutes. Their movements are recorded by a camera for subsequent analysis. The evaluated parameters include the Time Spent on Open Arms (TOA) and the Entries into Open Arms (EOA). These parameters demonstrate an inverse correlation with the anxiety level [41]. The apparatus underwent cleaning between each examination, utilizing a 7% ethanol solution.
Novelty suppressed feeding test
We used the novelty-suppressed feeding test to further assess anxiety [3]. The test is carried out in an open field under bright illumination conditions. This test subjects rats to a 24-hour feeding restriction. Following food restriction, the rats are positioned in an open field near a food pellet in the center of the arena. The anxiety-related metric is the time it takes to approach and eat the food.
Depression-like measurement
Forced Swimming Test (FST)
The FST is used to assess depressive-like behaviour in rodents [46]. Rats are forced to swim for 5 minutes in glass cylinder (height = 50 cm,diameter = 30 cm) which contains 30 cm of water at (23°C ± 2°C) while the camera records observations for later analysis to determine immobility times (TIM). Depressive behavior is indicated by an increasing TIM [58].
Biochemical assays
After being subjected to the behavioral tests, all rats were anesthetized with chloral solution (100 mg/kg). Organs such as the brain, liver, and spleen were excised. The brain was carefully dissected to obtain the prefrontal cortex (PFC), and hippocampus (HP) of each animal. The different brain sections were then homogenized, as previously described [3, 34].
Determination of lipid peroxidation levels
LPO levels were determined following the procedure described by Draper and Hadley [23, 28]. The quantification of oxidized lipids was performed by assessing Thiobarbituric Acid Reactive Substances (TBARS). The reaction mixture included tissue homogenates, 10% Trichloroacetic Acid (TCA), and 0.67% Thiobarbituric Acid (TBA), and was subjected to a 15-minute incubation in a boiling water bath. Subsequently, butanol (2:1 V/V) was introduced, followed by centrifugation at 800 g for 5 minutes. The resulting reaction product was measured at 532 nm.
Determination of nitric oxide levels
NO levels were measured using Griess reagent [16]. In short, 150 μL of sample was incubated at room temperature with 20 mL of Griess reagent and 130 μL of distilled water for 30 minutes. NO levels were determined at 540 nm. Tissue NO levels were measured in μmol/g tissue.
Tumor Necrosis Factor-alpha (TNF-α) assay
TNF-ɑ levels were measured using a commercial rat TNF-ɑ ELISA kit according to the manufacturer’s protocols (CRE0003; Biotechnics). The TNF-α level in tissue homogenates was expressed as picogram/mg of protein [3].
Data analysis
The data underwent analysis through a two-way analysis of variance (ANOVA), followed by Bonferroni's post hoc test for group comparisons. Results were presented as mean ± SEM, and statistical significance was determined at a level of p-value < 0.05.
Results
Locomotor activity
This parameter was affected by LPS treatment (F(3.24) = 50.25, p < 0.001), and no effect of sex (F(1.24) = 0.7, p > 0.05) was noted. Our results showed, in the OFT, rats in the LPS P1 group displayed a statistically significant increase in the total number of visited squares in comparison with control groups (p < 0.001 for both genders) and only male rats in the LPS P3 group exhibited a statistically significant decrease in the total number of visited squares (p < 0.01). The LPS P5 group did not behave differently from controls. Gender differences in this parameter were generally absent, except for females in the LPS P1 group, who demonstrated a noteworthy increase in the total number of visited squares compared to their male counterparts (p < 0.05) (Fig. 2a).
Behavioral performances of male and female rats in the open field test. A Number of total squares visited (NTS). B Number of returns into center (NRC) or revisited central squares. C Total amount of time spent in the central area (TCA), measured in seconds. The data are presented as means ± SEM. *p < 0.05 vs. control group; and $p < 0.05 vs. gender counterpart
Measures of anxious behaviors
Open field test
In terms of numbers of returns to the center, the treatment factor (F(1.24) = 56.68, p < 0.001) and the sex factor (F(3.24) = 7.93, p < 0.001) significantly affected the NRC. As shown in Fig. 2b, male and female rats in the LPS P3 and LPS P5 groups displayed a statistically significant decrease in the number of returns to the central area (for males: p < 0.01 in the LPS P3 and p < 0.05 in the LPS P5 groups; for females: p < 0.05 in the LPS P3 and LPS P5 groups). This came in opposition to females in the LPS P1 group which displayed an elevated number of returns to the center in comparison with their female control and male counterparts (p < 0.001) (Fig. 2b). Similarly, the treatment factor (F(1.24) = 25.50, p < 0.0001) and the sex factor (F(3.24) = 10.57, p = 0.001) significantly affected the TCA. In concordance with these results, rats in the LPS P3 group in both genders spent less time than controls in the central area of the open field (p < 0.01 for males in the LPS P3 group and p < 0.05 for females), and males in the LPS P5 group also spent less time in the central area p < 0.05, whereas LPS P1 females spent the longest period as compared to controls and male counterparts (p < 0.001) (Fig. 2c). For the 2 parameters in this assessment, male rats in the LPS P1 group did not behave differently from controls. Gender differences were noted for these two parameters: females in the LPS P1 group exhibited a significant increase in the number of returns and the time spent in the central area in comparison with their male counterparts (p < 0.01). Also, males in the LPS P3 exhibited a significant decrease in these two parameters in comparison with their female counterparts (p < 0.05).
Elevated plus-maze test
Results of the other parameters assessing for anxious behaviors were more uniform. In the EPM test, statistical analysis showed that EOA was significantly affected by the LPS treatment (F(3.24) = 14.38, p < 0.0001). When compared to their respective controls, males and females in the LPS P3 and LPS P5 groups displayed a statistically significant decrease in the number of entries in the open arms (p < 0.001 for males in LPS P3 group and p < 0.05 in the LPS P5 group, p < 0.05 for females in the LPS P3 and LPS P5 groups) (Fig. 3a) and a decreased amount of time spent in these arms (p < 0.01 for males in the LPS P1 and LPS P3 groups, p < 0.05 for males in the LPS P5 group, and p < 0.05 for females in LPS P1 and LPS P3 groups) (Fig. 3b). No gender differences were noted in this experiment (F(1.24) = 02.07, p > 0.05), except for males in the LPS P3 group which exhibited a significant decrease in the two parameters as compared to their female counterparts (p < 0.05) (Fig. 3b).
Behavioral performance of male and female rats measured in the elevated plus maze test. A Number of entries in the open arms (EOA). B Total amount of time spent in the open arms (TOA), measured in seconds. The data are presented as means ± SEM. *p < 0.05 vs. control group; and $p < 0.05 vs. gender counterpart
Novelty-suppressed feeding test
Finally, in the novelty suppressed feeding test, the treatment factor (F(3.24) = 31.01, p < 0.001) and the sex factor (F(3.24) = 9.12, p = 0.0059) significantly affected the LF. Males in the three experimental groups showed a statistically significant increase in their latency feeding time (p < 0.01 in the LPS P1 and LPS P3 groups, p < 0.05 in the LPS P5 group) compared to control group, and for females only LPS P1 (p < 0.001) and LPS P3 (p < 0.01) groups displayed a statistically significant increase in their latency feeding time as shown in (Fig. 4). No gender differences were noted in this experiment.
Measure of depressive-like behaviors
The TIM in the FST (measured in seconds) was used to evaluate depressive-like behaviors. Statistical analysis showed that TIM was significantly affected by the treatment factor (F(3.24) = 31.30, p < 0.0001) and the sex factor (F(3.24) = 8.32, p = 0.0081) . A general pattern of prolonged TIM was observed in both genders subjected to LPS administration in comparison with controls (p < 0.001 for males in the LPS P1, p < 0.01 in the LPS P3 and p < 0.05 in the LPS P5 group; and for females: p < 0.05 in the LPS P1 and LPS P5 groups and p < 0.001 in the LPS P3) in comparison to controls (Fig. 5).
Behavioral performance of male and female rats measured in the forced swimming test. Total time of immobility (TI), measured in seconds. The results are represented as means ± the mean standard error (SEM). The data are presented as means ± SEM. *p < 0.05 vs. control group; and $p < 0.05 vs. gender counterpart
Statistically significant gender differences were noted at two time periods: at postnatal day 1, males had a higher time of immobility in comparison with their female counterparts (p < 0.05), whereas at postnatal day 3, females exhibited a more elevated increase on this parameter (p < 0.01) (Fig. 5).
Biochemical analyses
Lipid peroxidation in the prefrontal cortex and hippocampus
The treatment factor (F(3.24) = 29.70, p < 0.0001) and the sex factor (F(3.24) = 1.24, p = 0.089) significantly affected the LPO. In the three LPS groups, rats of both genders displayed higher MDA levels in their PFC (p < 0.01 for males in the three LPS P1 and LPS P3 groups, p < 0.05 in the LPS P5 group, p < 0.01 for females in the LPS P1 and P5 groups and p < 0.001 in the LPS P3 group) as compared to controls (Fig. 6a), in the HP also the three groups in both genders exhibited higher MDA levels (p < 0.01 for males in the three LPS groups, p < 0.05 in the LPS P5 group, p < 0.001 for females in the LPS P3 group, p < 0.01 in the LPS P1 group and p < 0.05 in the LPS P5 group) as compared to controls (Fig. 6b). A statistically notable distinction between genders was observed (F(1.24) = 9.54, p < 0.005): female rats had more elevated MDA levels in opposition to their male counterparts in the LPS P3 group in the PFC and HP p < 0.05.
Detection of lipid peroxidation (MDA) level by TBARS in the prefrontal cortex (PFC), and hippocampus (HP) of male and female rats. A LPO level in the PFC. B LPO level in the HP. Levels are expressed in nmol/mg of protein. The data are presented as means ± SEM. *p < 0.05 vs. control group; and $p < 0.05 vs. gender counterpart
Levels of nitric oxide and superoxide dismutase activity in the prefrontal cortex and hippocampus
NO levels were affected by LPS treatment (F(3.24) = 15.36, p < 0.0001), and no effect of sex (F(1.24) = 7.92, p = 0.15) was noted. NO levels were significantly more elevated in male and female rats subjected to LPS administration in comparison with controls; this was observed in both the PFC (p < 0.01 for both genders in the LPS P1 and LPS P3 groups and p < 0.05 in the LPS P5 group) (Fig. 7a) and the HP (p < 0.01 for males in the three LPS groups, p < 0.001 for females in the LPS P3 group and p < 0.01 in the LPS P1 and LPS P5 groups) (Fig. 7b) (p < 0.001). A gender effect was noted (F(1.24) = 7.92, p = 0.0096, as female rats in the LPS P1 and LPS P3 exhibited a statistically significant increase in NO levels in both brain parts as compared to males (p < 0.05).
Concentration of nitric oxide (NO) level in the prefrontal cortex (PFC) and hippocampus (HP) of male and female rats. A NO level in the PFC. B NO level in the HP. Levels are expressed in μmol/mg of protein. The results are represented as means ± the mean standard error (SEM). The data are presented as means ± SEM. *p < 0.05 vs. control group; and $p < 0.05 vs. gender counterpart
Alternatively, SOD activity was affected by LPS treatment (F(3.24) = 31.78, p < 0.0001), there was a statistically significant reduction in SOD activity observed in the PFC of rats of both genders in the three LPS groups (p < 0.01 for males in the LPS P1 and LPS P3 groups, p < 0.05 in the LPS P5 group, p < 0.001 for females in the LPS P3 group (Fig. 8a) and HP (p < 0.01 for males and females in the LPS P1 and LPS P3 groups, and p < 0.05 in the LPS P5) compared to controls (Fig. 8b). Compared to controls, Females in the LPS P3 group had a characteristically lower SOD activity and higher NO level in their PFC compared to males (p < 0.05).
Superoxide dismutase (SOD) activity in the prefrontal cortex (PFC) and hippocampus (HP) of male and female rats. A SOD activity in the PFC. B SOD activity in the HP. Activity is expressed in units/min/mg of protein. The results are represented as means ± the mean standard error (SEM). The data are presented as means ± SEM. *p < 0.05 vs. control group; and $p < 0.05 vs. gender counterpart
Tumor necrosis factor alpha level in the prefrontal cortex and hippocampus
TNF-α levels were affected by LPS treatment (F(3.24) = 22.34, p < 0.0001), and no effect of sex (F(1.24) = 0.01, p = 0.89) was noted. All rats in the experimental groups demonstrated a statistically significant increase in their TNF-α levels in the PFC (p < 0.01 for males in the LPS P1 and LPS P3 groups, p < 0.05 in the LPS P5 group, p < 0.001 for females in the LPS P3 group and p < 0.01 in the LPS P1 and LPS P5 groups) (Fig. 9a) and HP (p < 0.001 for males in the LPS P1 group, p < 0.01 LPS P3 and LPS P5 groups, p < 0.001 for females in the LPS P1 and LPS P3 groups and p < 0.01 in the LPS P5) (Fig. 9b) compared to controls. No gender variations were noted (F(1.24) = 2.82, p = 0.14). Except for LPS P3 females had a higher HP TNF-α level as opposed to their male counterparts (p < 0.05).
Tumor Necrosis Factor-alpha (TNF-α) level in the prefrontal cortex (PFC) and hippocampus (HP) of male and female rats. A TNF-α level in the PFC. B TNF-α level in the HP. Levels are expressed in pg/mg of protein. The data are presented as means ± SEM. *p < 0.05 vs. control group; and $p < 0.05 vs. gender counterpart
Discussion
Stress can be considered as a real or perceived threat to an individual's physiological integrity, leading to behavioral reactions [32]. In rodents, most of the brain growth occurs in the first two weeks postnatally, with the growth spurt reaching its peak around the eighth day [57]. The administration of LPS in rats during this critical period of development is considered a prototype of neonatal neuroinflammation, with lifelong molecular and behavioral changes [17, 19, 52]. It also impairs the process of producing long-term potentiation in hippocampal tissue sections [52]. As such, It was suggested that LPS played an essential role in neurodegenerative and psychiatric disorders, not only in rodents [38, 60] but also in humans [59].
In this experiment, we evaluated the affective and cognitive disorders in male and female Wistar rats following the application of LPS at different postnatal periods (PND 1, 3 and 5). To the best of our knowledge, no work has previously examined the impact of LPS administration at different postnatal periods, as outlined in our methodology, on depression and anxiety levels in adulthood. We noted that, in general, LPS administration produces uniform anxiogenic behavior, a depressive response, and increased oxidative and inflammatory stress across all groups as compared to controls. Males receiving an LPS injection at postnatal day 1 and female rats receiving the injection at days 3 and 5 displayed more anxiogenic and depressive-like behaviors than their gender counterparts. Females displayed higher oxidative and inflammatory biomarker levels as compared to males, with results being more pronounced in groups receiving the LPS injection at days 3 and 5. Our findings align with earlier preclinical research emphasizing the significance of sex differences at baseline. This suggests that females may be more susceptible to the adverse effects of stress on mood and anxiety-related behaviors [1]. The locomotor activity test, which measures the exploratory behavior and general activity of animals, provides an initial screen for anxiety-related behaviors in the open field and elevated plus maze (avoiding the open arm) tests in rodents [47]. Brain activity leads to increased cerebral metabolism and central neuronal excitation, which in turn causes locomotion activation [13]. Therefore, early life exposure to LPS can stir anxiety-like behaviors in adult rats increasing locomotor activity. In our study, this was more pronounced in females. In the forced swim test, the indicator of depression is immobility time [35]. Depression in the female LPS groups was more pronounced at almost all postnatal exposure periods compared to controls as indicated by the performance on the forced swim test. This may have been underlined by various disruptions in physiological processes. Reported harmonious results, pointing towards a more depressive pattern in females compared to males. An overwhelmed oxidative state (increased MDA and decreased SOD activity) in the PFC and HP throughout all groups, along with a heightened nitric oxide level, can explain the observed neurobehavioral display. The redox and nitric oxide status in females revealed that they had higher levels of oxidative stress as compared to their male counterparts, further corroborating the heightened depressive-like response in the female groups [9, 33].
Cytotoxicity, transcription factor activation, antiviral activity, and immune response regulation are just a few of the consequences that TNF-alpha exhibits. Measuring the levels of the TNF-alpha after early exposure to E-coli LPS showed an increase in TNF-alpha levels at both PFC and HP tissue levels with no noted gender variation. Our data, therefore, suggest the involvement of TNF-α in the pathophysiology of depression-like activity displayed by the exposed rats; this range aligns with the report from, in which increased TNF-α in the PFC and HP followed an early day infection with pneumococcal meningitis. The inflammatory activation process may be underlined by the overwhelmed antioxidant levels in these tissues [2].
Alternatively, the outcomes of this study correlate with those of Walker et al. In his study, both male and female Wistar rats exposed to 500 μg/kg of LPS (Salmonella enterica, serotype Enteritidis) on days 3 and 5 postnatally exhibited an increase in hippocampal interleukin-1β and TNF-α levels following adulthood (85 days) restraint stress [54]. Hippocampal TNF-α levels showed a gender-based variation, with higher levels being observed in males. This finding has yet been limited by a possible transient increase in TNF-α content in the female group, which diminished before central nervous tissues were collected for analysis [54]. The biochemical findings of this investigation are consistent with our observations of a persistent rise in inflammation and oxidative stress, revealing that postnatal LPS exposure causes persistent injuries to the immune and central inflammatory systems, which ultimately results in long-lasting neuropsychiatric disabilities. Contributing to this view, our previous work showed that neonatal exposure to LPS (PND1) increased anxiety-like and depression-like behaviors during adulthood, which were associated with increased oxidative stress and neuroinflammation in the hippocampus, reflected by increased levels of free radicals and TNFα [3]. As known, high oxygen metabolism can produce the overproduction of free radicals, which, in excess, could be damaging many essential biomolecules vital to the normal activity of neuronal cells (e.g. proteins, DNA and membrane lipids) [30], and triggering the overexpression of pro-inflammatory cytokines causing neuroinflammation, such as TNFα [18]. On the other hand, inflammation can also intensify oxidative stress by recruiting leukocytes and generating free radicals.
Some other recent projects that further corroborate the results from this study, although experimented at different dose and age of exposure, include evidence that LPS administration in rodents not only activates the immune system, but stimulates the production of inflammatory cytokines (interleukin 1-β, interleukin 6, and TNF-α) in the central nervous system [44] and also alters the central monoamines [20]. For instance, studies conducted in vivo have demonstrated that an injection of LPS raises the extracellular levels of serotonin in the HP and PFC, two central nervous system areas that have been implicated in the pathophysiology of depression and anxiety [36]. Postnatal exposure to LPS can also injure the nigrostriatal dopaminergic system, as indicated by a decrease in the expression of tyrosine hydroxylase in neurons in the substantia nigra, along with an impairment of the nigrostriatal neuronal connectivity and axonal injury to the dorsal HP [26]. Analogous results have been described by Bland et al. who showed that an E-coli-based LPS injection on PND 4 causes a decrease in the total number of neurons in the PFC of adult rats [11].
Reported gender differences in behavioral and biochemical responses after LPS administration may stem from the sexual dimorphism and differences in the pathophysiological systems between males and females [27]. For instance, there have been records of sex-based physiological differences in depression [51]. Female susceptibility to mood disorders has been linked to surges in gonadal hormones [1] and further supported by the differences in the path of interaction between serotonin and estrogen. A combination of any of these fundamental events may underline the sexual dimorphism observed in the anxiety- and depressive-like behaviors observed in our results.
When comparing the outcomes of LPS administration in studies employing rodent models, the time of administration, the dose of LPS, and the strain of associated bacteria must be considered. In the current experiment, we used LPS extracted from E. coli, which is commonly used in this type of rodent-based protocols. The period of outcome assessment (i.e., acutely after the exposure to the injection versus chronically) denotes another critical element that influences the interpretation of any obtained results. Keeping in mind both factors, we present in Table 1 a comparison of the studies assessing the neuropsychiatric effects of LPS administration in rat models, in the function of the dose of the injection, its time of administration (up to 14 days after birth), the derived strain, and the period of outcome assessment. Most of the reported studies show a unified pattern of increase in anxiety and depressive-like behaviors as a consequence of this subtype of early-life adversity. Gender dimorphism in susceptibility to stress-induced anxiety and depression is also probably mediated by the abovementioned differences in methodology (Table 1).
Conclusions
The study suggests evidence for a uniform increase in anxious parameters, depressive-like behaviors, and central nervous system oxidative stress and inflammation in adult Wistar rats after postnatal exposure to LPS. This is coupled with a pattern of dimorphic gender response, with more severe outcomes observed in female rats. Response to LPS administration seems to be modulated by several variables, including the gender of the animal model and the timing of the exposure, among other variables. Further investigations of the interplay between LPS administration and the timeline of the injection would strongly benefit from a head-to-head comparison between the different exposure groups. This would help identify the most vulnerable window period during which the application of an inflammatory stressor triggers worst future outcomes. This can, therefore, provide a fundamental basis for understanding future consequences of inflammation required to trigger neuropsychiatric sequelae.
Data availability
The manuscript has no associated data.
Change history
21 June 2024
A Correction to this paper has been published: https://doi.org/10.1007/s11055-024-01621-x
References
Bale TL, Epperson CN (2015) Sex differences and stress across the lifespan. Nat Neurosci 18(10):1413–1420. https://doi.org/10.1038/nn.4112
Barichello T, Dos Santos I, Savi GD, Florentino AF, Silvestre C, Comim CM, Feier G, Sachs D, Teixeira MM, Teixeira AL, Quevedo J (2009) Tumor necrosis factor alpha (TNF-α) levels in the brain and cerebrospinal fluid after meningitis induced by Streptococcus pneumoniae. Neuroscience Letters 467(3):217–219. https://doi.org/10.1016/j.neulet.2009.10.039
Benmhammed H, El Hayek S, Nassiri A, Bousalham R, Mesfioui A, Ouichou A, El Hessni A (2019) Effects of lipopolysaccharide administration and maternal deprivation on anxiety and depressive symptoms in male and female Wistar rats: Neurobehavioral and biochemical assessments. Behavioural Brain Research 362:46–55. https://doi.org/10.1016/j.bbr.2019.01.005
Bennet L, Gunn AJ (2006) The Fetal Origins of Adult Mental Illness. In: Wintour EM, Owens JA (eds) Early Life Origins of Health and Disease. Springer US, Boston, MA, pp 204–218
Benson S, Brinkhoff A, Lueg L, Roderigo T, Kribben A, Wilde B, Witzke O, Engler H, Schedlowski M, Elsenbruch S (2017) Effects of acute systemic inflammation on the interplay between sad mood and affective cognition. Transl Psychiatry 7(12):1281. https://doi.org/10.1038/s41398-017-0043-0
Berkiks I, Benmhammed H, Mesfioui A, Ouichou A, El Hasnaoui A, Mouden S, Touil T, Bahbiti Y, Nakache R, El Hessni A (2018a) Postnatal melatonin treatment protects against affective disorders induced by early-life immune stimulation by reducing the microglia cell activation and oxidative stress. International Journal of Neuroscience 128(6):495–504. https://doi.org/10.1080/00207454.2017.1398156
Berkiks I, Boulbaroud S, Garcia-Segura LM, Mesfioui A, Ouichou A, Mouden S, Benmhammed H, El Hasnaoui A, Nakache R, Bahbiti Y, El Hessni A (2018b) Thymelaea lythroides extract attenuates microglial activation and depressive-like behavior in LPS-induced inflammation in adult male rats. Biomedicine & Pharmacotherapy 99:655–663. https://doi.org/10.1016/j.biopha.2018.01.125
Berkiks I, Garcia-Segura LM, Nassiri A, Mesfioui A, Ouichou A, Boulbaroud S, Bahbiti Y, Lopez-Rodriguez AB, Hasnaoui E, El Hessni A (2019) The sex differences of the behavior response to early Life immune stimulation: Microglia and astrocytes involvement. Physiology & Behavior 199:386–394. https://doi.org/10.1016/j.physbeh.2018.11.037
Bernardi MM, Teixeira LP, Ligeiro-de-Oliveira AP, Tavares-de-Lima W, Palermo-Neto J, Kirsten TB (2014) Neonatal lipopolysaccharide exposure induces sexually dimorphic sickness behavior in adult rats. Psychology & Neuroscience 7(2):113–123. https://doi.org/10.3922/j.psns.2014.007
Bilbo SD (2009) Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci 3. https://doi.org/10.3389/neuro.08.014.2009
Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, Bilbo SD (2010) Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain, Behavior, and Immunity 24(3):329–338. https://doi.org/10.1016/j.bbi.2009.09.012
Bourin M, Hascoët M (2003) The mouse light/dark box test. European Journal of Pharmacology 463(1–3):55–65. https://doi.org/10.1016/S0014-2999(03)01274-3
Brown P (2007) Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Current Opinion in Neurobiology 17(6):656–664. https://doi.org/10.1016/j.conb.2007.12.001
Brunton PJ (2015) Programming the Brain and Behaviour by Early-Life Stress: A Focus on Neuroactive Steroids. J Neuroendocrinol 27(6):468–480. https://doi.org/10.1111/jne.12265
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti J-F, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R (2007) Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 56(7):1761–1772. https://doi.org/10.2337/db06-1491
Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK (1992) Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol 149(8):2736–2741
Claypoole LD, Zimmerberg B, Williamson LL (2017) Neonatal lipopolysaccharide treatment alters hippocampal neuroinflammation, microglia morphology and anxiety-like behavior in rats selectively bred for an infantile trait. Brain, Behavior, and Immunity 59:135–146. https://doi.org/10.1016/j.bbi.2016.08.017
Csiszar A, Wang M, Lakatta EG, Ungvari Z (2008) Inflammation and endothelial dysfunction during aging: role of NF-κB. Journal of Applied Physiology 105(4):1333–1341. https://doi.org/10.1152/japplphysiol.90470.2008
Custódio CS, Mello BSF, Filho AJMC, De Carvalho Lima CN, Cordeiro RC, Miyajima F, Réus GZ, Vasconcelos SMM, Barichello T, Quevedo J, De Oliveira AC, De Lucena DF, Macedo DS (2017) Neonatal Immune Challenge with Lipopolysaccharide Triggers Long-lasting Sex- and Age-related Behavioral and Immune/Neurotrophic Alterations in Mice: Relevance to Autism Spectrum Disorders. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0616-1
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9(1):46–56. https://doi.org/10.1038/nrn2297
Depino AM (2015) Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience 299:56–65. https://doi.org/10.1016/j.neuroscience.2015.04.065
Doenni VM, Song CM, Hill MN, Pittman QJ (2017) Early-life inflammation with LPS delays fear extinction in adult rodents. Brain, Behavior, and Immunity 63:176–185. https://doi.org/10.1016/j.bbi.2016.11.022
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431. https://doi.org/10.1016/0076-6879(90)86135-i
El Brouzi MY, Lamtai M, Zghari O, Ouakki S, Azizoun I, El Hessni A, Mesfioui A, Ouichou A (2021) Intrahippocampal Effects of Nickel Injection on the Affective and Cognitive Response in Wistar Rat: Potential Role of Oxidative Stress. Biol Trace Elem Res 199(9):3382–3392. https://doi.org/10.1007/s12011-020-02457-5
Engler H, Benson S, Wegner A, Spreitzer I, Schedlowski M, Elsenbruch S (2016) Men and women differ in inflammatory and neuroendocrine responses to endotoxin but not in the severity of sickness symptoms. Brain, Behavior, and Immunity 52:18–26. https://doi.org/10.1016/j.bbi.2015.08.013
Fan L-W, Tien L-T, Lin RCS, Simpson KL, Rhodes PG, Cai Z (2011) Neonatal exposure to lipopolysaccharide enhances vulnerability of nigrostriatal dopaminergic neurons to rotenone neurotoxicity in later life. Neurobiology of Disease 44(3):304–316. https://doi.org/10.1016/j.nbd.2011.07.011
Fish EN (2008) The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 8(9):737–744. https://doi.org/10.1038/nri2394
Freitas RM, Sousa FCF, Vasconcelos SMM, Viana GSB, Fonteles MMF (2004) Pilocarpine-induced status epilepticus in rats: lipid peroxidation level, nitrite formation, GABAergic and glutamatergic receptor alterations in the hippocampus, striatum and frontal cortex. Pharmacology Biochemistry and Behavior 78(2):327–332. https://doi.org/10.1016/j.pbb.2004.04.004
Garcia AMB, Cardenas FP, Morato S (2005) Effect of different illumination levels on rat behavior in the elevated plus-maze. Physiology & Behavior 85(3):265–270. https://doi.org/10.1016/j.physbeh.2005.04.007
Harman D (1956) Aging: A Theory Based on Free Radical and Radiation Chemistry. Journal of Gerontology 11(3):298–300. https://doi.org/10.1093/geronj/11.3.298
Harvey L, Boksa P (2012) Prenatal and postnatal animal models of immune activation: Relevance to a range of neurodevelopmental disorders. Devel Neurobio 72(10):1335–1348. https://doi.org/10.1002/dneu.22043
Hostinar CE (2015) Recent developments in the study of social relationships, stress responses, and physical health. Current Opinion in Psychology 5:90–95. https://doi.org/10.1016/j.copsyc.2015.05.004
Kokras N, Antoniou K, Mikail HG, Kafetzopoulos V, Papadopoulou-Daifoti Z, Dalla C (2015) Forced swim test: What about females? Neuropharmacology 99:408–421. https://doi.org/10.1016/j.neuropharm.2015.03.016
Lamtai M, Ouakki S, Zghari O, Hamzaoui AE, Benmhammed H, Azirar S, Hessni AE, Mesfioui A, Ouichou A (2020) Neuroprotective effect of melatonin on nickel-induced affective and cognitive disorders and oxidative damage in rats. Environ Anal Health Toxicol 35(4):e2020025. https://doi.org/10.5620/eaht.2020025
Liao J-C, Tsai J-C, Liu C-Y, Huang H-C, Wu L-Y, Peng W-H (2013) Antidepressant-like activity of turmerone in behavioral despair tests in mice. BMC Complement Altern Med 13(1):299. https://doi.org/10.1186/1472-6882-13-299
Linthorst AC, Reul JM (1998) Brain neurotransmission during peripheral inflammation. Ann N Y Acad Sci 840:139–152. https://doi.org/10.1111/j.1749-6632.1998.tb09558.x
Lu Y-C, Yeh W-C, Ohashi PS (2008) LPS/TLR4 signal transduction pathway. Cytokine 42(2):145–151. https://doi.org/10.1016/j.cyto.2008.01.006
Ma M, Ren Q, Yang C, Zhang J, Yao W, Dong C, Ohgi Y, Futamura T, Hashimoto K (2017) Antidepressant effects of combination of brexpiprazole and fluoxetine on depression-like behavior and dendritic changes in mice after inflammation. Psychopharmacology 234(4):525–533. https://doi.org/10.1007/s00213-016-4483-7
Majidi-Zolbanin J, Azarfarin M, Samadi H, Enayati M, Salari A-A (2013) Adolescent fluoxetine treatment decreases the effects of neonatal immune activation on anxiety-like behavior in mice. Behavioural Brain Research 250:123–132. https://doi.org/10.1016/j.bbr.2013.05.003
Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI (2015) Sex Differences in Depressive and Socioemotional Responses to an Inflammatory Challenge: Implications for Sex Differences in Depression. Neuropsychopharmacol 40(7):1709–1716. https://doi.org/10.1038/npp.2015.17
Naïla N, Makthar W, Lamtai M, Zghari O, Aboubaker EH, Abdelhalem M, Ali O (2021) Effect of intra-hippocampal lead injection on affective and cognitive disorders in male WISTAR rats: Possible involvement of oxidative stress. E3S Web Conf 319:02017. https://doi.org/10.1051/e3sconf/202131902017
O’Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR (2009) To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain, Behavior, and Immunity 23(7):887–897. https://doi.org/10.1016/j.bbi.2009.04.005
O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho A-M, Quigley EMM, Cryan JF, Dinan TG (2009) Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses. Biological Psychiatry 65(3):263–267. https://doi.org/10.1016/j.biopsych.2008.06.026
Pitossi F, del Rey A, Kabiersch A, Besedovsky H (1997) Induction of cytokine transcripts in the central nervous system and pituitary following peripheral administration of endotoxin to mice. J Neurosci Res 48(4):287–298. https://doi.org/10.1002/(sici)1097-4547(19970515)48:4<287::aid-jnr1>3.0.co;2-7
Pitychoutis PM, Papadopoulou-Daifoti Z (2010) Of depression and immunity: does sex matter? Int J Neuropsychopharm 13(05):675–689. https://doi.org/10.1017/S1461145710000465
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: A new model sensitive to antidepressant treatments. European Journal of Pharmacology 47(4):379–391. https://doi.org/10.1016/0014-2999(78)90118-8
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European Journal of Pharmacology 463(1–3):3–33. https://doi.org/10.1016/S0014-2999(03)01272-X
Sominsky L, Walker AK, Ong LK, Tynan RJ, Walker FR, Hodgson DM (2012) Increased microglial activation in the rat brain following neonatal exposure to a bacterial mimetic. Behavioural Brain Research 226(1):351–356. https://doi.org/10.1016/j.bbr.2011.08.038
Spatz Widom C, DuMont K, Czaja SJ (2007) A Prospective Investigation of Major Depressive Disorder and Comorbidity in Abused and Neglected Children Grown Up. Arch Gen Psychiatry 64(1):49. https://doi.org/10.1001/archpsyc.64.1.49
Spencer SJ, Heida JG, Pittman QJ (2005) Early life immune challenge—effects on behavioural indices of adult rat fear and anxiety. Behavioural Brain Research 164(2):231–238. https://doi.org/10.1016/j.bbr.2005.06.032
Sramek JJ, Murphy MF, Cutler NR (2016) Sex differences in the psychopharmacological treatment of depression. Dialogues Clin Neurosci 18(4):447–457. https://doi.org/10.31887/DCNS.2016.18.4/ncutler
Tishkina A, Stepanichev M, Kudryashova I, Freiman S, Onufriev M, Lazareva N, Gulyaeva N (2016) Neonatal proinflammatory challenge in male Wistar rats: Effects on behavior, synaptic plasticity, and adrenocortical stress response. Behavioural Brain Research 304:1–10. https://doi.org/10.1016/j.bbr.2016.02.001
Walker AK, Nakamura T, Byrne RJ, Naicker S, Tynan RJ, Hunter M, Hodgson DM (2009) Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: Implications for the double-hit hypothesis. Psychoneuroendocrinology 34(10):1515–1525. https://doi.org/10.1016/j.psyneuen.2009.05.010
Walker AK, Nakamura T, Hodgson DM (2010) Neonatal lipopolysaccharide exposure alters central cytokine responses to stress in adulthood in Wistar rats. Stress 13(6):506–515. https://doi.org/10.3109/10253890.2010.489977
Walker FR, March J, Hodgson DM (2004) Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behavioural Brain Research 154(1):63–69. https://doi.org/10.1016/j.bbr.2004.01.019
Wang K-C, Fan L-W, Kaizaki A, Pang Y, Cai Z, Tien L-T (2013) Neonatal lipopolysaccharide exposure induces long-lasting learning impairment, less anxiety-like response and hippocampal injury in adult rats. Neuroscience 234:146–157. https://doi.org/10.1016/j.neuroscience.2012.12.049
Watson RE, DeSesso JM, Hurtt ME, Cappon GD (2006) Postnatal growth and morphological development of the brain: a species comparison. Birth Defect Res B 77(5):471–484. https://doi.org/10.1002/bdrb.20090
Zghari O, Azirar S, Lamtai M, El Hessni A, Ouichou A, Mesfioui A (2023) Melatonin counteracts aluminum-induced affective and cognitive disorders and oxidative damage in male wistar rats. Neurosci Behav Physi 53(6):917–928. https://doi.org/10.1007/s11055-023-01465-x
Zhan X, Stamova B, Jin L-W, DeCarli C, Phinney B, Sharp FR (2016) Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 87(22):2324–2332. https://doi.org/10.1212/WNL.0000000000003391
Zhang X-Y, Cao J-B, Zhang L-M, Li Y-F, Mi W-D (2015) Deferoxamine attenuates lipopolysaccharide-induced neuroinflammation and memory impairment in mice. J Neuroinflammation 12(1):20. https://doi.org/10.1186/s12974-015-0238-3
Acknowledgments
We would like to thank Miss Natalia Yanguas Casás and Doctor Luis Miguel Garcia Segura for providing us with the appropriate protocol for tissue homogenization for ELISA and TNF-α assay.
Funding
No fund was received or no funding organization was involved in this study.
Author information
Authors and Affiliations
Contributions
Hajar Benmhammed and Mouloud Lamtai performed the experiments, analyzed the data and wrote the paper. Abdelhalem Mesfioui and Abdeljabbar Nassiri participated in behavioral analysis and statistical significance. Samira Mouden and Samir Bikri reviewed and provided comments on the content and interpretation of the manuscript. Aboubaker El Hessni supervised the work, revised and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Ethics Committee (Local Institutional Research Committee)
Conflict of interest
There are no conficts of interests with authors or other organizations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Benmhammed, H., Lamtai, M., Mesfioui, A. et al. Effects of lipopolysaccharide administration at different postnatal periods on behavioral and biochemical assessments in Wistar rats. Neurosci Behav Physi 54, 357–373 (2024). https://doi.org/10.1007/s11055-024-01584-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-024-01584-z