Abstract
Tripterygium Wilfordii Hook F has been exploited as a treatment for several diseases due to its neuroprotective, anti-tumor, and anti-inflammatory effects. Triptolide is one of its key bioactive compounds. Currently, the role of triptolide in cognitive dysfunction remains unclear. Here, the role of triptolide on cognitive dysfunction was investigated using chronic cerebral hypoperfusion-induced vascular dementia (VD) rat model. SD rats were administrated with Triptolide (5 μg/kg) for 6 weeks after undergoing permanent bilateral common carotid artery occlusion. The results show that triptolide treatment conferred neuroprotective effects in VD rats. Intraperitoneal injection of triptolide attenuated oxidative stress, learning and memory deficits, and neuronal apoptosis in the hippocampi. Moreover, triptolide enhanced the expression of SIRT1, PGC-1α, ZO-1, Claudin-5, and decreased the serum levels of NSE and S100B significantly. It also improved CCH-induced learning and memory deficits, and this is attributed to its capacity to promote SIRT1/PGC-1α signaling, confer antioxidant effects, and inhibit neuronal apoptosis. These findings indicate that triptolide may be an effective therapeutic agent for vascular cognitive dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vascular dementia (VD) is a clinical condition characterized by cognitive impairment due to hemorrhagic or ischemic cerebrovascular disease [1, 2]. It accounts for 17–25% of all dementia cases globally, and is the 2nd most common type of dementia [3]. Although the precise cause of VD is not well-understood, previous studies have shown that its development is closely related to chronic cerebral hypoperfusion (CCH) [4]. Several experimental studies indicated that chronic cerebral hypoperfusion can be caused by permanent bilateral common carotid artery occlusion (BCCAo). This principle is used to establish a model upon which investigations into the molecular mechanisms and pathological changes in cognitive dysfunction in VD can be carried out [5, 6].
Oxidative stress has been recognized to be one of the factors which cause neurodegeneration in vascular dementia and Alzheimer’s disease [7, 8]. During CCH, oxidative stress destabilizes the ratio of pro-oxidant systems and endogenous antioxidants systems leading to oxidative damage to the phospholipids, nucleic acids and proteins [9]. As an intracellular endogenous regulator of oxidative stress and mitochondrial energy regulation, peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) plays a critical role in the pathogenesis of neurodegenerative diseases [7, 10]. PGC-1α inhibits the NADPH oxidase-induced oxygen free radical release, and up-regulates antioxidant enzymes e.g. SOD and GPX1 [11]. A nicotinamide-adenine dinucleotide (NAD+)-regulated deacetylase, SIRT1, modulates various physiological processes such as cellular metabolism, oxidative stress, inflammation and senescence [12, 13]. SIRT1 also activates and increases the expression of PGC-1α through deacetylation, up-regulates antioxidant enzymes, inhibits apoptosis, and reduces oxidative stress-induced cellular injury [14]. Other functions of SIRT1 include synaptic plasticity and memory formation, prevention of neurodegeneration and cognitive dysfunction [15, 16]. Here, we hypothesized that attenuating oxidative stress and neuronal apoptosis by activating SIRT1/PGC-1α signaling may improve cognitive dysfunction in rats with vascular dementia.
Triptolide is a bioactive diterpene constituent of Tripterygium Wilfordii Hook F (a Chinese herb) with low molecular weight, high fat solubility and easy to cross the blood–brain barrier [17, 18]. It is commonly used to treat many diseases because it possesses anti-inflammatory, anti-tumor and neuroprotective properties [19,20,21]. It was demonstrated that triptolide can decrease inflammation and oxidative stress, and reduce neuronal apoptosis induced by LPS [22]. In hippocampal neurons, triptolide increases the expression of synaptophysin, deceases β-amyloid production, and effectively slows down neuronal degeneration [23, 24]. However, the effects of triptolide on vascular dementia remain elusive.
Here, we hypothesized that triptolide may attenuate oxidative stress and neuronal apoptosis by activating SIRT1/PGC-1α pathway leading to CCH-induced vascular dementia, an effect that improves the cognitive dysfunction. Thus, the permanent bilateral common carotid artery occlusion model was used to investigate the protective roles of triptolide on cognitive function.
Materials and Methods
Animals
Male SD rats (6–8 weeks old, weighing 200 ± 20 g, provided by the Department of Laboratory Animals of Nanchang University, China) were raised in the experimental animal room of Nanchang University Medical College. Rats were fed and allowed free access to water in a house with the following conditions: temperature (24 ± 2) °C, humidity 30–50%, 12 h light/dark conditions. All animal handling protocols and experiments conformed to the Animal Ethics Committee of Nanchang University (Nanchang, China) guidelines.
Animal Surgeries
Rat models of vascular dementia were developed via permanent bilateral common carotid artery occlusion [6]. Animals were anesthetized with sodium pentobarbital (60 mg/kg), injected intraperitoneally, and the head and limbs were fixed in supine position. Thereafter, a median incision (1.5–2.0 cm) was made after which the muscles and fascia were separated. 4-0 silk sutures was used to double-ligate the proximal and distal ends of the artery, and the incision was sutured after sterilizing. During the surgical procedure, the body temperature was regulated by a heating lamp at 36.0 ± 2 °C. A sham operation was performed using the same surgical procedure except for the occlusion of common carotid arteries.
Animals were randomly allocated into: sham operated group (Sham group, n = 12), vascular dementia group (permanent bilateral common carotid artery occlusion, VD group, n = 12), triptolide ((Sigma, USA) (5 μg/kg/day) treatment group (VD + TPL group, n = 12)), EX527 ((MedChemExpress, USA) (SIRT1 inhibitor, 5 mg/kg/day) treatment group (VD + EX527 group, n = 12)), and Triptolide + EX527 treatment group (VD + TPL + EX527 group, n = 12). Dimethyl sulfoxide (DMSO) (Sigma) was used to dissolve EX527 and triptolide which were then diluted in 0.9% saline to a final DMSO concentration of 1% (v/v). Rats in sham group were intraperitoneally injected with triptolide vehicle once a day for 6 successive weeks from the second day after surgery.
Morris Water Maze
Six weeks after the model was established, the Morris water maze test was performed on the animals to assess their spatial memory and learning abilities. The animals were tested for swimming 1 day in advance, and each animal was tested four times to find the hidden platform. If the rat was struggling or not moving in the water, it would be removed for subsequent testing.
On the platform trial days, each rat was trained 4 times daily for 4 successive days. Rat were placed in water and then allowed 120 s to locate the hidden platform (escape latency). For rats that could not find the platform within 120 s, they were gently guided to the platform and the highest score of 120 s was given to such rats. After finding the platform, animals remained there for 15 s. 24 h after the last platform trial, the platform was subjected to spatial probe trial. The rats were then placed in water opposite to the target quadrant. Thereafter, the duration they stayed in the target quadrant within 120 s were recorded.
Histological Examination
Immediately after the Morris water maze test, the rats were intraperitoneally injected with sodium pentobarbital and their blood samples were collected, followed by perfusion with 4% paraformaldehyde fixative and 0.9% saline. Subsequently, and all brains were collected for subsequent experiments. Whole brain specimens were excised and fixed in 4% paraformaldehyde for 24 h before they were embedded in paraffin. The specimens were eventually sectioned into 5-μm thick slices by a microtome.
To perform immunohistochemistry (IHC) assay, antigens were retrieved from the deparaffinized sections by soaking them in sodium citrate buffer at 100 °C for 15 min. The specimen were then rinsed with 0.01 M PBS three times (5 min for each) before they were treated with 3% hydrogen peroxide for 20 min at RT. Thereafter, they were blocked with PBS solution containing 10% goat serum for 15 min. Next, polyclonal antibodies raised in rabbit against SIRT1 (Abcam Trading Shanghai Company) were added to the specimen and incubated at 4 °C overnight. Subsequently, the specimen were washed 3 times (5 min for each) in PBS, and then treated with secondary antibodies for 1 h at RT. Finally, the horseradish peroxidase (HRP)-conjugated streptavidin (Boster, China) was added and the specimen were stained with DAB and counterstained with hematoxylin.
For histological analysis, brain tissue sections were deparaffinized and stained with hematoxylin and eosin (H&E) to detect morphological changes. Based on histopathological analysis of 3 non-overlapping horizons at ×200 magnification, histopathological injury of neurons degeneration was evaluated by pleomorphism and hyperchromasia.
TdT-Mediated dUTP-Biotin Nick-end Labeling (TUNEL)
The paraffin-embedded brain sections were stained with the TUNEL-based cell death assay kits (Roche, Germany) according to the manufacturer’s instructions. The percentage of TUNEL positive cells in the selected microscopic fields of hippocampi were counted and calculated by an investigator blinded to the treatment groups.
Enzyme-Linked Immunosorbent Assay (ELISA)
Immediately after the blood samples were collected, the samples were centrifuged at 3000 rpm for 15 min to obtain a supernatant. Then, a commercial ELISA kit was used to determine the serum levels of S100B and neuron specific enolase (NSE) according to the protocols provided by the manufacturer (Elabscience, China).
Measurement of SOD Activity and Concentration of Malondialdehyde (MDA)
Immediately after the hippocampi were collected, the specimens were homogenized in 10% solution of physiological saline, and then centrifuged at 4000 rpm for 15 min to obtain a supernatant. The activity of SOD and concentration of MDA were assessed by a Thermo Scientific Microplate Reader according to the manufacturer’s protocol (Nanjing Jiancheng Bioengineering institute, China).
Western Blot
To measure the protein expression, the hippocampi were harvested and total proteins were extracted using the protocols provided by the manufacturer. The protein concentration was determined using the bicinchoninic acid method (BCA) kit (Boster). Equal amount of protein samples (40 μg) were separated on 12% SDS-PAGE gels electrophoresis after which they were transferred to a PVDF membrane. 5% (w/v) skim milk in TBST was used to block the membrane. Proteins were then incubated overnight at 4 °C with rabbit polyclonal antibody to SIRT1, PGC-1α, ZO-1, Claudin-5 (Abcam Trading Shanghai Company) and β-actin (Beijing Golden Bridge Biotech, China), followed by HRP-conjugated goat polyclonal secondary antibody (Abcam) for 1 h at RT. Finally, the blots were analyzed and quantified with a ChemiDoc™ MP System (Bio-Rad Laboratories, USA). The densities of the bands were normalized with respect to the values of β-actin.
Data Analysis
Data are presented as mean ± SD. One-way analysis of variance (ANOVA) was used to measure differences between groups, followed by the post hoc Duncon’s test. The two-way ANOVA followed by LSD test was used to analyze the MWT data. P values < 0.05 were considered to be statistically significant. Data analysis was performed using SPSS 13.0 software.
Results
Triptolide Improves the CCH-Induced Spatial Memory and Learning Deficits
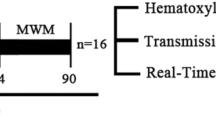
As is shown in Fig. 1a, the Morris water maze test was performed to determine the effect of triptolide on the CCH-induced cognitive impairment at the 7th week after surgery. In the swimming test 1 day in advance, each animal tried to find the hidden platform and no animals were removed from subsequent experiment. Data showed that the escape latencies of VD rats were markedly longer (43.8 ± 3.1 vs. 15.2 ± 2.5 s) and the rats spent shorter duration in the target quadrant relative to Sham rats (46.8 ± 3.7 vs. 23.8 ± 2.6 s) from the 3rd day (trial day 3 and 4, P < 0.05) (Fig. 1b, c). After treatment with triptolide for 6 weeks, the escape latency of VD + TPL group was improved (27.7 ± 2.6 vs. 43.8 ± 3.1 s) and the duration rats spent in the target quadrant was markedly longer (38.8 ± 3.1 vs. 21.0 ± 3.9 s) than that of VD group (P < 0.05), suggesting that triptolide may partially promote spatial memory and learning impairment caused by CCH. However, these improvements were significantly smaller in Triptolide + EX527 treatment group (P < 0.05).
Triptolide promotes spatial memory and learning deficits triggered by CCH and reduces neuronal degeneration in the hippocampi. a Flowchart of the experimental protocol. b, c Spatial memory and learning abilities were assessed by Morris Water Maze, escape latency was the mean value of each platform trial for 4 consecutive days and time the rats spent in target quadrant was measured during the probe trials. d Sections of hippocampi underwent HE staining at 6 weeks after BCCAo. Histopathological injury of neurons degeneration was evaluated by pleomorphism and hyperchromasia. Arrowheads indicate normal neurons, arrows indicate damaged neurons. Values are expressed as mean ± SD, n = 12 rats per group. *P < 0.05 versus Sham group, #P < 0.05 versus VD group, $P < 0.05 versus VD +TPL group
Triptolide Reduces Oxidative Stress, Apoptosis and Neuronal Pathology Damage in Rat Hippocampus During VD
To determine whether triptolide improves neuropathological damage after CCH, the pyramidal neurons in the hippocampal CA1 of rats was examined. Figure 1d showed that sections from Sham group showed normal morphological structure, while those from VD group and VD + EX527 group had degenerative changes characterized mainly with pleomorphism and hyperchromasia. Triptolide treatment for 6 week caused remarkable reduction in degenerative changes, which were significantly reversed by EX527 treatment.
The concentration of MDA and activity of SOD in the hippocampus specimens were detected after Morris water maze test. As is shown in Fig. 2a, b, the MDA content in VD group was significantly higher compared with that of Sham group (4.46 ± 0.43 vs. 1.30 ± 0.14 nmol/mg prot), whereas the SOD activity was decreased (142.63 ± 13.56 vs. 54.94 ± 5.88 U/mg prot) (P < 0.05). Triptolide treatment for 6 weeks increased the SOD activity (97.63 ± 8.27 vs. 54.94 ± 5.88 U/mg prot) and decreased the MDA content significantly (2.65 ± 0.23 vs. 4.46 ± 0.43 nmol/mg prot) (P < 0.05), suggesting that triptolide suppresses oxidative stress caused by CCH. Interestingly, these antioxidant effects were partially decreased in VD + TPL + EX527 group (approx. 40%) (P < 0.05).
Effect of triptolide on apoptosis, SOD activity, MDA level in the hippocampi of rats. a, b The levels of MDA and SOD in Sham and experimental groups. c Representative microscopic images of TUNEL staining in hippocampal CA1 (magnification: 200×). d The percentage of TUNEL positive cells. Values are expressed as mean ± SD, n = 3 rats in each group. *P < 0.05 versus Sham group, #P < 0.05 versus VD group, $P < 0.05 versus VD +TPL group
Furthermore, the TUNEL assay was used to measure apoptosis level in the hippocampi. Data showed that the percentage of TUNEL positive cells in the VD + TPL group was significantly lower than that in the VD group (5.91 ± 0.87 vs. 12.67 ± 1.08%) (P < 0.05), suggesting that triptolide can prevent CCH-induced hippocampal neuronal apoptosis. High number of TUNEL-positive cells were recorded in VD + TPL + EX527 group relative to rats treated with triptolide for 6 weeks (17.05 ± 1.54 vs. 5.91 ± 0.87%) (P < 0.05) (Fig. 2c, d).
Triptolide Attenuates Blood Brain Barrier (BBB) Hyperpermeability Induced by CCH in Rats
To determine whether triptolide can reduce the BBB hyperpermeability induced by CCH, biomarkers of brain damage (S100B and NSE) [25] and tight junction proteins (ZO-1 and Claudin-5) were measured after the Morris water maze test. Figure 3a, b shows that serum concentration of S100B (63.98 ± 6.11 vs. 27.32 ± 3.85 pg/ml) and NSE (7.51 ± 0.61 vs. 2.27 ± 0.27 ng/ml) were markedly higher in VD groups relative to those in Sham group and the protein level of Claudin-5 and ZO-1 significantly decreased (approx. 65%) (P < 0.05). In contrast, triptolide treatment for 6 weeks decreased the level of the biomarkers and proteins significantly (P < 0.05), indicating that triptolide can attenuate BBB hyperpermeability in VD rats. These results are consistent with the antioxidant effects of triptolide but could not be observed in VD + TPL + EX527 treatment group.
Effect of triptolide on serum NSE and S100B levels, and the expression of Claudin-5 and ZO-1 in rats. a, b Serum NSE and S100B concentration in sham and experimental group were measured by ELISA. c Western blot assay for ZO-1 and Claudin-5 expression. d, e Quantitative analysis of Claudin-5 and ZO-1 expression, the densities of the bands were normalized with respect to the values of β-actin. Values are expressed as mean ± SD, n = 6 rats in each group. *P < 0.05 versus Sham group, #P < 0.05 versus VD group, $P < 0.05 versus VD +TPL group
Triptolide Increases SIRT1 Expression in Rats with VD
IHC and western blot assays were performed to examine the expression status of SIRT1 in rats with VD. In line with the results of IHC assays, the protein level of SIRT1 was decreased in VD group compared to Sham group (0.65 ± 0.06 vs. 0.26 ± 0.04) (P < 0.05) (Fig. 4b, d). After treatment with triptolide for 6 weeks, the protein level of SIRT1 increased in VD + TPL group (0.43 ± 0.06 vs. 0.26 ± 0.04) (P < 0.05). Moreover, in contrast with triptolide-induced SIRT1 overexpression, we observed statistically significant difference in the SIRT1 expression between VD + TPL group and VD + TPL + EX527 group (0.43 ± 0.06 vs. 0.22 ± 0.02) (P < 0.05). These findings imply that triptolide abolishes the CCH-induced decrease in SIRT1 expression.
Triptolide regulates PGC-1α and apoptosis through SIRT1. a Representative IHC staining microscopic images of SIRT1 protein in hippocampal CA1. b IHC staining for SIRT1 protein. c Protein bands of SIRT1, PGC-1α, Bcl-2, Bax, Caspase-3 and β-actin. d The protein levels of Caspase-3, Bax, Bcl-2, PGC-1α and SIRT1 were calculated as % of the β-actin expression. Values are expressed as mean ± SD. n = 6 rats in each group, *P < 0.05 versus Sham group, #P < 0.05 versus VD group, $P < 0.05 versus VD +TPL group
Triptolide Regulates PGC-1α and Apoptosis Through SIRT1 Signaling
The expression of PGC-1α and apoptosis-related proteins were further evaluated. The protein levels of PGC-1α and Bcl-2 were significantly deceased in VD group, whereas the levels of Caspase-3 and Bax were increased (P < 0.05) (Fig. 4d). The levels of PGC-1α and Bcl-2 were significantly higher in VD + TPL group compared with VD group. Additionally, the levels of Bax and Caspase-3 were lower in VD group compared to VD + TPL group (P < 0.05). However, TPL + EX527 treatment decreased PGC-1α expression and abolished the inhibitory effects of triptolide on apoptosis in VD + TPL group. These results suggest that triptolide attenuates CCH-induced neuronal apoptosis and oxidative stress through SIRT1 signaling.
Discussion
This is the first study revealing that triptolide improves chronic cerebral hypoperfusion-induced vascular dementia by regulating oxidative stress signaling. Triptolide treatment decreased ROS levels by activating SIRT1/PGC-1α pathway. Moreover, triptolide decreased blood–brain barrier hyperpermeability by promoting the expression of tight junction proteins (Claudin-5 and ZO-1). In addition, triptolide treatment exerted a therapeutic effect on vascular cognitive dysfunction following chronic cerebral hypoperfusion. This effect was attributed to the inhibitory effect of triptolide on oxidative stress which increased the level of tight junction proteins, SIRT1, and PGC-1α.
Central inflammation and oxidative stress are the two main mechanisms in the early stages of BCCAo-induced brain injury, leading to vascular endothelial cell injury, intracellular calcium overload, mitochondrial dysfunction and elevated pro-inflammatory factors. Meanwhile, several experimental studies indicated that cognitive impairment and neurodegeneration became apparent beginning at 4–6 weeks after BCCAo [26,27,28], and degenerated neurons after CCH may be caused by cholinergic dysfunction and severe oxidative stress. Furthermore, oxidative stress may damage glial cells, neuronal and vessel endothelia, thereby enhancing neuronal apoptosis, BBB hyperpermeability, neuronal degeneration and neurovascular uncoupling, all of which may cause vascular dementia [29]. It was reported that antioxidant therapy has a neuroprotective effect due to its capacity to decrease ROS levels and increase antioxidant enzymes levels [10, 11]. Superoxide dismutase (SOD) plays antioxidant roles. On the other hand, malondialdehyde (MDA) is produced by lipid peroxidation and oxygen free radicals. The concentration of SOD and MDA can reflect the oxidative stress level of brain. Central nervous system injury is often accompanied by nervous tissue and cellular damage, decreased tight junction proteins (Claudin-5 and ZO-1) and high levels of biomarkers of brain damage (NSE and S100B) in cells, cerebrospinal fluid and blood [30]. In this study, the level of MDA was elevated while that of SOD was reduced in rats with VD. Moreover, impaired memory and learning abilities together with increased BBB hyperpermeability and neuronal degeneration were observed. These data indicate that the imbalance between antioxidant species and the production of ROS may lead to cognitive dysfunction and increased BBB hyperpermeability in VD.
Triptolide is one of the most bioactive constituents of Tripterygium Wilfordii Hook F extracts. It is exploited as a drug for several diseases. In the recent past, triptolide has been shown to modulate inflammatory responses [31] and oxidative stress imbalance [18, 32], indicating that triptolide may serve as an antioxidative agent in vascular dementia. Here, the permanent bilateral common carotid artery occlusion condition was used to model oxidative stress and cognitive dysfunction. Consistent with previous studies, triptolide treatment decreased MDA levels, increased the activity of SOD and improved the learning and memory ability in VD rats, indicating an antioxidative role of triptolide in VD rats. In addition, triptolide decreased the levels of S100B and NSE and increased the expression of tight junction proteins (Claudin-5 and ZO-1) suggesting that triptolide can alleviate BBB hyperpermeability. Further western blot, TUNEL assays and HE staining showed that triptolide prevented neuronal apoptosis and neurodegeneration.
Numerus studies have demonstrated that SIRT1 (a nicotinamide-adenine dinucleotide-regulated deacetylase) has some therapeutic effects in neurodegenerative diseases [15, 30]. PGC-1α is a SIRT1-deacetylating protein that mediates oxidative stress in response to VD and AD [16, 33]. SIRT1 activates and increases PGC-1α expression by reducing the acetylation of PGC-1α. In turn, activated PGC-1α up-regulates the expression of antioxidant enzyme. In this study, we found the protein level of SIRT1 and PGC-1α were significantly decreased after CCH in Sham group, while triptolide treatment increased SIRT1 expression in VD rats, which increased the levels of PGC-1α and SOD. Moreover, these effects were partially abolished by SIRT1 inhibitor EX527, implying that triptolide decreases the levels of oxidative stress in the hippocampi during CCH by regulating the SIRT1/PGC-1α pathway. By doing so, it decreases the expression of Caspase-3, increases Bcl-2/Bax ratio, and reduces hippocampal neuronal apoptosis. Therefore, these results indicate that triptolide reduces oxidative stress, neuronal apoptosis and neuronal degeneration in rats with vascular dementia by regulating the SIRT1/PGC-1α pathway.
However, the present study has several limitations. It has been reported that nuclear factor kappa B pathway, the energy sensing AMPK signaling pathways and some other oxidative stress-related pathways are possibly associated with vascular cognitive dysfunction [26, 34], besides, triptolide has been shown to modulate inflammatory responses and oxidative stress in several previous studies, while we only discussed PGC-1α-mediated oxidative stress that may be involved in the progression of VD. Future experiments should delve deeper into other signaling pathways or mechanisms associated with vascular dementia. Second, in this study, we used 6–8 weeks old of SD rats to establish a VD model. Based on evidence from the literature [28, 35, 36], neuronal degeneration in long-term VD models was more likely to be observed in adult (8–12 weeks old) rats. Future experiments should use adult rats to establish a model of vascular dementia. Third, the experiments in this study were performed in vivo, so further researches on the mechanism of protective effect of triptolide on hippocampal neurons are needed in vitro. Nevertheless, this study proposes new insights into the protective effects of triptolide on cognitive dysfunction in rats with vascular dementia.
In conclusion, this is the first report showing that triptolide significantly promotes the expression of SIRT1 in cognitive function and reduces BBB hyperpermeability in rats with vascular dementia. These effects were due to its antioxidant effects. The findings of this study demonstrate that triptolide may be a promising treatment for vascular dementia.
References
Khan A, Kalaria RN, Corbett A et al (2016) Update on vascular dementia. J Geriatr Psychiatry Neurol 29(5):281–301
Kalaria RN, Akinyemi R, Ihara M (2016) Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta 5:915–925
Wiesmann M, Kiliaan AJ, Claassen JA (2013) Vascular aspects of cognitive impairment and dementia. J Cereb Blood Flow Metab 33(11):1696–1706
Yang T, Sun Y, Lu Z et al (2017) The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res Rev 34:15–29
Du SQ, Wang XR, Xiao LY et al (2017) Molecular mechanisms of vascular dementia: what can be learned from animal models of chronic cerebral hypoperfusion? Mol Neurobiol 54(5):3670–3682
Tsuchiya M, Sako K, Yura S et al (1993) Local cerebral glucose utilisation following acute and chronic bilateral carotid artery ligation in Wistar rats: relation to changes in local cerebral blood flow. Exp Brain Res 95(1):1–7
Bennett S, Grant MM, Aldred S (2009) Oxidative stress in vascular dementia and Alzheimer’s disease: a common pathology. J Alzheimers Dis 17(2):245–257
Gustaw-Rothenberg K, Kowalczuk K, Stryjecka-Zimmer M (2010) Lipids’ peroxidation markers in Alzheimer’s disease and vascular dementia. Geriatr Gerontol Int 10(2):161–166
Zhang X, Wu B, Nie K et al (2014) Effects of acupuncture on declined cerebral blood flow, impaired mitochondrial respiratory function and oxidative stress in multi-infarct dementia rats. Neurochem Int 65:23–29
Zhang XY, Zhang XJ, Xv J et al (2018) Crocin attenuates acute hypobaric hypoxia-induced cognitive deficits of rats. Eur J Pharmacol 818:300–305
Fu B, Zhang J, Zhang X et al (2014) Alpha-lipoic acid upregulates SIRT1-dependent PGC-1alpha expression and protects mouse brain against focal ischemia. Neuroscience 281:251–257
Corbi G, Conti V, Russomanno G et al (2013) Adrenergic signaling and oxidative stress: a role for sirtuins? Front Physiol 4:324
Helisalmi S, Vepsalainen S, Hiltunen M et al (2008) Genetic study between SIRT1, PPARD, PGC-1alpha genes and Alzheimer’s disease. J Neurol 255(5):668–673
Koo JH, Cho JY (2017) Treadmill exercise attenuates alpha-synuclein levels by promoting mitochondrial function and autophagy possibly via SIRT1 in the chronic MPTP/P-induced mouse model of Parkinson’s disease. Neurotox Res 32(3):473–486
Donmez G, Wang D, Cohen DE et al (2010) SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell 142(2):320–332
Zhou Y, Wang S, Li Y et al (2017) SIRT1/PGC-1alpha signaling promotes mitochondrial functional recovery and reduces apoptosis after intracerebral hemorrhage in rats. Front Mol Neurosci 10:443
Li XJ, Jiang ZZ, Zhang LY (2014) Triptolide: progress on research in pharmacodynamics and toxicology. J Ethnopharmacol 155(1):67–79
Liu Q (2017) Triptolide and its expanding multiple pharmacological functions. Int Immunopharmacol 11(3):377–383
Hao M, Li X, Feng J et al (2015) Triptolide protects against ischemic stroke in rats. Inflammation 38(4):1617–1623
Li R, Lu K, Wang Y et al (2017) Triptolide attenuates pressure overload-induced myocardial remodeling in mice via the inhibition of NLRP3 inflammasome expression. Biochem Biophys Res Commun 485(1):69–75
Zhang YQ, Shen Y, Liao MM et al (2019) Galactosylated chitosan triptolide nanoparticles for overcoming hepatocellular carcinoma: enhanced therapeutic efficacy, low toxicity, and validated network regulatory mechanisms. Nanomedicine 15(1):86–97
Lu Y, Bao X, Sun T et al (2012) Triptolide attenuate the oxidative stress induced by LPS/D-GalN in mice. J Cell Biochem 113(3):1022–1033
Nie J, Zhou M, Lu C et al (2012) Effects of triptolide on the synaptophysin expression of hippocampal neurons in the AD cellular model. Int Immunopharmacol 13(2):175–180
Cheng S, Leblanc KJ, Li L (2014) Triptolide preserves cognitive function and reduces neuropathology in a mouse model of Alzheimer’s disease. PLoS ONE 9(9):e108845
Hajdukova L, Sobek O, Prchalova D et al (2015) Biomarkers of brain damage: S100B and NSE concentrations in cerebrospinal fluid—a normative study. Biomed Res Int 2015:379071
Chen Y, Guo Z, Peng X et al (2018) Nimodipine represses AMPK phosphorylation and excessive autophagy after chronic cerebral hypoperfusion in rats. Brain Res Bull 140:88–96
Farkas E, Luiten PG, Bari F (2007) Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54(1):162–180
Ma X, Xu W, Zhang Z et al (2017) Salvianolic acid B ameliorates cognitive deficits through IGF-1/Akt pathway in rats with vascular dementia. Cell Physiol Biochem 43(4):1381–1391
Dias IH, Polidori MC, Griffiths HR (2014) Hypercholesterolaemia-induced oxidative stress at the blood-brain barrier. Biochem Soc Trans 42(4):1001–1005
Stamatovic SM, Martinez-Revollar G, Hu A et al (2019) Decline in sirtuin-1 expression and activity plays a critical role in bloodbrain barrier permeability in aging. Neurobiol Dis 126:105-116.
Zheng CX, Chen ZH, Zeng CH et al (2008) Triptolide protects podocytes from puromycin aminonucleoside induced injury in vivo and in vitro. Kidney Int 74(5):596–612
Fan D, He X, Bian Y et al (2016) Triptolide modulates TREM-1 signal pathway to inhibit the inflammatory response in rheumatoid arthritis. Int J Mol Sci 17(4):498
Aguirre-Rueda D, Guerra-Ojeda S, Aldasoro M et al (2015) Astrocytes protect neurons from Abeta1-42 peptide-induced neurotoxicity increasing TFAM and PGC-1 and decreasing PPAR-gamma and SIRT-1. Int J Med Sci 12(1):48–56
He XL, Wang YH, Bi MG et al (2012) Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur J Pharmacol 680(1–3):41–48
Kwon KJ, Lee EJ, Kim MK et al (2015) Diabetes augments cognitive dysfunction in chronic cerebral hypoperfusion by increasing neuronal cell death: implication of cilostazol for diabetes mellitus-induced dementia. Neurobiol Dis 73:12–23
Kim MS, Bang JH, Lee J et al (2015) Salvia miltiorrhiza extract protects white matter and the hippocampus from damage induced by chronic cerebral hypoperfusion in rats. BMC Complement Altern Med 15:415
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81560193), Science and Technology Research Project of Education Department of Jiangxi Province (No. GJJ180019), Science and Technology Research Project of Health and Health committee Department of Jiangxi Province (20195231).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yao, P., Li, Y., Yang, Y. et al. Triptolide Improves Cognitive Dysfunction in Rats with Vascular Dementia by Activating the SIRT1/PGC-1α Signaling Pathway. Neurochem Res 44, 1977–1985 (2019). https://doi.org/10.1007/s11064-019-02831-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02831-3