Abstract
Vascular dementia is the second most common cause of dementia in older people and is characterized by the sudden onset of impairments in thinking skills and behavior, which generally occur following a stroke. Unfortunately, effective therapy for vascular dementia remains inadequate. Erythropoietin (EPO) is a glycoprotein hormone that controls erythropoiesis, or red blood cell production. Recently, a prominent role for EPO has been defined in the nervous system, and there is growing interest in the potential therapeutic use of EPO for neuroprotection. However, whether it is protective from memory impairments and the underlying mechanisms of vascular dementia (VD) remains unknown. In the current study, we reported that supplements with exogenous erythropoietin (EPO) for 4 weeks could restore impaired memory in 2-vessel occlusion (2VO) rats, a well-established vascular dementia animal model. EPO also rescued impairments in dendritic spines and cholinergic dysfunctions in the hippocampus. Moreover, EPO suppressed the overactivation of GSK-3β in the hippocampus by stimulating the JAK2/STAT5/PI3K/Akt signal pathway. Furthermore, we found that genetic knockdown of the EPO receptor (EPO-R) by shRNA blocks the neuroprotection conferred by EPO on memory in VD. We hypothesized that EPO treatment is able to rescue the memory impairments in VD by stimulating the EPO-R/JAK2/STAT5/PI3K/Akt/GSK-3β pathway and suggest the potential usage of EPO in the therapy for VD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular dementia refers to a mild and gradual worsening of learning/memory and other cognitive functions presumed to be due to vascular disorders within the brain. It is the second most common cause of dementia, and patients often present with symptoms similar to those of Alzheimer’s disease (AD). However, the related changes in the brain are not due to AD pathology but to chronic reduced blood flow in the brain, eventually resulting in dementia [1]. Unlike AD, there are no licensed treatments for vascular dementia in the world.
Chronic cerebral hypoperfusion (CCH) has been considered to be a critical reason for the pathogenesis of cognitive decline in VD [2]. CCH mimics the pathological initiation of VD by performing the permanent occlusion of bilateral common carotid arteries. Previous studies have revealed that CCH leads to cognitive deficits, as assessed by the water maze task and step-down inhibitory avoidance [3]. In addition to apparent neuronal loss in the hippocampus, determined by Nissl staining, NeuN immunostaining, and cleaved caspase-3 immunostaining [4], CCH is able to induce cholinergic abnormalities, such as a reduction in the levels of acetylcholine (ACh) and the number of muscarinic acetylcholine receptors [5,6,7]. Furthermore, CCH increased hyperphosphorylation of the microtubule-associated protein tau and caused imbalances in the phosphorylation system by activating glycogen synthase kinase 3β (GSK-3β) and Akt [8]. Thus, suppression of GSK-3β activity is a promising therapeutic approach for CCH-related memory deficit.
Erythropoietin (EPO) is a glycoprotein hormone that controls erythropoiesis by acting as a cytokine (protein signaling molecule) for erythrocyte, or red blood cell, precursors in the bone marrow, which are primarily found to be secreted from the kidneys [9]. In addition to hematopoietic cells, expression of the EPO receptor (EPO-R) and an EPO response are observed in other cell types, including neurons [10]. Recently, numerous experimental studies have suggested that erythropoietin (EPO) is an endogenous mediator of neuroprotection in various central nervous system disorders. For example, the expression of EPO and the EPO-R in the adult brain can be stimulated by stress and is regulated by oxygen supply because the receptor and ligand are upregulated after hypoxia or ischemia [11]. In a previous study, we reported that EPO attenuates memory deficits in aging rats by rescuing impairments due to oxidative stress and inflammation and by promoting BDNF release [12], which suggested that it is also able to protect against the memory decline observed in aged rats.
Here, we report that supplementing CCH rats with EPO for 4 consecutive weeks is able to rescue the memory decline in CCH rats. EPO also restores the loss of dendritic spines and dysfunction in cholinergic neurons in the hippocampus. In addition, we found that EPO inhibits the activation of GSK-3β by stimulating the EPO-R/JAK2/STAT5/PI3K/Akt/GSK-3β signaling pathway. These data suggest that EPO could play a neuroprotective role in vascular dementia.
Materials and Methods
Animals and Drug Treatment
A total of 40 male Wistar rats (280–300 g), 4–5 months old (Experimental Animal Center of Zhengzhou University), were kept at a temperature of 22 ± 3 °C in a 12-h light/dark cycle and were maintained under a maintenance rodent diet ad libitum throughout the study. The rats were randomly divided into the following four groups: sham operation plus oral vehicle treatment (Sham), 2-VO surgery plus oral vehicle treatment (2VO), 2-VO surgery plus oral EPO (5000 IU/Kg, Shanghai Kehua Biopharmacy Inc. Shanghai, China) treatment (2VO + EPO), and sham operation plus EPO treatment (EPO). The Institutional Animal Care and Use Committee approved this animal study. All procedures in this study were carried out in accordance with the recommendations in the guide for the care and use of laboratory animals of Zhengzhou University. All rats were sacrificed for biochemical studies 24 h after a step-down inhibitory avoidance behavioral task, as described below.
2-VO Surgery
2-VO surgery was performed as previously described [13, 14]. Briefly, the rats were anesthetized with 10% chloral hydrate (350 mg/kg, i.p.), and the bilateral common carotid arteries were gently separated from the carotid sheath and vagal nerves. Each artery was bi-ligated with 6–0 silk sutures, and then the arteries were cut between the two ligatures. The sham-operated controls received the same operation without ligation. During the surgical procedure, the animal’s body temperature was maintained at 36.5 ± 0.5 °C using a heating pad.
Morris Water Maze Test
The rats were tested in a Morris water maze (MWM) according to previous reports [15]. To exclude the impact of vision on this task, a visible platform task of the MWM was performed before the regular MWM task. The pool consisted of a circular tank (120 cm in diameter, 50 cm high) and was divided into four quadrants. It was filled with water (30 cm in height) made opaque by Chinese ink, and the water temperature was maintained at 21–22 °C. A black platform (10 cm in diameter) was located in the center of quadrant 3 (2 cm below the water surface). Prominent external cues attached to the curtain surrounding the wall were used to indicate the location of the platform. During the training stage, rats were required to locate the submerged platform by using external cues in the room. They were tested for four trials per day for 5 consecutive days. The rats were released from one of four possible starting points and allowed to search the platform for 60 s. The rats that could not find the platform within 60 s were guided to the platform and were allowed to stay on the platform for 30 s. The time to reach the platform was recorded using a video camera linked to an electronic imaging system. Training trials on day 7 were followed by a probe trial. For this, the platform was removed, and the search time in the quadrant previously containing the platform was measured for over 60 s. This provides a measurement for the retention of spatial memory and indicates whether a spatial strategy was used during the hidden platform training.
Step-Down Passive Avoidance Test
One day after the MWM test, the step-down passive avoidance test was performed according to a previously described method [16, 17]. Briefly, on the first day of the experiment, the rats were acclimated to the acquisition chamber. On the second day, the rats were gently placed on the wooden platform, and the latency to step-down (SDL) was recorded as the learning phase. When all four paws touched the grid, a low-level electric shock (0.3 mA, 3 s) was delivered. On days 1, 3, and 7 after shock delivery, the rats’ step-down latencies were measured (maximum 300 s) while no shock was applied.
Golgi Staining
Golgi staining was performed as previously described [15]. Briefly, tissue slices (approximately 4 mm thick) were placed in the solution containing 5% chloral hydrate, 5% potassium dichromate, and 10% formalin in ddH2O for 3 days and were then subjected to 1% silver nitrate under a continuous vacuum for 4 days. After that, the brain tissues were cut into 40 μm thick sections with a vibratome and analyzed using a light microscope (Olympus BX60, Tokyo, Japan) with a 100X objective lens. The number of dendritic spines and the percentage of mushroom-like spines on the hippocampal CA1 pyramidal neurons were analyzed blindly. For each experimental group, a minimum of 40 cells per animal (n = 4) was analyzed by Image-Pro Plus 6.0 software. The criteria for the spine analysis were defined according to a previous study [18].
Western Blot
Protein lysates of the hippocampus were subjected to 10% SDS-PAGE and transferred onto a nitrocellulose membrane. After blocking with 5% skim milk, the membranes were incubated overnight at 4 °C with primary antibodies to phospho-Ser9-GSK-3β (1:1000, ab107166, Abcam, Shanghai, China), GSK-3β (1:1000, ab93926, Abcam, Shanghai, China), pTyr1007/1008-JAK2 (1:5000, ab32101, Abcam, Shanghai, China), JAK2 (1:5000, ab39636, Abcam, Shanghai, China), pTyr694-STAT5 (1:5000, ab32364, Abcam, Shanghai, China), and STAT5 (1:500, ab68465, Abcam, Shanghai, China). The membranes were then incubated with secondary horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) and anti-biotin antibody (1:2000, Cell signaling, USA) for 1 h at room temperature. The blots were detected using the enhanced chemiluminescence (ECL, Amersham, UK) reaction, and the protein bands were quantified using ImageJ software.
ELISA Assay
The activities of GSK-3β, PI3K and Akt were determined by the GENMED kit (GMS50161.4, GMS50058.2, GMS50054.2, Genmed, MA, USA) according to the manufacturer’s instructions. The activity of JAK2 was assayed by a commercial kit (Uscn Life Science Inc. #E95494Ra).
Lentivirus Construction and Injection
The shRNA of the rat EPO-R (NM_017002) was constructed and synthesized by Shanghai GeneChem Co., Ltd. (Shanghai, China). The target sequence used against the rat EPO-R was as follows: 5′-CAGCGCTTGGAAGACTTGGTGTGTT-3′. Recombinant lentivirus Lenti-EPO-R-shRNA-GFP was produced by cotransfecting 293 T cells with the lentivirus expression plasmid and packaging plasmids using Lipofectamine 2000. Intrahippocampal microinjection of recombinant lentivirus was carried out using a stereotaxic instrument (RWD, Shenzhen, China) according to the following coordinates: 3.5 mm posterior to bregma; 3.5 mm lateral to sagittal suture; 3.5 mm ventral [19].
Immunofluorescence
The immunofluorescence assay was performed as previously reported [20]. The brains were fixed by transcardial perfusion of 4% paraformaldehyde and sectioned coronally. The sections were first incubated with rabbit anti-phospho-Ser9-GSK-3β polyclonal antibody (1:200, ab107166, Abcam, Shanghai, China). Subsequently, the sections were incubated with fluorescein Rhodamine-conjugated goat anti-rabbit (1:200; cat. no. sc-2012; Santa Cruz Biotechnology, Inc.) The nucleus was stained with 4′,6-diamidino-2-phenylindole (DAPI; Beyotime Institute of Biotechnology, Inc., Dalian, China; 1:100). A total of 5 sections per rat were analyzed under a microscope (DTX500; Nikon Corporation, Tokyo, Japan).
Statistical Analysis
All data were analyzed using SPSS 14.0 with either Student’s t test or a one-way analysis of variance (ANOVA) followed by post hoc Student’s t test. The data are expressed as mean ± S.E.M. (standard error of the mean) and deemed statistically significant when P < 0.05.
Results
EPO Supplement Rescues the Spatial Memory Decline in 2VO Rats
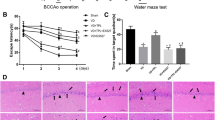
The Morris water maze test was used to evaluate the spatial learning and memory ability of the rat. This task utilized a visible platform task before the training period, followed by a 5-day training period, and finally a probe trial on day 7. In the visible platform swimming test, the escape latencies and path lengths of the rats were not statistically different between the 2VO and sham groups (one-way ANOVA, p > 0.05), suggesting that surgery did not affect the animal’s visual ability (Fig. 1a, b). During the 5 days of probe navigation training, the 2VO rats displayed a markedly longer escape latency compared with the control rats from the third day, and the EPO supplement significantly shortened the escape latency (Fig. 1c). After the probe trial test on the 7th day, the platform was removed. As expected, the 2VO rats displayed an increase in the time until the first crossing of the platform region, fewer platform-passing times, and less time spent in the target quadrant (Fig. 1d–f). Treatment with EPO is able to attenuate those impairments, as indicated by a reduction in the time until the first crossing, more crossing times, and a longer duration in the target quadrant. The above data suggest that EPO treatment could prevent spatial memory impairment in the 2VO rats. No significant difference in the swimming speed or total distance was observed among four groups during the probe trial test (Fig. 1g, h).
EPO supplementation rescues spatial memory decline in 2VO rats. The Morris water maze was used to evaluate the spatial memory of rats. a, b The latency (a) and the swimming distance (b) of the visible platform task in the Morris water maze. c The escape latency during the 5-day learning stages of the invisible platform task. d–f The first crossing latency (d), the total crossing times (e), and the total time spent in the target quadrant (f) in the probe trial. g, h The swimming speed (g) and the total swimming distances of the four groups. **P < 0.01, compared with the sham rats; ## P < 0.01, compared with the 2VO rats. N = 10
EPO Supplement Attenuates the Fear Memory Decline in 2VO Rats
Previous studies suggested that fear memory is impaired in the CCH rats [16]. Thus, the current study also examined the effect of EPO supplementation on fear memory in 2VO rats. As shown in Fig. 2, the step-down latency (SDL) was significantly decreased during memory trials on the 1st, 3rd, and 7th day after shock delivery to the foot paw in the 2VO rats. Treatment with EPO significantly extended the SDL in 2VO rats. No obvious difference was found between EPO-treated alone rats when compared to sham rats. Meanwhile, there were no significant differences between all groups during the learning phase (Fig. 2). Thus, we conclude that EPO could attenuate the fear memory impairment induced by chronic cerebral hypoperfusion.
EPO Supplement Attenuates Dendritic Spine Abnormalities in 2VO Rats
The loss of dendritic spines plays an important role in memory impairment in CCH rats [21]. We used Golgi staining to examine the dendritic spine density. Compared with the control group, the dendritic spine density in the hippocampal CA1 region of the 2VO rats significantly decreased. Meanwhile, the percentage of mushroom-type spines, the matured-type spine, is also reduced in the 2VO rats. However, treatment with EPO effectively restored the dendritic spine density and percentage of mushroom-type spines in 2VO rats. No obvious change was found between the EPO-treated alone rats and the sham rats (Fig. 3a–c). Thus, EPO supplement is able to attenuate synaptic function.
EPO supplementation restores dendritic spine abnormalities in 2VO rats. The representative images of dendritic spines (a) from the Golgi staining of the CA1 region, the quantitative analysis of the density of the spines (b), and the percentage of mushroom types (c) were obtained using ImageJ software. **P < 0.01, compared with the sham rats; ## P < 0.01, compared with the 2VO rats. N = 3
EPO Supplement Rescues Cholinergic Dysfunction in 2VO Rats
Previous studies have revealed that cholinergic abnormalities are associated with a disturbance in cognitive function in patients with VaD [22]. We then measured the effect of EPO supplement on the activities of AchE and ChAT and the level of Ach in the hippocampal homogenate of 2VO rats. Consistent with previous reports [14], the level of Ach and the activity of ChAT decreased and the activity of AchE increased significantly in the 2VO rats, suggesting a dysfunction in the cholinergic system in the CCH. Supplementation with EPO restored the activity of ChAT and the level of Ach and inhibited the activity of AchE in 2VO rats (Fig. 4a–c). These data support the behavioral test results and suggest that EPO may exert its neuroprotection by rescuing cholinergic function.
EPO Supplementation Inhibits GSK-3β Activity via the PI3K-Akt Signal Pathway
The PI3K-Akt-GSK-3β signaling pathway plays an important role in the EPO-stimulated proliferation of human erythroid progenitors [23], and GSK-3β is a key signaling molecule that induces neurodegeneration and deficits in memory formation related to dementia [24]. We then measured the GSK-3β activity by a commercial kit as previously described [25]. As indicated in Fig. 5a, the activity of GSK-3β is dramatically increased in 2VO rats, and EPO is able to reduce the increased GSK-3β activity. Western blot analysis also verified the inhibition of GSK-3β activity by EPO because the phosphorylation level of serine 9 is restored by EPO treatment (Fig. 5b, c). Meanwhile, EPO treatment significantly stimulated the activity of PI3K and Akt (Fig. 5d, e). These data suggest that EPO reduces the activity of GSK-3β via the PI3K-Akt signal pathway.
EPO supplementation inhibits GSK-3β activity via the PI3K-Akt signaling pathway. a The activity of GSK-3β was measured by commercial kits. b The representative western blots for the phosphorylation of Ser9-GSK-3β and total GSK-3β and quantitative analysis. c, d The activities of PI3K and Akt were measured by commercial kits **P < 0.01, compared with the young rats; ## P < 0.01, compared with the aged rats. N = 5
EPO Supplementation Stimulates the EPO-R/JAK2/STAT5 Pathway
Previous studies have suggested that the majority of the biological function of EPO is processed by the EPO receptor. We then examined whether EPO receptor knockout could block the neuroprotection conferred by EPO on memory in vivo. We injected the lenti-EPO-R-shRNA-GFP into the hippocampus after 2VO surgery and then treated the rats with or without EPO for 4 weeks. As expected, the EPO-R level was reduced to 56% in lenti-EPO-R-shRNA-GFP virus-injected rats when compared to the vector virus-injected rats (data not shown). The Morris water maze task was used to evaluate learning and memory. Representative paths taken by the control and treated animals are shown in Fig. 6a. It was observed that during the first 5 days of maze trials and compared to 2VO rats treated with EPO and the lenti-vector, the rats injected with lenti-EPO-R-shRNA-GFP showed a slower learning response (Fig. 6b). In the probe trial task, the rats treated with lenti-EPO-R-shRNA-GFP plus EPO displayed a lengthened time until the first crossing, fewer crossing times, and a shorter duration in the target quadrant than the rats treated with lenti-vector plus EPO (Fig. 6c–e). The above data suggests that EPO-R is necessary for the neuroprotection conferred by EPO on memory impairment induced by 2VO. No significant difference in the swimming speed was observed between the four groups during the probe trial test (Fig. 6f).
Knockout of the EPO-R blocks the memory restoration by EPO in 2VO rats. a The swimming traces of rats on the 5th day of training in the Morris water maze. b The escape latency during the 5-day learning stages of the invisible platform task. c–e The first crossing latency (c), the total crossing times (d), and the total time spent in the target quadrant (e) in the probe trial. f The swimming speed of the four groups. **P < 0.01, compared with the sham rats; ## P < 0.01, compared with the 2VO rats; $$ P < 0.01, compared with the 2VO + EPO + Vec rats. N = 10
Finally, we examined the JAK2/STAT5 pathway, which is important in mediating the activation of the EPO receptor [26]. We examined the phosphorylation of JAK2 and STAT5 at tyrosine-1007/1008 and tyrosine-694, respectively, in the hippocampus by western blot analysis. We found that the 2VO surgery dramatically reduced, while EPO supplementation restored, the phosphorylation levels of Tyr-1007/1008 of JAK2 and Tyr-694 of STAT5 (Fig. 7a, b). Using the commercial activity assay kit, we found that 2VO rats displayed lower JAK2 activity compared with the sham rats, and the EPO supplement recovered the JAK2 activity in 2VO rats (Fig. 7c). Silencing of the EPO-R blocks the activation of the JAK2/STAT5 pathway by EPO (Fig. 7a–c). This suggests that the neuroprotection of EPO in VD rats is mediated by the EPO-R/JAK2/STAT5/PI3K/Akt/GSK-3β pathway.
EPO supplementation activates the JAK2/STAT5 pathway. a, b The representative western blots for the phosphorylation of Tyr1007/1008-JAK2, total JAK2, Tyr694-STAT5, and total STAT5 from two independent experiments (a) and quantitative analysis (b). c The activity of JAK2 was measured by commercial kits. **P < 0.01, compared with the sham rats; ## P < 0.01, compared with the 2VO rats; $$ P < 0.01, compared with the 2VO + EPO + Vec rats. N = 10
Discussion
Previous studies have suggested that EPO has neuroprotective effects after ischemic, hypoxic, metabolic, neurotoxic and excitotoxic stress in the nervous system. EPO operates at several levels within the central nervous system, including limiting the production of reactive oxygen species and glutamate, modulating neurotransmission, reversing vasospasm, promoting angiogenesis, preventing apoptosis, reducing inflammation and recruiting stem cells [27, 28]. We reported previously that EPO supplementation is able to rescue cognitive decline in aged rats [12], and in the present study, we found that treating the 2VO rats with EPO for 4 weeks additionally rescued spatial and fear memory decline. EPO treatment also restored the loss of dendritic spines and the cholinergic dysfunction in the hippocampus. Importantly, EPO inhibited GSK-3β activity in the hippocampus by stimulating the EPO-R/JAK2/STAT5/PI3K/Akt/GSK-3β pathway.
The effects of EPO on cognition were first observed when it was clinically used for the treatment of anemia in chronic kidney disease [29]. In that study, EPO is able to improve cognitive performance by increasing red blood cells/hemoglobin with a subsequent enhancement of tissue oxygenation. In the animal study, chronic EPO treatment for 19 weeks in healthy mice induces a slight improvement in hippocampal learning and memory as measured using the Morris water maze [30]. Given that the EPO receptor is widely expressed in the nervous system and that EPO easily crosses the intact blood–brain barrier [31], EPO likely affects cognition by directly acting on the brain. For example, in human studies, no correlation between the blood values and cognitive enhancement was found [32], and non-hematopoietic EPO variants (e.g., CEPO = carbamoylated erythropoietin) were found to exert specific actions on the nervous system [33]. A high-dose EPO treatment in young mice results in an improvement in hippocampus-associated learning and memory, as well as a highly significant enhancement of long-term potentiation in the hippocampus [34]. The protective role of EPO on cognition has been used in the treatment of neuropsychiatric disorders with cognitive impairments, such as schizophrenia [35]. In our previous study, EPO supplementation is able to rescue cognitive decline in aged rats [12]. Here, supplementation of EPO for 4 weeks effectively restored impaired memory in the vascular dementia rat model.
The hippocampus receives cholinergic projections from the basal forebrain cholinergic complex [36]. In neurodegenerative diseases, the dysfunction of the cholinergic system was reported to play an important role in cognitive deficits [37]. In particular, in vascular dementia, the postmortem examinations revealed significant reductions in ChAT activity in the hippocampus [38]. In patients with Binswanger or multi-infarct dementia (MID), an apparent reduction in CSF ACh concentration was also reported [39]. Furthermore, one study reported a loss of cholinergic neurons in 40% of VaD patients, accompanied by reduced ACh activity in the cortex, hippocampus, striatum, and CSF [40]. In the VaD animal models, persistent reductions in several cholinergic markers were also reported. For example, 2VO surgery was shown to result in the loss of cholinergic neurons, as demonstrated by decreased choline acetyltransferase (ChAT) and AChE activities [5, 6], as well as reduced mRNA expression of the m3 and m5 muscarinic acetylcholine (ACh) receptors [41]. In addition, decreased ACh content and the corresponding impairments in learning and memory were also found in rats with 4-vessel occlusion [42]. In the present study, administration of EPO for 4 weeks was able to rescue the cholinergic dysfunction by restoring Ach levels and ChAT activity, suppressing AchE activity.
One of the earliest detectable signaling events initiated by EPO-R activation is phosphorylation at tyrosine residues of several intracellular proteins, and the Jak2 protein tyrosine kinase was first identified to serve as the principal kinase involved in mediating Epo-responsive signal transduction [43]. Tyrosine-phosphorylated Jak2 also appears to interact directly with STAT5A and STAT5B, leading to tyrosine phosphorylation and activation of STAT5 [44]. The activation of STAT5 directly enhances the transcriptional activation of PI3K and Akt1 [45], and Stat5 binds with the p85 regulatory subunit of PI3K via the Gab2 scaffolding adapter and then activates PI3K [46]. Furthermore, Stat5 could serve as a transcription factor for both Pik3r1 and Pik3ca encoding p85α and p110α, which are important for the activation of PI3K. Thus, the PI3K/Akt1 pathway is a downstream effector of Jak2/Stat5 signaling. Here, we knocked out the EPO-R by siRNA and found that the neuroprotective effect of EPO on 2VO rats was dramatically reduced. Meanwhile, the activation of EPO on JAK2/STAT5 was also blocked by the EPO-R knockdown. These data strongly suggest that EPO-R/JAK2/STAT5 is essential for the activation of PI3K/Akt by EPO.
Thus, our study provides the in vivo experimental basis for the application of EPO in the attenuation or retardation of cognitive impairments of vascular dementia.
Change history
20 June 2017
An erratum to this article has been published.
References
O'Brien JT, Thomas A (2015) Vascular dementia. Lancet 386(10004):1698–1706. doi:10.1016/S0140-6736(15)00463-8
Gupta S, Singh P, Sharma BM, Sharma B (2015) Neuroprotective effects of agomelatine and vinpocetine against chronic cerebral hypoperfusion induced vascular dementia. Curr Neurovasc Res 12(3):240–252
de la Torre JC, Fortin T, Park GA, Butler KS, Kozlowski P, Pappas BA, de Socarraz H, Saunders JK et al (1992) Chronic cerebrovascular insufficiency induces dementia-like deficits in aged rats. Brain Res 582(2):186–195
Choi DH, Lee KH, Kim JH, Seo JH, Kim HY, Shin CY, Han JS, Han SH et al (2014) NADPH oxidase 1, a novel molecular source of ROS in hippocampal neuronal death in vascular dementia. Antioxid Redox Signal 21(4):533–550. doi:10.1089/ars.2012.5129
Ni JW, Matsumoto K, Li HB, Murakami Y, Watanabe H (1995) Neuronal damage and decrease of central acetylcholine level following permanent occlusion of bilateral common carotid arteries in rat. Brain Res 673(2):290–296
Tanaka K, Ogawa N, Asanuma M, Kondo Y, Nomura M (1996) Relationship between cholinergic dysfunction and discrimination learning disabilities in Wistar rats following chronic cerebral hypoperfusion. Brain Res 729(1):55–65
Wang J, Zhang HY, Tang XC (2009) Cholinergic deficiency involved in vascular dementia: possible mechanism and strategy of treatment. Acta Pharmacol Sin 30(7):879–888. doi:10.1038/aps.2009.82
Yao ZH, Zhang JJ, Xie XF (2012) Enriched environment prevents cognitive impairment and tau hyperphosphorylation after chronic cerebral hypoperfusion. Curr Neurovasc Res 9(3):176–184
Souma T, Suzuki N, Yamamoto M (2015) Renal erythropoietin-producing cells in health and disease. Front Physiol 6:167. doi:10.3389/fphys.2015.00167
Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R (1997) Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience 76(1):105–116
Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E (1999) A potential role for erythropoietin in focal permanent cerebral ischemia in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 19(6):643–651. doi:10.1097/00004647-199906000-00007
Jia Z, Xue R, Ma S, Xu J, Guo S, Li S, Zhang E, Wang J et al (2016) Erythropoietin attenuates the memory deficits in aging rats by rescuing the oxidative stress and inflammation and promoting BDNF releasing. Mol Neurobiol 53(8):5664–5670. doi:10.1007/s12035-015-9438-1
Nie C, Nie H, Zhao Y, Wu J, Zhang X (2016) Betaine reverses the memory impairments in a chronic cerebral hypoperfusion rat model. Neurosci Lett 615:9–14. doi:10.1016/j.neulet.2015.11.019
Chen C, Zheng Y, Wu T, Wu C, Cheng X (2016) Oral administration of grape seed polyphenol extract restores memory deficits in chronic cerebral hypoperfusion rats. Behav Pharmacol. doi:10.1097/FBP.0000000000000276
Wang X, Wang LP, Tang H, Shan WY, Wang X, Liu D, Wu YY, Tian Q, Wang JZ, Zhu LQ (2014) Acetyl-L-carnitine rescues scopolamine-induced memory deficits by restoring insulin-like growth factor II via decreasing p53 oxidation. Neuropharmacology 76 Pt A:80-87 doi:10.1016/j.neuropharm.2013.08.022
Sarkaki A, Rafieirad M, Hossini SE, Farbood Y, Motamedi F, Mansouri SM, Naghizadeh B (2013) Improvement in memory and brain long-term potentiation deficits due to permanent hypoperfusion/ischemia by grape seed extract in rats. Iranian journal of basic medical sciences 16(9):1004–1010
Zhou P, Chen Z, Zhao N, Liu D, Guo ZY, Tan L, Hu J, Wang Q et al (2011) Acetyl-L-carnitine attenuates homocysteine-induced Alzheimer-like histopathological and behavioral abnormalities. Rejuvenation Res 14(6):669–679. doi:10.1089/rej.2011.1195
Liu D, Tang H, Li XY, Deng MF, Wei N, Wang X, Zhou YF, Wang DQ et al (2017) Targeting the HDAC2/HNF-4A/miR-101b/AMPK pathway rescues Tauopathy and dendritic abnormalities in Alzheimer’s disease. Molecular therapy : the journal of the American Society of Gene Therapy 25(3):752–764. doi:10.1016/j.ymthe.2017.01.018
Yin YY, Liu H, Cong XB, Liu Z, Wang Q, Wang JZ, Zhu LQ (2010) Acetyl-L-carnitine attenuates okadaic acid induced tau hyperphosphorylation and spatial memory impairment in rats. Journal of Alzheimer’s disease: JAD 19(2):735–746. doi:10.3233/JAD-2010-1272
Liu D, Wei N, Man HY, Lu Y, Zhu LQ, Wang JZ (2015) The MT2 receptor stimulates axonogenesis and enhances synaptic transmission by activating Akt signaling. Cell Death Differ 22(4):583–596. doi:10.1038/cdd.2014.195
Wang Z, Fan J, Wang J, Li Y, Duan D, Du G, Wang Q (2016) Chronic cerebral hypoperfusion induces long-lasting cognitive deficits accompanied by long-term hippocampal silent synapses increase in rats. Behav Brain Res 301:243–252. doi:10.1016/j.bbr.2015.12.047
Gottfries CG, Blennow K, Karlsson I, Wallin A (1994) The neurochemistry of vascular dementia. Dementia 5(3–4):163–167
Schmidt EK, Fichelson S, Feller SM (2004) PI3 kinase is important for Ras, MEK and Erk activation of Epo-stimulated human erythroid progenitors. BMC Biol 2:7. doi:10.1186/1741-7007-2-7
Giese KP (2009) GSK-3: a key player in neurodegeneration and memory. IUBMB life 61(5):516–521. doi:10.1002/iub.187
Pan X, Gong N, Zhao J, Yu Z, Gu F, Chen J, Sun X, Zhao L et al (2010) Powerful beneficial effects of benfotiamine on cognitive impairment and beta-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain : a journal of neurology 133(Pt 5):1342–1351. doi:10.1093/brain/awq069
Shi Z, Hodges VM, Dunlop EA, Percy MJ, Maxwell AP, El-Tanani M, Lappin TR (2010) Erythropoietin-induced activation of the JAK2/STAT5, PI3K/Akt, and Ras/ERK pathways promotes malignant cell behavior in a modified breast cancer cell line. Molecular cancer research : MCR 8(4):615–626. doi:10.1158/1541-7786.MCR-09-0264
Ponce LL, Navarro JC, Ahmed O, Robertson CS (2013) Erythropoietin neuroprotection with traumatic brain injury. Pathophysiology: the official journal of the International Society for Pathophysiology 20(1):31–38. doi:10.1016/j.pathophys.2012.02.005
Robertson CS, Cherian L, Shah M, Garcia R, Navarro JC, Grill RJ, Hand CC, Tian TS et al (2012) Neuroprotection with an erythropoietin mimetic peptide (pHBSP) in a model of mild traumatic brain injury complicated by hemorrhagic shock. J Neurotrauma 29(6):1156–1166. doi:10.1089/neu.2011.1827
Ehrenreich H, Bartels C, Sargin D, Stawicki S, Krampe H (2008) Recombinant human erythropoietin in the treatment of human brain disease: focus on cognition. Journal of renal nutrition: the official journal of the Council on Renal Nutrition of the National Kidney Foundation 18(1):146–153. doi:10.1053/j.jrn.2007.10.029
Hengemihle JM, Abugo O, Rifkind J, Spangler E, Danon D, Ingram DK (1996) Chronic treatment with human recombinant erythropoietin increases hematocrit and improves water maze performance in mice. Physiol Behav 59(1):153–156
Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A (2000) Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A 97(19):10526–10531
Wustenberg T, Begemann M, Bartels C, Gefeller O, Stawicki S, Hinze-Selch D, Mohr A, Falkai P et al (2011) Recombinant human erythropoietin delays loss of gray matter in chronic schizophrenia. Mol Psychiatry 16(1):26–36, 21. doi:10.1038/mp.2010.51
Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M et al (2004) Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science 305(5681):239–242. doi:10.1126/science.1098313
Adamcio B, Sargin D, Stradomska A, Medrihan L, Gertler C, Theis F, Zhang M, Muller M et al (2008) Erythropoietin enhances hippocampal long-term potentiation and memory. BMC Biol 6:37. doi:10.1186/1741-7007-6-37
Ehrenreich H, Hinze-Selch D, Stawicki S, Aust C, Knolle-Veentjer S, Wilms S, Heinz G, Erdag S et al (2007) Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol Psychiatry 12(2):206–220. doi:10.1038/sj.mp.4001907
Schliebs R, Arendt T (2011) The cholinergic system in aging and neuronal degeneration. Behav Brain Res 221(2):555–563. doi:10.1016/j.bbr.2010.11.058
Liberini P (1997) The cholinergic system in Alzheimer’s disease and dementia with Lewy bodies: from animal models to neuropathological data. Funct Neurol 12(3–4):153–157
Waller SB, Ball MJ, Reynolds MA, London ED (1986) Muscarinic binding and choline acetyltransferase in postmortem brains of demented patients. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques 13(4 Suppl):528–532
Tohgi H, Abe T, Kimura M, Saheki M, Takahashi S (1996) Cerebrospinal fluid acetylcholine and choline in vascular dementia of Binswanger and multiple small infarct types as compared with Alzheimer-type dementia. J Neural Transm 103(10):1211–1220. doi:10.1007/BF01271206
Court JPE, Kalaria R (2002) Neurotransmitter control of the cerebral vasculature and abnormalities vascular cognitive impairment. Martin Dunitz, London
Zhao Q, Murakami Y, Tohda M, Obi R, Shimada Y, Matsumoto K (2007) Chotosan, a kampo formula, ameliorates chronic cerebral hypoperfusion-induced deficits in object recognition behaviors and central cholinergic systems in mice. J Pharmacol Sci 103(4):360–373
Zhang LLWJ, Liu SW, Chen ME (2004) Changes of somatostatin and acetylcholine contents in vascular dementia rats. Acta Acad Med Milit Tert 26(8):3
Bogdarin Iu A (1994) The role of sex steroid hormones and nerobolyl in regulating fatty and amino acid metabolism. Biull Eksp Biol Med 117(6):602–605
Johnston JA, Bacon CM, Finbloom DS, Rees RC, Kaplan D, Shibuya K, Ortaldo JR, Gupta S et al (1995) Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc Natl Acad Sci U S A 92(19):8705–8709
Schmidt JW, Wehde BL, Sakamoto K, Triplett AA, Anderson SM, Tsichlis PN, Leone G, Wagner KU (2014) Stat5 regulates the phosphatidylinositol 3-kinase/Akt1 pathway during mammary gland development and tumorigenesis. Mol Cell Biol 34(7):1363–1377. doi:10.1128/MCB.01220-13
Nyga R, Pecquet C, Harir N, Gu H, Dhennin-Duthille I, Regnier A, Gouilleux-Gruart V, Lassoued K et al (2005) Activated STAT5 proteins induce activation of the PI 3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding adapter. The Biochemical journal 390(Pt 1):359–366. doi:10.1042/BJ20041523
Acknowledgments
This study was supported by the National Science Foundation of China (81600944) and the Youth Innovation Fund of The First Affiliated Hospital of Zhengzhou University.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at https://doi.org/10.1007/s12035-017-0657-5.
Rights and permissions
About this article
Cite this article
Ma, S., Chen, J., Chen, C. et al. Erythropoietin Rescues Memory Impairment in a Rat Model of Chronic Cerebral Hypoperfusion via the EPO-R/JAK2/STAT5/PI3K/Akt/GSK-3β Pathway. Mol Neurobiol 55, 3290–3299 (2018). https://doi.org/10.1007/s12035-017-0568-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0568-5